A poly(2-(dimethylamino)ethyl methacrylate-co-methacrylic acid) complex induced route to fabricate a super-hydrophilic hydrogel and its controllable oil/water separation
Tingting Li,
Jie Shen,
Zheng Zhang,
Sui Wang* and
Danyi Wei
Faculty of Materials Science and Chemical Engineering, State Key Laboratory Base of Novel Functional Materials and Preparation Science, Ningbo University, Ningbo 315211, P. R. China. E-mail: wjydjy2011@163.com; Fax: +86-574-87609987; Tel: +86-574-87600798
First published on 11th April 2016
Abstract
Smart hydrogels, or stimulus–responsive (SR) hydrogels which are composed of three-dimensional networks with crosslinked hydrophilic polymer chains can display dramatic volume changes in response to external environments, such as temperature, pH and certain stimuli. Controllable and significant responses to environmental stimuli and super-hydrophilicity are crucial for versatility in terms of oil/water separation, especially. Thermo and pH dual-controllable oil/water separation materials are successfully fabricated by free radical polymerization of 2-(dimethylamino)ethyl methacrylate (DMAEMA) and methacrylic acid (MAA). The substrate of the stainless steel mesh was coated with the super-hydrophilic hydrogel film by simple immersion in an aqueous solution. The as-prepared mesh showed high hydrophobicity with an oil contact angle of about 151° underwater. More importantly, it can be used to separate oil/water mixtures like silicone oil. As shown, water can pass through the processed mesh in temperatures of less than 55 °C (pH 7) and pH values under pH 13 (T = 25 °C) when oil is intercepted on the mesh. Otherwise, the water retention capacity of the hydrogel is significantly reduced. The separation efficiency (98.35%) remains high after 15 uses and the mesh can be easily cleaned, stored, and reused. These properties render such thin films useful for applications to achieve various functional separation materials.
Introduction
Smart hydrogels are able to dramatically swell or shrink, namely, changing their volume in response to external stimuli, such as temperature, ion strength, pH, light, electricity, magnetism and chemical substances.1–6 Such hydrogels, which set perception, driver and information processing at an organic whole, are developed with functional materials of the same category like an organism with intelligent properties. Responsive dendrimers also have a myriad of potential applications in many other fields, including smart components for information storage,7 optical fiber biosensors,5 scaffolds for tissue engineering,8,9 carriers for protein immobilization or recognition,10–13 matrices for biomolecules or cell separation as well as vehicles for drug delivery.14–17 Rapid and prominent responses to environmental stimuli and hydrophilicity are crucial objectives for versatility on account of remarkable and lasting feedback after collecting incoming signals.18 It is noteworthy that DMAEMA has been chosen as a superior candidate for fabricating smart hydrogels because of its hydrophilicity, biocompatibility, biodegradability, low toxicity and adhesiveness. The chemical synthesis procedure of conventional hydrogels, nevertheless, is usually complicated in terms of using physical separation. For instance, it is reported that some representative amphiphilic macromolecules are chosen to fabricate pH-sensitive surfaces by adjusting the pH including using a kind of chitosan hydrogel or poly(acrylic acid) (PAA) hybrid to show excellent encapsulation efficiency with reversibly switchable wettability.2,15,19–21 Amine-containing conjugated polymers have been comprehensively investigated as water purification agents because of their plentiful functional groups and powerful coordination ability.22–25Oil/water separation has been an urgent and necessary issue due to oil spill accidents in maritime transportation, wastewater brought to the surface during oil drilling and refinery wastewater from oil industry products.26,27 To solve this problem, it is significant to develop emerging membrane-making techniques and advanced controllable separation materials to improve the permeability and selectivity, depending on their outstanding properties. Various approaches for active preparation such as chemical cross-linking, interfacial polymerization, modification with polymeric additives, and UV photo-grafting28–35 have been developed to compound the smart responsive separation materials. More and more smart hydrogels have been committed to enhancing oil removal efficiency, decreasing surface energy and simplifying separation devices.36–38 However, to date, few works have reported smart double stimuli–responsive controllable separation materials.
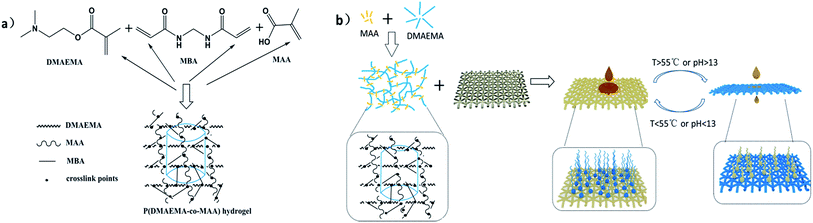
In this present research, we propose a novel and simple gelation route to synthesize a poly(2-(dimethylamino)ethyl methacrylate)-co-methacrylic acid p(DMAEMA-co-MAA) hydrogel based on free radical polymerization. It contains DMAEMA monomer, MAA as the cross-linking monomer, N,N′-methylene bisacrylamide (MBA) as the chemical crosslinker, and potassium persulfate (K2S2O8) as the initiator. The stainless steel mesh, as a porous substrate, was dip-coated with a mixed pregel solution. Moreover, the composites can exhibit a superoleophilic–superhydrophobic characteristic on the surface under alkaline conditions at room temperature. PDMAEMA, as a new type of intelligent hydrogel, has broad development prospects owing to its thermo and pH dual-responsive characteristic. Two groups including hydrophilic tertiary amine groups, carbonyl groups and hydrophobic alkyl groups match with each other on the spatial structure. Because of this, PDMAEMA gradually came to be the most preferred SR polymer in the separation system. The polymers can consequentially turn from relative stretches in the state of random strings into water-insoluble particles in aqueous solution when the temperature exceeds the lower critical solution temperature (LCST) of PDMAEMA which ranges from 30 to 50 °C with different pH, molecular weight, salt concentration and so on. It is worth stressing that the varying protonation degree of the tertiary amino group will induce a change in the hydrophilic–lipophilic balance and the phase transition temperature of PDMAEMA under the conditions of varying pH value.
Our smart hydrogels show an extraordinary large response swelling ratio, rapid response rate and excellent selectivity underwater as required for liquid–liquid separation. The ultrafiltration coating-modified stainless steel mesh surface exhibits high affinity to water but not to oils at less than 55 °C for rapid and efficient oil–water separation. In addition, reversible switching between high hydrophilicity at pH 3 and high hydrophobicity at pH 13 can be well realized. Oil can permeate through the as-prepared mesh because the higher temperature or pH cannot give rise to influence on a better water retention capacity and the swelling volume of p(DMAEMA-co-MAA) hydrogels. Since this dual-responsive property is a result of the combined effect of the chemical variation of the surface and the surface roughness, water can be persistently collected through the mesh by adjusting temperature and pH value.
Experimental
Materials and instruments
2-(Dimethylamino)ethyl methacrylate (DMAEMA, 99%) and N,N′-methylene bisacrylamide (MBA, 97%), provided by Aladdin industrial corporation (Shanghai, China) were used as received. Methacrylic acid (MAA, ≥98%) and potassium persulfate (K2S2O8) were purchased from Sinopharm Chemical Reagent Com, Ltd (China). The stainless steel mesh with pore diameter of 38.5 μm (400 mesh size) was obtained from Taizhou chemical apparatus factory (China). All agents and solvents were used directly without further purification. Ultrapure water was used throughout all the experiments.Photo images were obtained using a camera (Canon EOS 600D). SEM images of the p(DMAEMA-co-MAA) hydrogel-coated mesh were collected via a field-emission scanning electron microscope (S4800, Japan). The contact angles of the superhydrophobic surfaces underwater or in air were measured on an OCA machine (DSA100, Germany) at ambient temperature. The average value of three measured results was adopted at different positions within the same sample. TGA analysis was performed using a TA Instrument (TG/DTA 7300) under a nitrogen atmosphere (air pressure 200) at a heating rate of 10 °C min−1 from ambient temperature to 130 °C and kept isothermal for 5 min. Fourier transform infrared spectroscopy (FT-IR) was employed at a resolution of 4 cm−1. The rheological studies were measured on a modular advanced rheometer system at 25 °C (HAAKE MARS, Germany).
The absorption property was also evaluated by calculating the swelling ratio. Firstly, the weight of dried hydrogel was measured. And then the dried hydrogel was immersed in the various PBS buffers until the weight was definite. The swelling ratio of the hydrogel was calculated according to eqn (1):
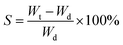 | (1) |
The as-prepared mesh demonstrated super-hydrophobicity and super-hydrophilicity simultaneously. Utilizing these properties, a setup was designed using the mesh to filter water down through to a collector and detain oil, realizing continuous oil/water mixture (30 v/v%) separation. Mixtures of silicone oil/water were separated. The separation efficiency was recorded via determining the concentration of oil before and after separation. The filtration interception coefficient (I(%)) was calculated according to eqn (2):
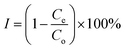 | (2) |
Preparation of the p(DMAEMA-co-MAA) mesh
The stainless steel mesh used as the substrate was firstly cleaned. DMAEMA solution and MAA as the cross-linking monomer (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by mol) were stirred at ambient temperature for 2 h. MBA solution, subsequently, as the cross-linker (1% by monomer mass) was added and then stirred for 4 h to form a homogeneous solution. The initiator K2S2O8 (0.5% by monomer mass) dissolved in deionized water was then added to the sample dropwise. The stainless steel mesh was immediately immersed in the mixed solution and then directionally taken out with sticky solution adhered on the surface of the steel wires, while forming the pregel. Under the protection of nitrogen in the whole experiment, the formed gel was obtained on the treated mesh at 70 °C for 6 h by free radical polymerization.
1 by mol) were stirred at ambient temperature for 2 h. MBA solution, subsequently, as the cross-linker (1% by monomer mass) was added and then stirred for 4 h to form a homogeneous solution. The initiator K2S2O8 (0.5% by monomer mass) dissolved in deionized water was then added to the sample dropwise. The stainless steel mesh was immediately immersed in the mixed solution and then directionally taken out with sticky solution adhered on the surface of the steel wires, while forming the pregel. Under the protection of nitrogen in the whole experiment, the formed gel was obtained on the treated mesh at 70 °C for 6 h by free radical polymerization.
Results and discussion
The preparation of p(DMAEMA-co-MAA) hydrogel
Fig. 1 shows the macroscopic images of the pregel solution (A) and p(DMAEMA-co-MAA) hydrogel (B). In a typical synthesis, MAA and DMAEMA were stirred well in the solution and reacted sufficiently, producing the pregel solution. DMAEMA and MAA can form the polymers. This feature will be discussed in this section. The potassium persulfate solution was cautiously added, dropwise, into the pregel hydrogel solution. Subsequently, gel quickly coated the stainless steel mesh. The preparation of p(DMAEMA-co-MAA) hydrogel may yield just the three-dimensional network structure. And the possible mechanism involved is discussed in Scheme 1. | ||
| Fig. 1 Digital images of the pregel solution (A) and p(DMAEMA-co-MAA) hydrogel (B) were obtained using a camera. | ||
Swelling kinetics
It is known that DMAEMA and MAA exhibit pH dependent swelling behaviour because they consist of ionisable groups. This feature was also observed during the swelling experiments in this study at both temperatures (Fig. 2b). The pKa values of DMAEMA and MAA are about 7.3 and 5.4, respectively. It is evident that the swelling ratios are changed apparently, that is, at pH < 6.6 or pH > 8.0 (Fig. 2a). From the results, it is clear that the shift comes after the neutralization of the MAA segment and before the deprotonation of the DMAEMA segment. On the basis of the chemical structures and observed phenomena described above, the swelling values are decreased at low pH (below pH 6.6) since the –COO– groups interact with the protonated DMAEMA segment through electrostatic attraction, giving rise to intermolecular complexation via hydrogen bonds. At pH ranges of 6.6–8.0, the –COOH groups of the MAA segment are gradually neutralized. Besides, the protonation degree of the tertiary amine group in PDMAEMA decreases and the electrostatic balance is destroyed. Beyond pH 8.0, the electrostatic repulsive force between the charged sites causes a decrease in swelling. | ||
| Fig. 2 Equilibrium swelling ratios of pH-sensitive (a) and temperature-sensitive (b) p(DMAEMA-co-MAA) hydrogels. | ||
Poly(2-(dimethylamino)ethyl methacrylate) smart hydrogel is a typical temperature-sensitive hydrogel exhibiting a volume phase transition at about its LCST. Fig. 2b illustrates the temperature dependence of the equilibrium swelling ratio of the gel in various buffers (pH 7.0) when the temperature is increased from 20 to 70 °C. The equilibrium swelling ratio of the gel increased drastically up to 50 °C. Hydrogen bonds exist between water molecules and monomeric units in a swollen hydrogel. When the temperature increases above the LCST, the equilibrium swelling ratio decreases, owing to the interactions of the groups, and the hydrogel collapses.
Mesh wettability
The surface wettability of the p(DMAEMA-co-MAA) mesh has been characterized by contact angle measurement. Fig. 3A displays the shape of a silicone oil droplet with oil red O staining on the as-prepared mesh in a water environment, and in this case the oil contact angle is about 151.8 ± 0.6°. The oil contact angle in air is about 19.4 ± 0.4° (3B). Water can be absorbed by the hydrogel and trapped in the structure, resulting in great decrease of the contact area between the oil droplet and the mesh. In consequence, the coated mesh shows superhydrophobicity underwater and superoleophobic properties in the oil/water/solid three-phase system. This unique phenomenon of the mesh exhibiting both properties results in the oil/water separation capability.Mesh morphology
The microstructure of the stainless steel mesh and the mesh coated by p(DMAEMA-co-MAA) hydrogel was characterized by microscopy technology (Fig. 4). The SEM image in Fig. 4a shows the blank stainless steel mesh and the SEM image (Fig. 4b) of the as-prepared mesh shows very uniform coating. The freeze-dried hydrogel coating exhibited a wave shape which made the base film much denser and showed a randomly cross-linked network after the removal of the trapped water molecules (Fig. 4b and c). At the same time, Fig. 4d demonstrates that the p(DMAEMA-co-MAA) hydrogel coatings cover the steel uniformly and the hydrogel exists compactly in the pores of the mesh which has less influence on the passage of water through the as-prepared mesh.FT-IR spectra of hydrogels
We have reported on the synthesis of copolymers with reversible micellization properties of p(DMAEMA-co-MAA) hydrogel. PDMAEMA is a weak polybase, which can interact with anionic substances, such as PMAA, by electrostatic attraction at low pH values. However, the DMAEMA could be protonated at low pH, making this segment hydrophilic, whereas the MAA will be ionized and becomes soluble at high pH, because both have different pKa values and different chain lengths.1 The molecular structures of DMAEMA, MAA and freeze-dried hydrogel samples were characterized by FT-IR, as shown Fig. 5a.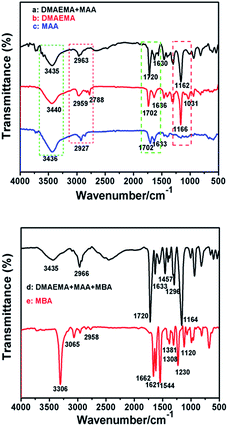 | ||
| Fig. 5 FT-IR spectra of DMAEMA-co-MAA (a), DMAEMA (b), MAA (c), hydrogel complex p(DMAEMA-co-MAA) (d) and MBA (e). | ||
Six typical bands were observed for MAA and DMAEMA, namely, O–H (∼3436 cm−1), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (∼1737 cm−1), C
O (∼1737 cm−1), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (∼1633 cm−1), C–N (∼1031 cm−1), C–O (∼1166 m−1) and N–H (∼3307 cm−1). The wide peak at 3422 cm−1 is the characteristic peak of –COOH. The peak at ∼2959 cm−1 and at ∼2778 cm−1 corresponds to methyl and methylene vibrations. The strong peak at ∼1166 cm−1 features asymmetric vibrations of C–O–C. Compared with curve b and c (presenting DMAEMA and MAA respectively), the attenuation of the peak at ∼1633 cm−1 assigned to C
C (∼1633 cm−1), C–N (∼1031 cm−1), C–O (∼1166 m−1) and N–H (∼3307 cm−1). The wide peak at 3422 cm−1 is the characteristic peak of –COOH. The peak at ∼2959 cm−1 and at ∼2778 cm−1 corresponds to methyl and methylene vibrations. The strong peak at ∼1166 cm−1 features asymmetric vibrations of C–O–C. Compared with curve b and c (presenting DMAEMA and MAA respectively), the attenuation of the peak at ∼1633 cm−1 assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in curve a indicates that the copolymerization of DMAEMA and MAA has taken place. Fig. 5d shows the micellar structure of the polymer. The peak intensity at ∼3307 cm−1 is assigned to the strong stretching vibration of N–H. Except for the typical peaks of MBA (Fig. 5e), Fig. 5d exhibited disappearing peak intensity at ∼3306 cm−1 for the vibration of N–H, suggesting that functional groups have reacted with each other. As predicted, the hydrogel peaks at ∼1720 cm−1, ∼1633 cm−1, ∼1456 cm−1 and ∼1296 cm−1 increased. These results show that peak signal heights were enhanced, which is probably because the polymer-based material MBA contains O–H active groups which react with the N–H groups to form cross-linked bonds, which influences the material’s formation into a networked structure.
C in curve a indicates that the copolymerization of DMAEMA and MAA has taken place. Fig. 5d shows the micellar structure of the polymer. The peak intensity at ∼3307 cm−1 is assigned to the strong stretching vibration of N–H. Except for the typical peaks of MBA (Fig. 5e), Fig. 5d exhibited disappearing peak intensity at ∼3306 cm−1 for the vibration of N–H, suggesting that functional groups have reacted with each other. As predicted, the hydrogel peaks at ∼1720 cm−1, ∼1633 cm−1, ∼1456 cm−1 and ∼1296 cm−1 increased. These results show that peak signal heights were enhanced, which is probably because the polymer-based material MBA contains O–H active groups which react with the N–H groups to form cross-linked bonds, which influences the material’s formation into a networked structure.
Thermogravimetric analysis
Subsequently, thermogravimetric analysis (TGA) spectra of p(DMAEMA-co-MAA) hydrogel-coated mesh samples were given in Fig. 6 to confirm the difference in ability of holding water. As shown in the full range spectra (Fig. 6a), the water content of the coated mesh from a 25 °C water bath is approximately 29.2%, whereas it is only 6.4% in the mesh from a 55 °C water bath. For comparison, as shown in the full range spectra (Fig. 6b), the values are 44% from a pH 3 HCl solution and just 0.6% from a pH 13 NaOH solution. What's more, some swelling property tests of columnar hydrogels have been carried out. The hydrogels have much better stimuli–responsive properties than normal hydrogels; however, their thermo-responsive equilibrium swelling ratio is still limited, for example, the thermo-responsive equilibrium swelling ratio in response to a temperature change from 55 °C to 25 °C is typically >600%.Rheological study
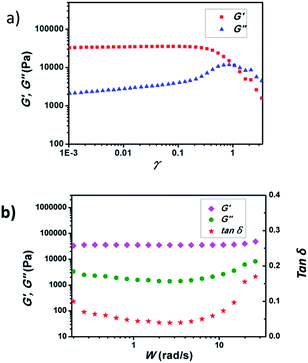
Before separation applications, the mechanical properties of the hydrogel were investigated. Fig. 7a shows the variation of the storage modulus (G′) and loss modulus (G′′) of the p(DMAEMA-co-MAA) hydrogel as a function of the strain amplitude γ from 0.0001–10. At γ < 1.0, G′ is much greater than G′′, indicating the formation of a viscoelastic hydrogel at 25 °C. The polymer gel showed an initial linear region. G′ and G′′ increase slightly with increasing strain amplitude, forming a plateau in the profile. Increasing the strain amplitude leads to gradual decrease of G′ and eventual crossover with G′′ and has a yield point. At γ = 1.0, the yield stress of the gel was measured as about 6 kPa. After the G′ and G′′ reach their maximum points, the values dramatically decrease with increasing γ values, which may be attributed to network dissociation. This is usually observed in systems with fast dissociation of the dynamic cross-links. Fig. 7b shows the ω dependence of the G′, G′′ and damping factor (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) at 25 °C obtained by performing a dynamic frequency sweep. In the linear viscoelastic region, the value of the dynamic G′ of the gel was larger than that of its corresponding dynamic G′′, indicating the formation of a typical soft solid-like gel-phase material. The hydrogel exhibited a G′ of >30
δ) at 25 °C obtained by performing a dynamic frequency sweep. In the linear viscoelastic region, the value of the dynamic G′ of the gel was larger than that of its corresponding dynamic G′′, indicating the formation of a typical soft solid-like gel-phase material. The hydrogel exhibited a G′ of >30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Pa, suggesting that the sample was of good mechanical strength. The mechanical strength of the gel increased, probably due to the interactions induced by the DMAEMA molecules. In the frequency scanning curve, its tan
000 Pa, suggesting that the sample was of good mechanical strength. The mechanical strength of the gel increased, probably due to the interactions induced by the DMAEMA molecules. In the frequency scanning curve, its tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ is close to 0.1, showing that the gel has a well developed network structure.
δ is close to 0.1, showing that the gel has a well developed network structure.
Separation of oil and water
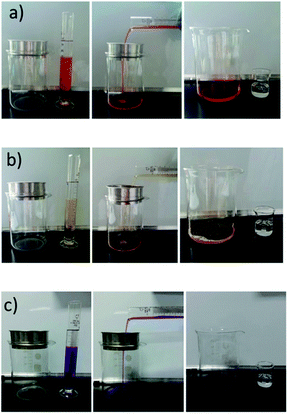
A series of proof-of-concept studies were carried out to test the oil/water separation capacity. The PDMAEMA-coated mesh shows different wettability with oil at different temperature and pH values because PDMAEMA is a thermo- and pH-sensitive polymer. The oil/water separation experiment procedure was performed as shown in Fig. 8a and b. The mesh was placed under a beaker. On pouring the mixture of silicone oil and water (dyed by oil red), water permeated though the mesh quickly with the driving force of gravity, while silicone oil was retained and kept in the upper mesh. The water was collected in the beaker for further analysis after the separation. The composition and environmental temperature greatly affect the separation properties of our hydrogels. When PDMAEMA reached its LCST, the swelling volume of the hydrogels was significantly decreased and the coated mesh's water retention capacity was greatly reduced. After separation, there is visible oil (dyed by oil red) in the water sample at high temperature in contrast to the sample at room temperature. Therefore, it turned out that the as-prepared mesh can selectively separate water from oil/water mixtures at room temperature and retain oil by just adjusting the temperature. | ||
| Fig. 8 The application performance of the temperature-controllable oil/water separation. (a and b) The as-coated mesh was placed under a beaker and a mixture of silicone oil and water (25 °C and 55 °C, dyed by oil red) was mixed well. As the mixture was poured into the as-coated mesh, water passed through quickly, but the oil was repelled in the upper mesh. In Fig. 8b, there is visible oil (dyed by oil red) in the water sample at high temperature in contrast to Fig. 8a, when poured into the as-coated mesh. The separations were finished and water samples were collected in the beaker below for further analysis. | ||
A series of oil/water mixtures with different pH values were made-up to evaluate the pH-responsivity. The aqueous solution was dyed by cresol red, which displays orange in about pH 3, yellow between 3.0 and 8.0, and purple above 8.0. Water can permeate the as-coated mesh at express speed, but the silicone oil was repelled in the upper mesh (Fig. 9a and b). Nevertheless, the alkali solution with silicone oil passed through the mesh at first separation (Fig. 9c). After separation, there is visible oil in the water sample in contrast to Fig. 9a and b. The water content of the coated mesh is about 44% in acidic conditions (silicone oil/HCl solution), which rises far above that in alkaline conditions, only 0.06% (silicone oil/NaOH solution). The transition corresponds to the protonation degree of the tertiary amine group in the hydrogel, decreasing from low pH to high pH conditions. This further confirmed that the preparative hydrogel-coated mesh is pH-responsive.
Mixtures of silicone oil/water, as an example, were also successfully separated with high efficiency. Almost no visible oil existed in the water after the separation, and the content of oil was assayed using an infrared spectrometer oil content analyzer. The separation efficiency of the as-prepared mesh is above 98.35% after a series of reuses, as shown in Fig. 10. The separation efficiency of silicone oil after 15 uses is a little lower than the first separation (98.47 ± 0.22%). In addition, the as-prepared meshes can be easily cleaned and stored for reuse. The meshes maintain high separation efficiency after 15 uses when using the silicone oil/water mixture as an example.
 | ||
| Fig. 10 The meshes maintain the high separation efficiency after 15 uses when using the silicone oil/water mixture as an example. | ||
Conclusions
As far as we are aware, an oil/water separation mesh has been successfully fabricated by combining DMAEMA-surface chemistry and polymerization reactions. It is shown that the activity difference with different temperature or pH values can give rise to different separation efficiencies in the networks. The as-prepared underwater superhydrophobic and superoleophobic mesh can selectively separate water from oil/water mixtures with high efficiency, and the mesh can be easily cleaned, stored, and reused. Responsible materials for controllable oil/water separation can also be realized by means of simultaneously conjugating two kinds of substances with different wettability with a p(DMAEMA-co-MAA) coated mesh. This modified material is an excellent candidate to be used in practical applications due to its thermo and pH dual-responsive properties and biological compatibility, making it more intelligent and diversified.Acknowledgements
This research is partly supported by National Natural Science Foundation of China (No. 81273130, 41576098), Zhejiang Provincial Natural Science Foundation of China (LY13B070013), the Science and Technology Plan Project of Ningbo City (2012C50043), and K. C. Wong Magna Fund in Ningbo University, P. R. China.References
- S. Dai, P. Ravi, K. C. Tam, B. W. Mao and L. H. Gang, Langmuir, 2003, 19, 5175–5177 CrossRef CAS.
- P. K. Jha, P. S. Desai, J. Y. Li and R. G. Larson, Polymers, 2014, 6, 1414–1436 CrossRef CAS.
- Y. Cao, N. Liu, C. Fu, K. Li, L. Tao, L. Feng and Y. Wei, ACS Appl. Mater. Interfaces, 2014, 6, 2026–2030 CAS.
- L. Hu, S. Gao, X. Ding, D. Wang, J. Jiang, J. Jin and A. L. Jiang, ACS Nano, 2015, 9, 4835–4842 CrossRef CAS PubMed.
- M. Tamura, F. Yanagawa, S. Sugiura, T. Takagi, K. Sumaru, H. Matsui and T. Kanamori, Sci. Rep., 2014, 4, 254 Search PubMed.
- P. Calvo-Marzal, M. P. Delaney, J. T. Auletta, T. Q. Pan, N. M. Perri, L. M. Weiland, D. H. Waldeck, W. W. Clark and T. Y. Meyer, ACS Macro Lett., 2012, 1, 204–208 CrossRef CAS.
- J. Cui, M. A. Lackey, A. E. Madkour, E. M. Saffer, D. M. Griffin, S. R. Bhatia, A. J. Crosby and G. N. Tew, Biomacromolecules, 2012, 13, 584–588 CrossRef CAS PubMed.
- K. Y. Lee and D. J. Mooney, Chem. Rev., 2001, 101, 1868–1879 Search PubMed.
- Y. P. Hou, C. A. Schoener, K. R. Regan, D. Munoz-Pinto, M. S. Hahn and M. A. Grunlan, Biomacromolecules, 2010, 11, 648–656 CrossRef CAS PubMed.
- M. Ramirez, D. Guan, V. Ugaz and Z. Chen, J. Am. Chem. Soc., 2013, 135, 5290–5293 CrossRef CAS PubMed.
- N. Adrus and M. Ulbricht, Polymer, 2012, 53, 4359–4366 CrossRef CAS.
- X.-W. H. Lei Qin, W. Zhang, W.-Y. Li and Yu-K. Zhang, Anal. Chem., 2009, 81, 7206–7216 CrossRef PubMed.
- A. Nematollahzadeh, P. Lindemann, W. Sun, J. Stute, D. Lutkemeyer and B. Sellergren, J. Chromatogr. A, 2014, 1345, 154–163 CrossRef CAS PubMed.
- S. Gupta, R. G. Alargova, P. K. Kilpatrick and O. D. Velev, Langmuir, 2010, 26, 3441–3452 CrossRef CAS PubMed.
- R. Jankaew, N. Rodkate, S. Lamlertthon, B. Rutnakornpituk, U. Wichai, G. Ross and M. Rutnakornpituk, Polym. Test., 2015, 42, 26–36 CrossRef CAS.
- K. Zhu, T. Ye, J. Liu, Z. Peng, S. Xu, J. Lei, H. Deng and B. Li, Int. J. Pharm., 2013, 441, 721–727 CrossRef CAS PubMed.
- P. Li, Y. Wang, Z. Peng, F. She and L. Kong, Carbohydr. Polym., 2011, 85, 698–704 CrossRef CAS.
- L. W. Xia, R. Xie, X. J. Ju, W. Wang, Q. Chen and L. Y. Chu, Nat. Commun., 2013, 4, 375–381 Search PubMed.
- M. H. Hsiao, K. H. Lin and D.-M. Liu, Soft Matter, 2013, 9, 2458–2466 RSC.
- O. D'Agostini-Junior, C. L. Petkowicz, A. G. Couto, S. F. de Andrade and R. A. Freitas, Mater. Sci. Eng., C, 2011, 31, 677–682 CrossRef.
- X. Jin and Y.-L. Hsieh, Polymer, 2005, 46, 5149–5160 CrossRef CAS.
- X. G. Li, M. R. Huang, W. Duan and Y. L. Yang, Chem. Rev., 2002, 102, 2925–3030 CrossRef CAS PubMed.
- L. Zhang, L. Chai, J. Liu, H. Wang, W. Yu and P. Sang, Langmuir, 2011, 27, 13729–13738 CrossRef CAS PubMed.
- L. Zhang, T. Wang, H. Wang, Y. Meng, W. Yu and L. Chai, Chem. Commun., 2013, 49, 9974–9976 RSC.
- L. Y. Chai, T. Wang, L. Y. Zhang, H. Y. Wang, W. C. Yang, S. Dai, Y. Meng and X. R. Li, Carbon, 2015, 81, 748–757 CrossRef CAS.
- L. Feng, Z. Zhang, Z. Mai, Y. Ma, B. Liu, L. Jiang and D. Zhu, Angew. Chem., 2004, 43, 2012–2014 CrossRef CAS PubMed.
- A. Asatekin and A. M. Mayes, Environ. Sci. Technol., 2009, 43, 4487–4492 CrossRef CAS PubMed.
- F. Schacher, T. Rudolph, F. Wieberger, M. Ulbricht and A. H. Muller, ACS Appl. Mater. Interfaces, 2009, 1, 1492–1503 CAS.
- Y. Cao, X. Zhang, L. Tao, K. Li, Z. Xue, L. Feng and Y. Wei, ACS Appl. Mater. Interfaces, 2013, 5, 4438–4442 CAS.
- H. Y. Wang, E. Q. Wang, Z. J. Liu, D. Gao, R. X. Yuan, L. Y. Sun and Y. J. Zhu, J. Mater. Chem. A, 2015, 3, 266–273 CAS.
- R. Du and J. Zhao, J. Membr. Sci., 2004, 239, 183–188 CrossRef CAS.
- J. R. Du, A. Chakma and X. Feng, Sep. Purif. Technol., 2008, 64, 63–70 CrossRef CAS.
- A. Pagidi, R. Saranya, G. Arthanareeswaran, A. F. Ismail and T. Matsuura, Desalination, 2014, 344, 280–288 CrossRef CAS.
- R. D. Suo-Hong Zhi, J. Xu and L.-S. Wan, React. Funct. Polym., 2015, 86, 184–190 CrossRef.
- X.-L. Li, L.-P. Zhu, Y.-Y. Xu, Z. Yi and B.-K. Zhu, J. Membr. Sci., 2011, 374, 33–42 CrossRef CAS.
- S. Y. Shi, J.-Y. Xu, H.-Q. Sun, J. Zhang, D.-H. Li and Y.-X. Wu, Chem. Eng. Res. Des., 2012, 90, 1652–1659 CrossRef CAS.
- C. H. Xue, P. T. Ji, P. Zhang, Y. R. Li and S. T. Jia, Appl. Surf. Sci., 2013, 284, 464–471 CrossRef CAS.
- B. X. Jing, H. T. Wang, K. Y. Lin, P. J. McGinn, C. Z. Na and Y. X. Zhu, Polymer, 2013, 54, 5771–5778 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |