Toxicity assessment of precise engineered gold nanoparticles with different shapes in zebrafish embryos†
Abstract
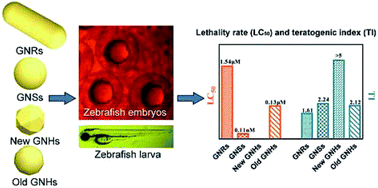
Gold nanoparticles demonstrate developmental toxicity in a shape dependent manner. In a zebrafish model, gold nanospheres exhibit more toxicity when compared with nanorods and nanopolyhedrons. After storage, nanopolyhedrons elicit obvious lethality. We believe that the results from this investigation could be used to track the toxicity of living organisms.

 Please wait while we load your content...
Please wait while we load your content...