Engineered biomimetic nanoabsorbent for cellular detoxification of chemotherapeutics†
Abstract
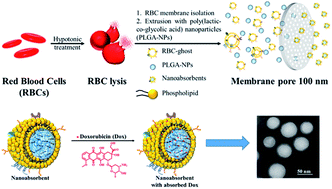
An approach to reduce the nonspecific cytotoxicity of chemotherapeutics has been put-forth using a biomimetic nanoabsorbent (NAb) as a detoxifying agent. The engineered NAb possesses a tunable drug absorption ability depending on the charge of the absorbing molecule, in which the cooperative absorption ability of the core and shell significantly reduces the cellular toxicity.

 Please wait while we load your content...
Please wait while we load your content...