Epitaxial growth and nanoscale electrical properties of Ce2Ti2O7 thin films†
Abstract
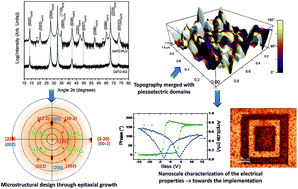
(00l) epitaxial Ce2Ti2O7 thin films with a layered perovskite/monoclinic structure were grown on (110)-oriented Nb-doped SrTiO3 substrates via pulsed laser deposition and a sol–gel method associated with spin-coating. Using the sol–gel method, the Ce2Ti2O7 films were obtained by annealing at 950 °C under a reductive Ar/H2 atmosphere. Employing the pulsed laser deposition technique, they were directly grown under vacuum (10−6 mbar) with a controlled re-oxidation during the cooling step. The pole figure measurements provide the in-plane crystallographic relationships between the film and substrate: [001]SrTiO3//[100]Ce2Ti2O7 and [1−10]SrTiO3//[010]Ce2Ti2O7. Piezoresponse force microscopy measurements highlight the local ferroelectric character of the films synthetized. The switching capability was more reliable for the film grown via pulsed laser deposition, which was explained by the lower mosaic spread. Higher local conductivity was also detected using conductive-atomic force microscopy of the physically deposited film and was attributed to its lower thickness. Such epitaxially deposited functional oxides may be considered as promising candidates for integration into advanced electronic devices.

 Please wait while we load your content...
Please wait while we load your content...