Synthesis and photoluminescence modulating of polypyrrole fluorescent nano-spheres/dots†
Ben Dong,
Mei Yang,
Shusheng Ge,
Yi Cao,
Baoyan Li and
Yun Lu*
Department of Polymer Science and Engineering, State Key Laboratory of Coordination Chemistry, Collaborative Innovation Center of Chemistry for Life Sciences, Key Laboratory of High Performance Polymer Materials and Technology (Nanjing University), Ministry of Education, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093, P. R. China. E-mail: yunlu@nju.edu.cn
First published on 25th February 2016
Abstract
Polypyrrole (PPy) nanospheres with an average diameter of 20 nm were successfully synthesized by simply using 3-chloroperbenzoic acid in pure ethanol. As-prepared PPy nanospheres showed a moderate photoluminescence signal with a quantum yield (QY) of 2.2% and excitation-dependent photoluminescence characteristics. To carry out the PPy nanosphere surface functionalization, PEG2000, 4,7,10-trioxa-1,13-tridecanediamine and ethylenediamine severed respectively as surface passivating agents, and accordingly 5.5 nm, 4.5 nm and 3 nm sized nanodots were obtained, which exhibited enhanced fluorescence intensity with maximum QYs of 3.1%, 13% and 40% respectively and tunable band gaps in the range of 0.78–1.53 eV. These results were attributed to the increased electron density in PPy fluorescent nanospheres/dots. These resultant PPy fluorescent nanodots could be appropriately applied as cell-imaging agents, fluorescent inks and pH sensors owing to their outstanding characteristics of low cytotoxicity, good biocompatibility and high luminescence stability.
1 Introduction
Highly fluorescent nanoparticles,1 including quantum dots (QDs),2 carbon nanodots (CDs)3 and so on, have attracted tremendous scientific and industrial interest due to their remarkable optical/fluorescence properties, motivated by various applications such as high-throughput screening, ultrasensitive assays, live cell imaging, and intracellular dynamics.4–7 Compared with the QDs made from toxic heavy metal elements, CDs are much superior in the aspects of high aqueous solubility, low or no toxicity, favorable biocompatibility and easy functionalization,8 and thus have become the preferred candidates for practical applications such as bioimaging and medical diagnosis.9 Go hand in hand with carbon nanomaterial research is the study on construction of the conducting polymer nanostructure, especially the polypyrrole (PPy) nanoparticles, since PPy possesses the similar π-conjugated structure with CDs, and the N heteroatom on pyrrole ring may cause the doping effect to tune the photoluminescence (PL) of the materials.8,10 Such nanospheres might be interested with various applications, for instance as nanocarriers for controlled drug delivery11 or a potentially electrically addressable tissue/cell support substrate.12 Up to now, a lot of efforts have been devoted to synthesis of PPy nanoparticles with the small size and the regular morphology as much as possible to explore the relationship between property of PPy and its size dimension/morphology. For example, PPy nanoparticles with a size of 100–300 nm have been prepared through an interfacial polymerization on the interface of water/chloroform mixture solvent in the presence of dodecyl trimethyl ammonium bromide or sodium dodecyl sulfate,13 or by using 3-chloroperbenzoic acid simultaneously as oxidant, structure-induced reagent and dopant in water/ethanol mixture,14 which showed a good potential as the electrode materials for high-performance supercapacitors. Rather smaller PPy nanoparticles with diameter of 30 nm to 5 nm have also been synthesized in water/amyl alcohol mixture in presence of sodium dodecyl sulfate,15 or in water by using the octyltrimethylammonium bromide as surfactant.16 However, for most of above mentioned systems, the reaction was achieved in mixed solvent of water/organic species and necessarily by employing different surfactants to control the size of products. Moreover, for the PL properties of these as-prepared PPy nanoparticles, very few reports are available. Therefore, continued efforts have to be made to control over the size and morphology of PPy with the simple and effective method, especially to modulate surface properties and photoluminescence of the PPy, which is still rarely investigated thoroughly thus far.On the other hand, surface passivation is an important route to modulate the PL properties of the carbon nanodots.17,18 Many organic molecules, oligomeric and polymers could be served as the surface passivating agents to prepare CDs with improved quantum yields (QY). For example, polyethylenimine and 4,7,10-trioxa-1,13-tridecanediamine have been demonstrated having a QY enhancement effect, for CDs, from the original value of 6.3% up to 12.0% and 15.3% respectively.19 There have been some studies revealing the possibility of tailoring the band gap or PL emission intensity of the functionalized graphene quantum dots with amine groups.20 However, the mechanism of fluorescent enhancement by surface passivation is still elusive. In the case of PPy nanoparticles, whether they are passivated or not and thus any novel property will emerge should be a focus for serious concern.
Here, on the basis of our existing works, we report a facile polymerization of pyrrole (Py) in pure ethanol to conveniently synthesize PPy fluorescent nanospheres with smaller size of about 20 nm by simply using 3-chloroperbenzoic acid (m-CPBA) as oxidant. The as-prepared PPy fluorescent nanospheres showed moderate photoluminescence signal with a quantum yield (QY) of 2.2% and excitation-dependence photoluminescence characteristics. Several chemicals with different structures such as PEG 2000 (polyethylene glycol (PEG) with a molecular weight of 2000 g mol−1), 4,7,10-trioxa-1,13-tridecanediamine (TTDDA, H2N(CH2)3O(CH2)2O(CH2)2O(CH2)3NH2) and ethylenediamine (EDA, H2NCH2CH2NH2) acted as the surface passivating agent was employed to treat the PPy fluorescent nanospheres, accordingly obtaining the PPy nanodots with an enhanced QY value of 3.1%, 13.3% and 40%, respectively. This result is beyond our expectation and comparable to that of common CDs.18,21–23 The role of surface passivating in fabrication of the highly luminescent PPy fluorescent nanodots and the effect of the varied structures of passivating agents on the surface property, PL intensity and HOMO–LUMO gaps (ΔECV) of these resultant PPy fluorescent nanodots were discussed in detail. Further, the potential of the resultant PPy fluorescent nanodots for biomedical imaging is proved by laser scanning confocal microscopy imaging of HL-60 cells with and without the PPy fluorescent nanodots labeling. Also, the applications as fluorescent ink was explored.
2 Experimental section
2.1. Materials
Py monomer and oxidant m-CPBA were purchased from Aladdin Reagent Co., and the former was distilled under reduced pressure prior to use and the latter was used without further purification. All other reagents are purchased from local commercial sources and used as received.2.2. Preparation of polypyrrole nanospheres and nanodots
In a typical experiment, PPy nanospheres were synthesized by adding 10 mmol Py and 10 mmol m-CPBA to 100 mL pure ethyl alcohol, and then polymerizing the Py monomers at room temperature (25 °C) under stirred state for 6 h. After that, the reaction system was treated first by removing the solvent in vacuo and then adding 200 mL DI water in for dispersing with stirring for 2 h at room temperature (25 °C). Finally, the supernatant containing PPy nanospheres was obtained and denoted as PPy nanospheres. Pure PPy nanospheres were obtained via freeze drying and had a yield of about 60%.Taking 20 mL supernatant containing PPy nanospheres to mix respectively with 0.1 g passivating agent (PEG2000, TTDDA and EDA), and heat severally at 60 °C for 6 hours. The supernatants containing PPy nanodots from above-mentioned three systems were separated and named as PPy/PEG2000 (7.62 mg mL−1), PPy/TTDDA (4.64 mg mL−1) and PPy/EDA (3.12 mg mL−1) respectively, and ready for subsequent test. Pure PPy/PEG2000, PPy/TTDDA and PPy/EDA were gained via freeze drying and had a yield of about 92%, 59% and 40% respectively.
2.3. Cellular toxicity, cellular imaging and fluorescence image tests
The biocompatibility and biomedical imaging of the resultant PPy fluorescent nanodots were tested by using HL-60 cells as test matter (see ESI† in detail). The fluorescence images wrote on paper by using the aqueous PPy/EDA nanodot solution (4 mg mL−1) as ink were observed under 365 nm UV light.2.4. Measurements
Sample morphologies were observed by scanning transmission electron microscopy (TEM, JEOL 2000FX). In each TEM measurement, one drop of diluted sample was placed on a copper grid covered with a nitrocellulose membrane and air dried before examination. DLS (dynamic light scattering) measurements were performed on a Brookhaven BI9000AT system (Brookhaven Instruments Corporation) with a wavelength of 533.0 nm and detection angle of 90° at 25 °C. Fourier-transform infrared spectra (FTIR) of solid-state samples (KBr matrix) were measured with a Bruker VECTOR22 spectrometer at 4 cm−1 resolutions. The elemental analysis was performed using the Elementar Vario MICRO analyzer (Heraeus, Germany) under a test temperature of 950 °C. X-ray photoelectron spectroscopy (XPS, ESCALB MK-II, VG Co., England) measurement was performed under a base pressure of 1 × 10−9 Torr using monochromatic Mg-Kα X-rays at hν = 1253.6 eV. 1H-NMR and 13C-NMR spectra were collected on a Bruker DRX-400 spectrometer using D2O as solvent. The UV-vis spectra were recorded on a Perkin Elmer Lambda 35 instrument with 1 nm resolution. The emission spectra were measured using a FluoroMax Spectro fluorometer (HORIBA Jobin Yvon, France) with 1 nm resolution. The luminescence decay profile of the PPy fluorescent nanospheres/nanodots was obtained by a time-correlated single-photon-counting (TCSPC) technique under a lifetime fluorescence spectrometer (DeltaFlex, HORIBA Jobin Yvon, France) with a laser excitation wavelength of 405 nm at room temperature. Phase structures were examined via powder X-ray diffraction (XRD) on a Shimadzu XD-3A instrument with Cu-Kα radiation (λ = 1.5418 Å) at room temperature. The quantum yield (Φ) of the PPy nanospheres/dots was measured by using the quinine sulfate solution (in 0.1 M H2SO4, literature quantum yield 54% at 360 nm) as the standard and calculated with the equation of Φ = ΦR × (I/IR) × (AR/A) × (n2/nR2). Where, Φ is the quantum yield, I is the measured integrated emission intensity, n is the refractive index, A is the optical density and the subscript R refers to the quinine sulfate. To estimate the HOMO and LUMO energy levels of PPy fluorescent nanospheres/dots, cyclic voltammetry (CV) was carried out by using a standard three-electrode system, which consists of platinum sheet drop-casted with PPy sample as the working electrode, a platinum wire as the counter electrode, Ag/AgCl as the reference electrode, and acetonitrile containing 0.1 M tetrabutylammonium hexafluorophosphate (TBAPF6, Aldrich) as the supporting electrolyte. CV curves were recorded and calibrated using ferrocene as the standards, whose HOMO is set at −4.8 eV with respect to zero vacuum level. The HOMO and LUMO energy levels in eV as well as the electrochemical energy gap (Eg in eV) of the samples were calculated according to the following equations:24| EHOMO = −e(Eonsetox − Eonsetferrocene + 4.8) (eV) | (1) |
| ELUMO = −e(Eonsetred − Eonsetferrocene + 4.8) (eV) | (2) |
3 Results and discussion
It is well known that most chemical polymerization of Py is accomplished in aqueous solution rather than in organic species since the water-soluble oxidant may initiate high-efficient polymerization of Py.25,26 When organic solvents, usually ethanol, were employed, the polymerization rate will be substantially slowed due to the complex interaction between oxidant and alcohol.27 During the polymerization process, the oxidized Py monomers went through nucleating and particle growing, and the final PPy particles formed in aqueous medium always possessed the bigger size than that gained in organic solvent owing to the aggregation of the unstable nucleus induced by fast polymerization.28 In other words, it is the characteristic of slow polymerization in organic solvent that could make the acquisition of PPy nanoparticles with subsize possible. In our case, pure ethanol was chosen as the solvent in which the metastable PPy nucleus could exist due to the slow polymerization caused by complexation of the oxidant with ethanol, and grow independently in a enough slow rate to form small particles.29According to TEM observation, the as-prepared PPy nanoparticles are spherical in shape, relatively uniform in size with a size distribution in the range of 16–26 nm and an average size of 20 nm, and well dispersed in water (shown in Fig. 1A). No lattice fringes and selected-area electron diffraction patterns were observed, suggesting that these PPy nanospheres are amorphous. XRD pattern (Fig. 1B) displayed a broad diffraction band with a maximum center 2θ = 23°, which is consistent with the diffracting from the polymer chains at the inter-planar spacing,30 demonstrating the amorphous state of PPy nanospheres. It is inferred that solvent nature such as different polarity may play an important role in PPy nanodots formation. Comparing with ethanol, the relatively weaker polar solvents such as THF and dioxane may be just allow to form weaker H-bonding with –NH in Py and/or –COOH in oxidant.27 Therefore, as a reference for checking the influence of solvent nature on PPy size, THF was also selected as the solvent to do the polymerization of Py. As we expected, because of weak complexation of the oxidant m-CPBA with THF, to get the desired product required longer reaction times about 54 h under the same conditions, and PPy nanospheres with smaller size of 10 nm diameter were obtained (Fig. S1†). These results demonstrated that the solvent nature does affect the polymerization rate of Py, thereby further affecting the size of PPy.
 | ||
| Fig. 1 (A) TEM images and size distribution, (B) XRD pattern and (C) FTIR spectrum of the PPy nanospheres. | ||
FTIR, XPS and NMR were carried out to investigate in detail the structure, surface composition and chemical state of PPy nanospheres. FTIR spectrum (Fig. 1C) showed series peaks at 1678 cm−1 (fundamental vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1253 cm−1 and 1073 cm−1 (the
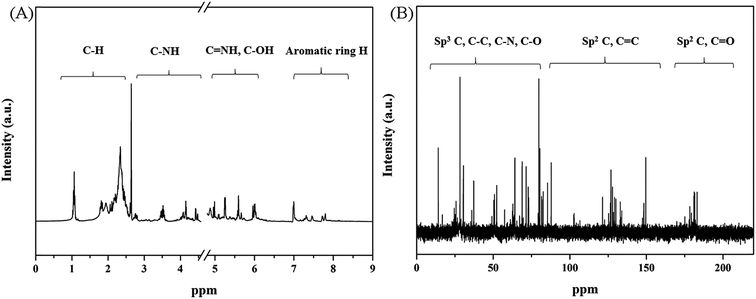
N), 1253 cm−1 and 1073 cm−1 (the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H in-plane vibrations), which verified the skeletal structure of PPy.31 The surface elementary composition of PPy nanospheres obtained from XPS indicated that the PPy nanospheres contains C, N, O and Cl elements, and their wt% are 68.08, 12.54, 18.74, 0.64, respectively. The element O, Cl and the relatively high carbon content were ascribed to the introduction of oxidant m-CPBA, which could act as dopant and be incorporated into the PPy chains.14 XPS spectra of PPy nanospheres (Fig. 2A and B) revealed that there are three characteristic peaks corresponding to C1s (284.0 eV), N1s related to C–N bond of Py (400.0 eV) and O1s related to C–O group (530.06 eV), which are consistent with the result of FTIR.32 Furthermore, NMR spectra (1H and 13C) provided the evidence for the existence of abundant carbon-base functional groups on the PPy nanospheres (Fig. 3A and B). With respect to 1H-NMR, the peaks around 1.5 ppm, 2.5 ppm and 4.0 ppm can be attributed to protons next to the carbon, nitrogen and oxygen atoms, respectively. Next, peaks in the range of 7–9 ppm may confirm the presence of aromatic ring hydrogen.33 In 13C-NMR, peaks in the range of 20–60 ppm and 120–150 ppm corresponded to C–C carbon atoms and C
C–H in-plane vibrations), which verified the skeletal structure of PPy.31 The surface elementary composition of PPy nanospheres obtained from XPS indicated that the PPy nanospheres contains C, N, O and Cl elements, and their wt% are 68.08, 12.54, 18.74, 0.64, respectively. The element O, Cl and the relatively high carbon content were ascribed to the introduction of oxidant m-CPBA, which could act as dopant and be incorporated into the PPy chains.14 XPS spectra of PPy nanospheres (Fig. 2A and B) revealed that there are three characteristic peaks corresponding to C1s (284.0 eV), N1s related to C–N bond of Py (400.0 eV) and O1s related to C–O group (530.06 eV), which are consistent with the result of FTIR.32 Furthermore, NMR spectra (1H and 13C) provided the evidence for the existence of abundant carbon-base functional groups on the PPy nanospheres (Fig. 3A and B). With respect to 1H-NMR, the peaks around 1.5 ppm, 2.5 ppm and 4.0 ppm can be attributed to protons next to the carbon, nitrogen and oxygen atoms, respectively. Next, peaks in the range of 7–9 ppm may confirm the presence of aromatic ring hydrogen.33 In 13C-NMR, peaks in the range of 20–60 ppm and 120–150 ppm corresponded to C–C carbon atoms and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C carbon atoms, respectively. In addition, peaks situated at 170–180 ppm were indicative of C
C carbon atoms, respectively. In addition, peaks situated at 170–180 ppm were indicative of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups.24 These results are consistent with the expected diverse carbon environment on the PPy nanospheres surface.34 The above mentioned spectroscopic data and NMR results indicated again that we have successfully obtained the surface-functionalized O-doped PPy nanospheres own to the doping of m-CPBA.
O groups.24 These results are consistent with the expected diverse carbon environment on the PPy nanospheres surface.34 The above mentioned spectroscopic data and NMR results indicated again that we have successfully obtained the surface-functionalized O-doped PPy nanospheres own to the doping of m-CPBA.
 | ||
| Fig. 2 XPS spectra of the PPy nanospheres. (A) Overall spectrum and (B) core region spectrum of N1s after peak-differentiate-fitting. | ||
In fact, the formation of resultant PPy nanospheres related not only to the solvent nature but also the oxidant species. In our case, the oxidant m-CPBA may occur self-assembly to form micelle-like-structure in the ethanol medium own to the balance between central hydrophobic benzene core and the outer hydrophilic –OH group of m-CPBA, the π–π interaction between m-CPBA molecules and the H-bonds possibly formed by –OH group of m-CPBA with NH of Py.27 The formed species could act as both structure-directing agent and oxidant in the synthesis process and promote homogeneous nucleation of Py.
Excellent optical properties are the most fascinating features of fluorescent nanospheres which stimulates research and development of fluorescent nanoparticles in various applications. As shown in UV-vis spectrum (Fig. 4A), the prepared PPy nanospheres in aqueous solution have two typical absorption peaks at 230 nm and 280 nm, corresponding to the π–π* transition in the benzenoid rings and the π–π* transition in the Py rings respectively,35 indicating that the PPy sample is in the doped state. As monomer and oxidant molecule involved into the polymerization, the Py and m-CPBA themselves all lack photoluminescent characteristics. While the common PPy shows extremely lower photoluminescence (PL) quantum yield of about 2.0%.36 Only for PPy nanoparticles or PPy dispersed in specific organic solvents, the intensive PL could be observed.36,37 In our case, the as-prepared PPy nanospheres exhibited a characteristic excitation wavelengths-dependent emission, that is, their emission peak shifted from 425 to 460 nm under 300 to 400 nm excitation, and the intensity of the emission peak increased firstly and then decreased with the increase of excitation wavelengths (Fig. 4B). The strongest emission peak was about 440 nm at the excitation wavelength of 360 nm, implying that the dominant energy gap has the lowest value at the moment.9 Such spectral appearance may be ascribed to the existence of different “chromophores” such as the C–N bonds offered by PPy and C–O groups resulted from m-CPBA and the interaction of PPy unit and m-CPBA, which may alter electronic structures and/or generate additional low-energy band-gaps in the PPy nanospheres.38,39 Also, these different “chromophores” may give expression to varied emission efficiency at the changed excitation wavelengths.40
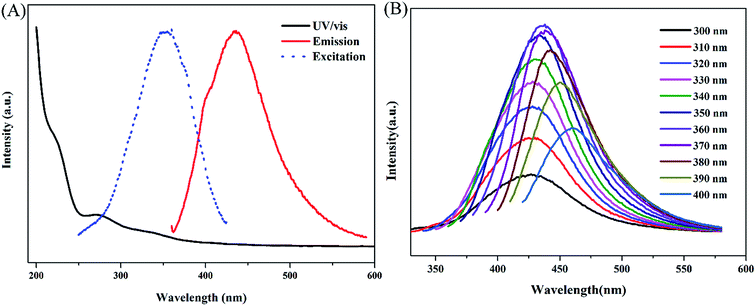 | ||
| Fig. 4 (A) UV-vis absorption, PL excitation and emission spectra of PPy nanospheres in aqueous solution. (B) Excitation-dependent PL for PPy nanospheres. | ||
The effects of ion strength, pH conditions and UV exposure time on the photoluminescent of PPy fluorescent nanospheres were investigated in details. There were no remarkable changes in PL intensity or peak characteristics at different ion strengths (Fig. 5A and B), showing the potential of PPy fluorescent nanospheres at various physical salt concentrations. Also it is observed that with the pH value changing from 2 to 13, PL intensity of the PPy nanospheres changed pronouncedly and the maximum emission wavelength shifted slightly from 430 nm to 440 nm (Fig. 5C and D), which may related to the protonation and deprotonation reaction taken place at the NH moiety of Py or the carboxyl groups of the dopant m-CPBA under the different pH conditions.41 Interestingly, the PL intensity at 360 nm excitation increased linearly as the pH increased from 8 to 12 (Fig. 6A and B), showing that the PPy nanospheres have potential as fluorescent sensors for pH quantitative determination. It is noteworthy that almost no bleaching was observed after continuous irradiation with UV light (6 W) for 24 h (Fig. 5E and F),17 indicating that the emission was quite stable, and this should be a consequence of PPy essential chemical structure.17,42
 | ||
| Fig. 5 Effect of different factors on fluorescence intensity of PPy nanospheres. (A and B) ionic strength (controlled by varied NaCl concentrations), (C and D) pH, (E and F) UV excitation time. | ||
 | ||
| Fig. 6 (A) PL spectra of the PPy nanospheres dispersion at different pH values (from 7.87 to 12.01) with excitation at 360 nm. (B) A linear relationship between PL intensity and pH (from 8 to 12). | ||
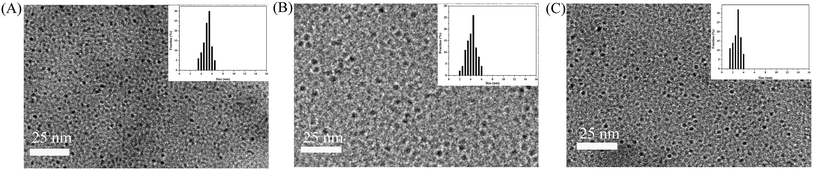
To further investigate the effect of the different surface structures of PPy nanospheres on PL property, PEG2000, TTDDA and EDA were used as surface passivating agents to treat the PPy nanospheres. From TEM and DLS results (Fig. 7) it is seen that the products obtained after surface passivating were homogeneously dispersed nanodots with an average diameter size of 5.5 nm (PPy/PEG2000), 4.5 nm (PPy/TTDDA) and 3.0 nm (PPy/EDA) respectively, which could be attributed to the enhanced intermolecular repulsive force and the out-of-balance between the π–π interaction and H-bond force in PPy nanospheres.14 Comparing with PEG2000, TTDDA has stronger electron-donating abilities owing to three alkoxyl and two amine group, and a more positive value of average charge. On the other hand, EDA shows a stronger electron transfer ability due to its shorter chain and the strongest electron-donating abilities among three. According the experimental results, we supposed that the enhanced intermolecular repulsive force of the PPy nanospheres came from the increased electron density on the surface, which afterwards disrupted the balance between the π–π interaction and H-bonds in PPy nanospheres, thus obtaining the smaller and well-dispersed (at 60 °C) PPy nanodots from their original assembly.27 This interpretation was confirmed by our further work on proceeding the surface passivating of PPy nanospheres at room temperature. We did not observe any change in the fluorescence intensity and the size of the PPy nanospheres, indicating that surface reaction of the PPy nanospheres was inhibited under room temperature. Moreover, the PPy nanospheres with bigger size were gained (Fig. S2†) after placing such system for several days at room temperature under the static condition, suggesting that the functionalized surfaces of PPy nanospheres could be conducive to the size control.28
Fig. 8A presents the PL emission spectra of the aqueous solutions of PPy nanospheres and three kinds of PPy nanodots with excitation of 360 nm laser. Compared with PPy nanospheres, PPy/PEG2000, PPy/TTDDA and PPy/EDA show the red-shift of the maximum absorption band (λmax) by 10 nm, 35 nm and 40 nm, respectively, which can be ascribed to surface interaction of PPy and the surface passivating agents. The optical band gaps (Eoptg, Table 1) of the four samples estimated from the UV-vis onset absorptions are 2.12, 2.10, 2.02 and 1.98 eV for PPy nanospheres, PPy/PEG2000, PPy/TTDDA and PPy/EDA, respectively. Clearly, the photophysical properties and energy levels of the resultant PPy nanospheres can be easily tuned by incorporating different electron-donating surface passivating agents.38 As can be seen, PL quantum yield of PPy was enhanced from the initial value of 2.2% to 3.1%, 13.3% and 40.0%, respectively. The corresponding time-resolved photoluminescence decay curves as shown in Fig. 8B can be fitted by a double-exponential function, and gave their fluorescence lifetime of 3.75, 3.85, 4.18 and 4.27 ns, respectively. For all samples, their fluorescence lifetime was in the nanosecond timescale, implying the singlet state nature of their emission.38 And the increased fluorescence lifetime of the different PPy nanospheres/dots should be connected with their surface states,43 possibly due to the suppression of non-radiative energy transitions induced by surface passivation.44 Moreover, the longer lifetime together with the larger red shift of the emission band are in agreement with lower-lying energy levels arising from the modified PPy nanospheres surface states by surface passivating agents.45 Furthermore, the increasing lifetimes of all four samples matched well with their decreasing sizes,46 indicating that the surface states of as-prepared PPy nanospheres/dots were greatly affected by the different surface passivating agents.
 | ||
| Fig. 8 (A) Photoluminescence intensity and (B) the exponential fitting curve of the PPy nanospheres and three kinds of PPy nanodots. | ||
In our case, the unpaired electrons of the surface passivating agents can contribute to the electron donation from the amine or alkoxy groups to the PPy nanospheres basing on the electron-withdrawing and electron-accepting behaviors of functional groups to benzene.47 Thus the electron density increases and the band gap of PPy nanospheres decreases, resulting in relatively longer luminescence lifetime and the enhanced PL emission.19,47,48 To support this hypothesis, the CV curves of all four samples were recorded to estimate their HOMO and LUMO energy levels (Fig. S3†), and the corresponding electrochemical data are summarized in Table 2. According to the respective onset potentials in their oxidation traces and reduction traces, the HOMO (highest occupied molecular orbital) energy levels were determined to be −5.14, −5.11, −4.83 and −4.61 eV, respectively, corresponding to the varied modulated charge transfer strengths in the presence of various surface passivating agents. In addition, the determined electrochemical HOMO–LUMO gaps (ΔECV) were 1.53, 1.43, 1.15 and 0.78 eV for PPy, PPy/PEG2000, PPy/TTDDA and PPy/EDA, respectively, being tuned effectively by incorporation of the different electron donating surface passivating agents.49 Since EDA molecules have the shorter chain length and smaller stereo-hindrance effect than PEG2000 and TTDDA, they could attach more on the PPy nanospheres and make them have much denser electron distribution, arousing the decrease of the band gap.50 Thus, it can be inferred that the luminescence mechanism is due to the generation of new energy levels in PPy nanospheres through different surface passivating agents.
| Sample | Eoxb (V) | Eredc (V) | HOMO (eV) | LUMO (eV) | Egd (eV) |
|---|---|---|---|---|---|
| a The ferrocene couple (Fc+/Fc) with a potential of 0.44 V was used as the internal reference and under our experimental conditions, E(Fc+/Fc) vs. Ag/AgCl.b Onset oxidation potential vs. Ag/AgCl.c Onset reduction potential vs. Ag/AgCl.d Band gaps derived from the difference between onset potentials of oxidation and reduction. | |||||
| PPy nanospheres | 0.74 | −0.79 | −5.14 | −3.61 | 1.53 |
| PPy/PEG2000 | 0.71 | −0.72 | −5.11 | −3.68 | 1.43 |
| PPy/TTDDA | 0.43 | −0.71 | −4.83 | −3.68 | 1.15 |
| PPy/EDA | 0.21 | −0.57 | −4.61 | −3.83 | 0.78 |
Due to the strong fluorescence, the PPy nanodots can be utilized for various applications such as using as cellular imaging agent or fluorescence ink. For instance, PPy/PEG2000 showed that relative cell viability is still about 90% even after a 24 h exposure, suggesting its low toxicity to the HL-60 cells. The nanodots were found only in the cell membrane and cytoplasmic area of the cell but very weak at the central region corresponding to the nucleus, indicating that the PPy/PEG2000 nanodots can easily penetrate into the cell but not enter the nuclei (Fig. S4C†) avoiding effectively genetic disruption.51 Moreover, no morphological cell damage was observed upon incubation with the PPy/PEG2000 nanodots, further exemplifying their low cytotoxicity. In the case of PPy/EDA, due to its high QY of 40%, the good imaging result and relative low toxicity could also be observed under the smaller dosage (20 μg mL−1) employed (Fig. S5†). On the other hand, when the colorless PPy/EDA aqueous solution with a very low concentration of 4 mg mL−1 was applied as an ink to the common commercially available paper by a pen tool, the images including Nanjing University logo, the Chinese words and the English words for Nanjing University could be seen clearly under a UV lamp and remained well defined after two months in an indoor environment (Fig. S6†),24 showing its charm possibly as the large-scale printing fluorescence inks.
4 Conclusion
PPy nanospheres with an average diameter of 20 nm have been successfully synthesized by controlling the slow oxidative polymerization of pyrrole in the presence of 3-chloroperbenzoic acid as the oxidizing agent and pure ethanol as the solvent. The as-prepared PPy nanospheres showed an excitation-dependence photoluminescence characteristics and the stable fluorescence intensity under varied ionic strengths and UV excitation time. Through the surface functionization by the different surface passivating agents such as PEG2000, TTDDA and EDA, the quantum yield of the polypyrrole fluorescent nanospheres could be enhanced from its initial value of 2.2% to 3.1%, 13% and 40% respectively, which was related to the increased electron density in samples. TEM, UV and CV results demonstrated that 5.5 nm to 3 nm sized PPy nanodots were gained after the surface passivation of the PPy nanospheres, and the decrease of sample size was consistent with the decrease of the band gap and the increase of fluorescence lifetime, which was attributed to different degrees of contribution of the electron donating strength of the passivating agents. This provides us with a valuable approach to predictably tune the Frontier molecular orbital energy levels and guide the synthesis of fluorescent emissive materials. Furthermore, these resultant PPy fluorescent nanospheres/dots can be appropriately applied as cell-imaging, and printing ink materials because of their outstanding characteristics of good biocompatibility and high luminescence stability.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21374046), Program for Changjiang Scholars and Innovative Research Team in University, Open Project of State Key Laboratory of Supramolecular Structure and Materials (SKLSSM201616) and the Testing Foundation of Nanjing University.References
- R. C. Y. King, M. Boussoualem and F. Roussel, Polymer, 2007, 48, 4047–4054 CrossRef CAS.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726–6744 CrossRef CAS PubMed.
- J. Fan and P. K. Chu, Small, 2010, 6, 2080–2098 CrossRef CAS PubMed.
- W. Lu, Y. Luo, G. Chang, X. Qin, F. Liao and X. Sun, Synth. Met., 2011, 161, 1766–1770 CrossRef CAS.
- P. Alivisatos, Nat. Biotechnol., 2004, 22, 47–52 CrossRef CAS PubMed.
- J. K. Jaiswal, H. Mattoussi, J. M. Mauro and S. M. Simon, Nat. Biotechnol., 2003, 21, 47–50 CrossRef CAS PubMed.
- L.-X. Wang, X.-G. Li and Y.-L. Yang, React. Funct. Polym., 2001, 47, 125–139 CrossRef CAS.
- Y.-Q. Zhang, D.-K. Ma, Y. Zhuang, X. Zhang, W. Chen, L.-L. Hong, Q.-X. Yan, K. Yu and S.-M. Huang, J. Mater. Chem., 2012, 22, 16714–16718 RSC.
- P. G. Luo, S. Sahu, S.-T. Yang, S. K. Sonkar, J. Wang, H. Wang, G. E. LeCroy, L. Cao and Y.-P. Sun, J. Mater. Chem. B, 2013, 1, 2116–2127 RSC.
- Y. Li, Y. Zhao, H. Cheng, Y. Hu, G. Shi, L. Dai and L. Qu, J. Am. Chem. Soc., 2012, 134, 15–18 CrossRef CAS PubMed.
- J. Ge, E. Neofytou, T. J. Cahill, R. E. Beygui and R. N. Zare, ACS Nano, 2012, 6, 227–233 CrossRef CAS PubMed.
- D. D. Ateh, H. A. Navsaria and P. Vadgama, J. R. Soc., Interface, 2006, 3, 741–752 CrossRef CAS PubMed.
- P. Dallas, D. Niarchos, D. Vrbanic, N. Boukos, S. Pejovnik, C. Trapalis and D. Petridis, Polymer, 2007, 48, 2007–2013 CrossRef CAS.
- C. Shen, Y. Sun, W. Yao and Y. Lu, Polymer, 2014, 55, 2817–2824 CrossRef CAS.
- Y. Liu, Y. Chu and L. Yang, Mater. Chem. Phys., 2006, 98, 304–308 CrossRef CAS.
- J. Jang and J. H. Oh, Chem. Commun., 2002, 2200–2201 RSC.
- Y.-P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. Wang, P. G. Luo, H. Yang, M. E. Kose, B. Chen, L. M. Veca and S.-Y. Xie, J. Am. Chem. Soc., 2006, 128, 7756–7757 CrossRef CAS PubMed.
- C. Liu, P. Zhang, F. Tian, W. Li, F. Li and W. Liu, J. Mater. Chem., 2011, 21, 13163–13167 RSC.
- C. Liu, P. Zhang, X. Zhai, F. Tian, W. Li, J. Yang, Y. Liu, H. Wang, W. Wang and W. Liu, Biomaterials, 2012, 33, 3604–3613 CrossRef CAS PubMed.
- X. Zhai, P. Zhang, C. Liu, T. Bai, W. Li, L. Dai and W. Liu, Chem. Commun., 2012, 48, 7955–7957 RSC.
- B. Han, W. Wang, H. Wu, F. Fang, N. Wang, X. Zhang and S. Xu, Colloids Surf., B, 2012, 100, 209–214 CrossRef CAS PubMed.
- A. B. Bourlinos, A. Stassinopoulos, D. Anglos, R. Zboril, M. Karakassides and E. P. Giannelis, Small, 2008, 4, 455–458 CrossRef CAS PubMed.
- R. Liu, D. Wu, S. Liu, K. Koynov, W. Knoll and Q. Li, Angew. Chem., Int. Ed., 2009, 48, 4598–4601 CrossRef CAS PubMed.
- S. Zhu, Q. Meng, L. Wang, J. Zhang, Y. Song, H. Jin, K. Zhang, H. Sun, H. Wang and B. Yang, Angew. Chem., Int. Ed., 2013, 52, 3953–3957 CrossRef CAS PubMed.
- K. J. Gilmore, M. Kita, Y. Han, A. Gelmi, M. J. Higgins, S. E. Moulton, G. M. Clark, R. Kapsa and G. G. Wallace, Biomaterials, 2009, 30, 5292–5304 CrossRef CAS PubMed.
- Y. Kudoh, Synth. Met., 1996, 79, 17–22 CrossRef CAS.
- M. L. Digar, S. N. Bhattacharyya and B. M. Mandal, Polymer, 1994, 35, 377–382 CrossRef CAS.
- T. K. Mandal and B. M. Mandal, J. Polym. Sci., Polym. Chem. Ed., 1999, 37, 3723–3729 CrossRef CAS.
- T. K. Mandal and B. M. Mandal, Langmuir, 1997, 13, 2421–2424 CrossRef CAS.
- T. Dai and Y. Lu, J. Mater. Chem., 2007, 17, 4797–4802 RSC.
- Y. Xia and J. Yang, Synth. Met., 2010, 160, 1688–1691 CrossRef CAS.
- P. Pfluger and G. B. Street, J. Chem. Phys., 1984, 80, 544–553 CrossRef CAS.
- M. J. Krysmann, A. Kelarakis, P. Dallas and E. P. Giannelis, J. Am. Chem. Soc., 2012, 134, 747–750 CrossRef CAS PubMed.
- G. E. LeCroy, S. K. Sonkar, F. Yang, L. M. Veca, P. Wang, K. N. Tackett, J.-J. Yu, E. Vasile, H. Qian, Y. Liu, P. Luo and Y.-P. Sun, ACS Nano, 2014, 8, 4522–4529 CrossRef CAS PubMed.
- S. Lamprakopoulos, D. Yfantis, A. Yfantis, D. Schmeisser, J. Anastassopoulou and T. Theophanides, Synth. Met., 2004, 144, 229–234 CrossRef CAS.
- P. Galář, B. Dzurňák, P. Malý, J. Čermák, A. Kromka, M. Omastová and B. Rezek, Int. J. Electrochem. Sci., 2013, 8, 57–70 Search PubMed.
- A. J. R. Son, H. Lee and B. Moon, Synth. Met., 2007, 157, 597–602 CrossRef CAS.
- S. Hu, A. Trinchi, P. Atkin and I. Cole, Angew. Chem., Int. Ed., 2015, 54, 2970–2974 CrossRef CAS PubMed.
- H. Zheng, Q. Wang, Y. Long, H. Zhang, X. Huang and R. Zhu, Chem. Commun., 2011, 47, 10650–10652 RSC.
- H. Tetsuka, R. Asahi, A. Nagoya, K. Okamoto, I. Tajima, R. Ohta and A. Okamoto, Adv. Mater., 2012, 24, 5333–5338 CrossRef CAS PubMed.
- X. Jia, J. Li and E. Wang, Nanoscale, 2012, 4, 5572–5575 RSC.
- Q. Liang, W. Ma, Y. Shi, Z. Li and X. Yang, Carbon, 2013, 60, 421–428 CrossRef CAS.
- S. Han, H. Zhang, J. Zhang, Y. Xie, L. Liu, H. Wang, X. Li, W. Liu and Y. Tang, RSC Adv., 2014, 4, 58084–58089 RSC.
- S. Yang, J. Sun, X. Li, W. Zhou, Z. Wang, P. He, G. Ding, X. Xie, Z. Kang and M. Jiang, J. Mater. Chem. A, 2014, 2, 8660–8667 RSC.
- K. Hola, A. B. Bourlinos, O. Kozak, K. Berka, K. M. Siskova, M. Havrdova, J. Tucek, K. Safarova, M. Otyepka, E. P. Giannelis and R. Zboril, Carbon, 2014, 70, 279–286 CrossRef CAS.
- C.-T. Chien, S.-S. Li, W.-J. Lai, Y.-C. Yeh, H.-A. Chen, I.-S. Chen, L.-C. Chen, K.-H. Chen, T. Nemoto, S. Isoda, M. Chen, T. Fujita, G. Eda, H. Yamaguchi, M. Chhowalla and C.-W. Chen, Angew. Chem., Int. Ed., 2012, 51, 6662–6666 CrossRef CAS PubMed.
- D. Billaud, E. B. Maarouf and E. Hannecart, Mater. Res. Bull., 1994, 29, 1239–1246 CrossRef CAS.
- Z.-A. Qiao, Y. Wang, Y. Gao, H. Li, T. Dai, Y. Liu and Q. Huo, Chem. Commun., 2010, 46, 8812–8814 RSC.
- X. Wang, L. Cao, S.-T. Yang, F. Lu, M. J. Meziani, L. Tian, K. W. Sun, M. A. Bloodgood and Y.-P. Sun, Angew. Chem., Int. Ed., 2010, 49, 5310–5314 CrossRef CAS PubMed.
- S. H. Jin, D. H. Kim, G. H. Jun, S. H. Hong and S. Jeon, ACS Nano, 2013, 7, 1239–1245 CrossRef CAS PubMed.
- F. Wang, Z. Xie, H. Zhang, C. Liu and Y. Zhang, Adv. Funct. Mater., 2011, 21, 1027–1031 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures of cellular toxicity test and cellular imaging, TEM images of PPy nanospheres using THF as solvents and bigger size PPy nanospheres, CV curves of PPy nanospheres, PPy/PEG2000, PPy/TTDDA and PPy/EDA, cellular toxicity and cellular imaging of PPy/PEG2000 and PPy/EDA fluorescent nanodots and symbols written on paper by using PPy/EDA nanodots fluorescence ink. See DOI: 10.1039/c6ra01468b |
| This journal is © The Royal Society of Chemistry 2016 |