DOI:
10.1039/C6RA00553E
(Paper)
RSC Adv., 2016,
6, 23746-23759
Olefin–paraffin separation performance of polyimide Matrimid®/silica nanocomposite membranes†
Received
7th January 2016
, Accepted 22nd February 2016
First published on 24th February 2016
Abstract
In this study, the performance of polyimide Matrimid® membranes in the separation of ethylene/ethane and propylene/propane was improved by silica nanoparticles. Further, the plasticization resistance of glassy polyimide was enhanced by silica nanoparticles. The silica nanoparticles were prepared using a sol–gel method. Pure and hybrid membranes were prepared by a solution-casting method and characterized using Fourier Transform Infrared Spectroscopy (FTIR), Scanning Electron Microscopy (SEM), Thermal Gravimetric (TGA) Analyses and differential scanning calorimetry (DSC). The results from membrane characterization indicated good distribution and connection of the particles to the polymer matrix. The findings from the gas separation tests showed that the addition of silica nanoparticles to the polymer caused an increase in olefin permeation and small changes in paraffin permeation, and consequently, an increase in the C2H4/C2H6 and C3H6/C3H8 selectivity. The selectivity of C3H6/C3H8 and C2H4/C2H6 was increased from 9.05 to 18.03, and from 3.25 to 6.13 respectively. The findings revealed that by adding silica nanoparticles up to 10 wt%, the plasticization pressure of propylene was delayed from 4 to 6 bar in the pure membrane.
1. Introduction
Light olefins including ethylene and propylene are the most consumed feedstock in the world, widely used for the production of plastics and chemicals. Separation of olefins from paraffins with the same number of carbons is a major expensive separation process in the petrochemical industry. Currently, this separation is carried out by a high-energy consumption cryogenic distillation method.1,2 A great deal of effort has been made by researchers to develop alternative methods. Membrane technology has many advantages such as simplicity, high efficiency and design flexibility and has the advantage of reducing the fixed and operation costs of olefin/paraffin separation.3–5 Therefore, in recent decades, extensive research has been done into the field of olefin and paraffin separation via membrane technology. Among the three main groups of membranes, i.e., polymeric, non-polymeric and facilitated transport, polymeric membranes have many advantages such as low cost, ease of processing, mechanical strength and high stability. These advantages make them an appropriate option for olefin and paraffin separation.4–11 The separation properties of polymeric membranes, such as cellulose acetate, polysulfone, silicone rubber, ethyl cellulose, poly(phenylene oxide), polydimethylsiloxane and polyimide, have been studied.12–16 The results of these studies have revealed that polyimide membranes can have the best performance for olefin–paraffin separation. In recent years, a lot of research has been done in order to improve the separation properties of theses membranes (permeation and the separation factor). A problem with polyimide membranes that prevents their effective use on an industrial scale in olefin/paraffin separation is plasticization of these membranes as a result of exposure to such condensable gases as propylene and propane.6,16,17 The plasticization phenomenon occurs as a result of swelling and increased free volume of polymer, leading to increased permeability and reduced selectivity of the membrane.18,19 Plasticization of polyimide membranes occurs at low feed pressure (2 to 4 bar).16,20,21 The increase in feed pressure to a point above the plasticization pressure results in a sharp decrease in selectivity. Therefore, increasing the plasticization pressure of polyimide membranes using such different methods as heat treatment,6,17,18 crosslinking and mixing with more resistant polymers22–24 constitutes an important area of inquiry. Using inorganic nanoparticles in polymer membranes leads to the formation of nanocomposite membranes with higher gas separation performance and higher stability.25 The literature has already well described the types, methods of preparation, features and mechanisms of gases passing through them.26 The research findings have indicated that nanocomposite membranes at the low percentage of nanoparticles and suitable interaction between particles and polymer media, have better mechanical strength and thermal and chemical stability. Also, they have better separation properties and finally, higher resistance against the undesirable plasticization phenomenon on the ground that nanoparticles can decrease flexibility of polymer chains and increase the plasticization pressure of membranes.27 Separation of oxygen, nitrogen, methane, carbon dioxide and hydrogen gases has been studied using a variety of nanocomposite membranes.28–33 In this respect, silica nanoparticle proved as a most useful particle in enhancing the gas separation properties of the polymeric membranes.34 Recently we evaluated the olefin/paraffin separation performance of the cellulose acetate/silica nanocomposite membranes.35 The findings confirmed the significant effect of silica nanoparticles on olefin/paraffin separation. Thus, in this study an attempt was made to improve the separation properties and also resistance to the plasticization of polyimide Matrimid® membrane in the separation of propylene/propane and ethylene/ethane by adding synthesized silica nanoparticles and restricting the movement of the polymer chains.
2. Experimental
2.1. Materials
Polyimide of Matrimid® (inherent viscosity = 0.62–0.68 dl g−1, density = 1.24 g cm−3, Tg = 302 °C) in powder form was provided by Huntsman Advanced Materials Americas Inc. The solvent N-methyl-2-pyrrolidone (NMP) was obtained from Merck Chemicals for synthesis of silica nanoparticles including tetraethoxysilane (TEOS), hydrochloric acid (HCl), ethanol from Merck, and a coupling agent (3-aminopropyl) trimethoxysilane (APTMOS) from Sigma-Aldrich. Research-grade ethane, ethylene, propane and propylene gases (purity > 99.95%) were purchased from Technical Gas Service.
2.2. Preparation of Matrimid® and Matrimid®–silica nanocomposite membranes
Pure Matrimid® membrane was prepared using solution casting and solvent evaporation method with a solution of 10 wt% of the polymer in NMP. To fabricate mixed matrix membranes, the silica particles, synthesized through the sol–gel process, were dispersed in NMP in an ultrasonic bath for 25 min. Then, the dispersed silica particles in NMP added to the polymer solution in different contents to obtain 10 wt% PI–silica solution. The polymer solution contained silica particles stirred vigorously to obtain a homogenous transparent solution. Then, the solution was cast on the glass plate and was heated to 75 °C for 12 h in a vacuum oven which has the privilege of evaporating the solvent so quickly that the silica particles hardly could descend to the glass side of the membrane. Dried, the membrane was removed from the glass plate and placed in a water bath for 30 min to remove the residual solvent. Finally, the membrane was dried in a vacuum oven at 65 °C for 2 h. The silica nanoparticles were synthesized from TEOS hydrolysis using the sol–gel process as fully described elsewhere.30,32,35 Matrimid®–silica nanocomposite membranes were also prepared by solution casting and solvent evaporation method. The casting solutions were prepared by mixing different ratios of the silica sol (with measured silica weight proportion) and 10 wt% polymer solutions. Samples with 5 (PI/S5), 10 (PI/S10), 15 (PI/S15) and 20 (PI/S20) wt% of silica were prepared. In order to measure the single-gas permeability characteristics of ethane, ethylene, propane and propylene with great accuracy casting knife was used to make sheet with the same thicknesses.
2.3. Membranes characterization
Fourier transform infrared spectroscopy (JASCO FT/IR-680 plus) was performed on membranes in the range of 400–4000 cm−1 to investigate the effect of silica particles on nanocomposite membranes and provide information on the functional groups in membranes. Thermogravimetric analyses (TGA) were performed using Thermal Gravimetric Analysis (TGA-50SHIMADZU, Japan). The samples were heated up to 900 °C at a rate of 5 °C min−1. The thermal behavior of Matrimid® and Matrimid®–silica hybrid membranes was investigated by differential scanning calorimetry (DSC) PerkinElmer DSC-7 from 20 to 600 °C at the heating rate of 10 °C min−1 and sample weights of about 2.5 mg. Scanning Electron Microscopy (KYKY-EM3200 SEM, China) was used to directly observe the morphological characteristics of the prepared membranes. The SEM samples were prepared by fracturing the membranes cryogenically in liquid nitrogen using two tweezers. The samples were sputter-coated with gold.
2.4. Gas permeability measurement
The single-gas permeability characteristics of ethane, ethylene, propane and propylene were measured using a constant volume/variable pressure permeation setup described in detail earlier.35,36 The gas permeability of membranes was calculated using the following equation:| |
 | (1) |
where P is the permeability in barrer (1 barrer = 1 × 10−10 cm3 (STP) cm cm−2 s−1 cm−1 Hg), V the volume of the downstream chamber (cm3), A the effective membrane area (cm2), L the membrane thickness (cm), T the experimental temperature (K), dp(t)/dt the steady rate of pressure measured by a pressure transducer in the downstream chamber and p0, the feed pressure using the same unit p(t). The diffusion coefficient (D) was determined using the vacuum time lag method:| |
 | (2) |
where θ is the time lag (s), the intercept obtained from extrapolating the linear region of the p(t) versus the time plot to the time axis, D is the diffusion coefficient (cm2 s−1).
The solubility coefficient (S) (cm3 (STP) cm−3 cm−1 Hg) was then calculated as:
| |
 | (3) |
The ideal selectivity of membranes (αA/B) was calculated from pure gas permeability as:
| |
 | (4) |
To test the reproducibility, at least three permeation tests were performed with each gas at a particular pressure and in the case of propylene and propane at higher feed pressure, three different films were tested. The results were within 5–8% error range.
3. Results and discussion
3.1. Membranes characterization
Fig. S1† shows the FTIR spectra of Matrimid®, synthesized silica, PI/S10 and PI/S20 nanocomposite membranes. As can be seen in Table 1, the peaks shown in the spectrum of pure Matrimid® were provided with the related bonds. The study of the spectra of nanocomposite membranes and pure polymer showed a strong absorption peak of 436 cm−1 in the nanocomposite membranes with the intensity increased by increasing the silica content. The peak was related to Si–O–Si rocking bonds not observed in the spectrum of the pure polymer (in Fig. S1,† the spectrum is specified with region (a)). In addition, the strong absorption peak at 790 cm−1 relating to the Si–O–Si symmetric stretch appeared in the nanocomposite membranes. Also, with the addition of silica nanoparticles, the width of the peak at 1090 cm−1 (C–N–C transverse stretch) for pure polymers (Table 1) was increased towards fewer wavelengths due to the peak of Si–O–Si asymmetric stretch bond at 1065 cm−1 (this increase in the width of the peak has been specified in part (b) of Fig. S1†). This indicated the presence of nanoparticles, on the ground that Si–O–Si asymmetric stretching bonds peak at 1065 cm−1 in the pure silica.27–29,35 Fig. 1 shows the spectrum for polyimide carbonyl groups and polyimide–silica nanocomposites separately. As can be seen, with the addition of nanoparticles into the nanocomposite membranes, the intensity of symmetric stretching peak of the carbonyls at 1714 cm−1 representative of the free carbonyl (groups without a hydrogen bond) was reduced and the intensity of the bonding carbonyl peak appearing at lower wavenumber within the range of 1680 cm−1 was increased. The reason for these changes was to create hydrogen bonds between the nanoparticles and the polyimide carbonyl group. The presence of hydrogen bonds, as shown in Fig. 2, indicated an appropriate interaction of particles and polymer which is one of the reasons causing uniform distribution of nanoparticles in the polymer matrix. Hydrogen bonding of polymer (carbonyl groups) to silica (OH groups of silica) also helps to avoid the formation of non-selective voids at the polymer/filler interface that is important to fulfill the selectivity enhancement.35,40,41 Also it has been observed that by increasing silica nanoparticles the peak related to the free carbonyl are slightly shifted. It confirms that by addition of silica nanoparticles in matrix the amount of hydrogen bonds between carbonyl groups and silica particles increase. Because the silica would lie between polymer chains the packing of the chains decrease and lead to decrement in the strength of the bonds between polymer chains and the absorption peaks shift to higher wave numbers. To observe the morphology of the cross-section of the membranes and examine the distribution of silica nanoparticles in the membranes, SEM test was conducted. As can be seen in Fig. 3–5, the SEM images of the cross-section of the membranes were prepared and shown with a magnification of 600, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 40
000 and 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. Fig. 3 shows the cross-section of the membranes with a magnification of 600 which represents a defect-free and uniform cross-section of the membranes. For a more accurate investigation of the distribution and dispersion of the particles in the polymer matrix, a higher magnifications of up to 20
000. Fig. 3 shows the cross-section of the membranes with a magnification of 600 which represents a defect-free and uniform cross-section of the membranes. For a more accurate investigation of the distribution and dispersion of the particles in the polymer matrix, a higher magnifications of up to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 40
000 and 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 was taken from the samples. As can be seen in Fig. 4 and 5, the silica particles with nanometric dimensions of smaller than 50 nm were distributed in the polymer matrix. The appropriate distribution of nanometric particles indicated establishment of a good particle–polymeric matrix compatibility which may be due to the occurrence of hydrogen bonding between the particles and the polymer. In nanocomposite membranes containing 15 and particularly 20 wt% silica, some groups of agglomerated particles could be observed, which, unlike the nanometric distributed particles, did not have an appropriate interaction with the polymer matrix. This lack of interaction led to the creation of voids at the agglomerated particle–polymeric matrix interface (Fig. 5). Therefore, it can be concluded that by adding particles up to 10 wt% only the nanometric distribution of the particles with a good polymer–particle interaction occurred, while with higher silica loading, agglomerated particles appeared in addition to the nanometric distributed particles.
000 was taken from the samples. As can be seen in Fig. 4 and 5, the silica particles with nanometric dimensions of smaller than 50 nm were distributed in the polymer matrix. The appropriate distribution of nanometric particles indicated establishment of a good particle–polymeric matrix compatibility which may be due to the occurrence of hydrogen bonding between the particles and the polymer. In nanocomposite membranes containing 15 and particularly 20 wt% silica, some groups of agglomerated particles could be observed, which, unlike the nanometric distributed particles, did not have an appropriate interaction with the polymer matrix. This lack of interaction led to the creation of voids at the agglomerated particle–polymeric matrix interface (Fig. 5). Therefore, it can be concluded that by adding particles up to 10 wt% only the nanometric distribution of the particles with a good polymer–particle interaction occurred, while with higher silica loading, agglomerated particles appeared in addition to the nanometric distributed particles.
Table 1 Band assignment and wavenumbers for Matrimid38,39
| Wavenumber (cm−1) |
Band assignment |
| 1752 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O symmetric stretch [imide I] O symmetric stretch [imide I] |
| 1720 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O anti-symmetric stretch [imide I] O anti-symmetric stretch [imide I] |
| 1696 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch of benzophenone carbonyl O stretch of benzophenone carbonyl |
| 1500 & 1488 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic stretching C aromatic stretching |
| 1374 |
CNC axial stretch [imide II] |
| 1097 |
CNC transverse stretch [imide III] |
| 721 |
CNC out-of-plane bending [imide IV] |
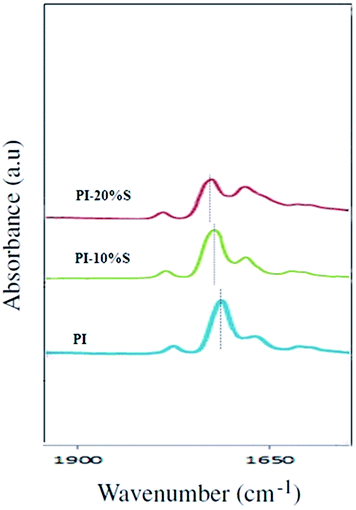 |
| | Fig. 1 FT-IR spectroscopy of prepared membranes in the carbonyl group range. It shows the effect of silica content on the position of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O symmetric stretch band. O symmetric stretch band. | |
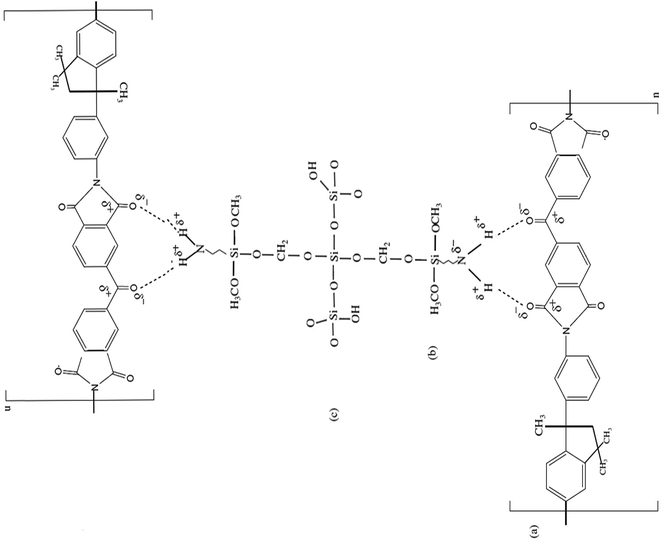 |
| | Fig. 2 Structures of (a) PI: Matrimid® 5218, (b) APTMOS (c) silica particles obtained from TEOS.37 | |
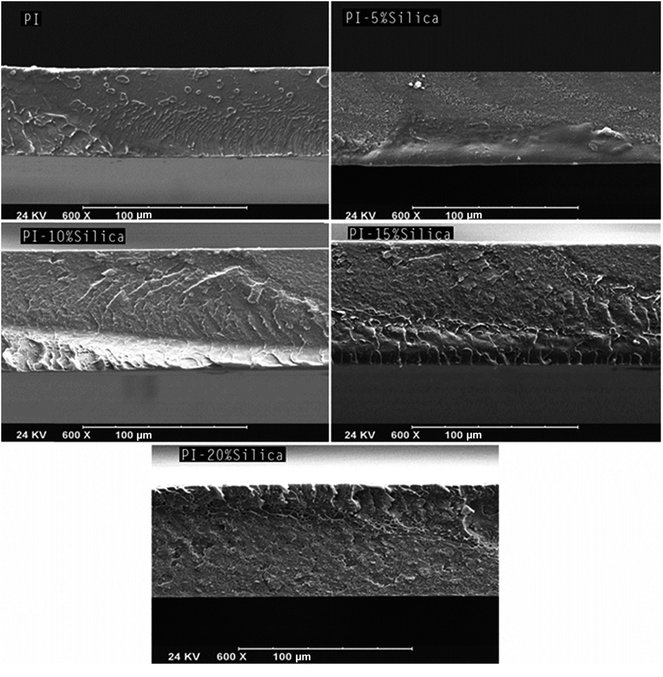 |
| | Fig. 3 SEM images of cross section of prepared membranes taken at a magnification of 600. | |
 |
| | Fig. 4 SEM images of cross section of prepared membranes taken at a magnification of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. 000. | |
 |
| | Fig. 5 SEM images of cross section of prepared membranes taken at a magnification of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. 000. | |
Fig. 6 shows the results of TGA test of the membranes. The test was conducted to detect the proportion of inorganic nanoparticles in the nanocomposite membranes and to investigate the effect of these materials on the thermal stability of pure polymer. As for Matrimid® membrane no significant weight loss is observed at temperatures below 170 °C. The first decay of the weight loss – degradation – starts at 170 °C and ends at 320 °C, which can be ascribed to low molecular weight macromolecules and other impurities within the polymer film. The second decay is started at 510 °C and ends at 670 °C and the chains of polyimide can undergo thermal degradation and exhibits the decomposition of the polymer fractions with imide groups. The last decay contained higher temperatures carbonizing the material into ash, leaving inorganic silica nanoparticles; in the carbonization process, the most weight loss of polyimides is induced by the expelling of noncarbon atoms (N, O) as different gases.27,29,35,42–45
 |
| | Fig. 6 Thermal degradation of prepared membranes. | |
This figure also shows that the presence of silica particles increased the temperature of the polymer degradation and by broadening the slope of membrane degradation, confirmed decelerated degradation of polyimide, thus improving its thermal properties. Therefore, the thermal stability of modified membrane is higher and this suggests a strong interaction between the matrix and filler. As the amount of silica in the membrane was increased, the complete decomposition temperature of the samples increased to 706 °C and 724 °C for PI–S10 and PI–S20 respectively and the residual weight of the sample was enhanced in accordance with the silica weight proportion.46 The DSC analysis of prepared film of Matrimid® by solution casting showed that its glass transition temperature to be 304 °C; while the glass temperature reported by Hunstman corporation is 302 °C and this deviation can be attributed to the different analytical equipment. Fig. 7 shows the glass temperature of pure and nanocomposite membranes. Results showed that by increasing nanoparticles loading in the membranes, their Tg values slightly increase and the Tg transition broadened slightly. The increase in Tg value by adding more silica nanoparticles can be attributed to the interaction between polymer chains and nanoparticles that restricted the movement and flexibility of the polymer chains.41,47
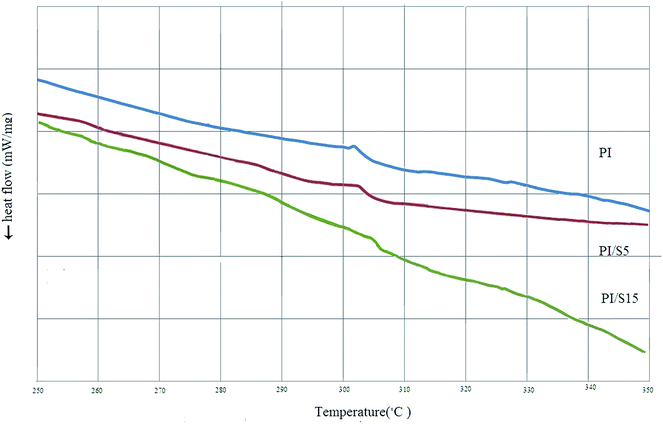 |
| | Fig. 7 DSC thermograms for pure Matrimid® and Matrimid®–silica hybrids. | |
3.2. Olefin/paraffin gas separation results
3.2.1. Effect of silica nanoparticles on the gas permeability. The permeability of the C3H8, C3H6, C2H4, and C2H6 gases in pure and nanocomposite membranes was measured at the feed pressure of 3 bar and a temperature of 30 °C. The results are reported in Fig. 8. It was revealed that the order of ethylene, ethane, propylene and propane permeation in pure and nanocomposite membranes was as follows:
| PC2H4 > PC3H6 > PC2H6 > PC3H8 |
 |
| | Fig. 8 Permeability of studied gases in PI and composite membranes at 30 °C and 3 bar. | |
It should be noted that in all prepared membranes, olefins had higher permeation than the corresponding paraffin and the selectivity of propylene/propane was higher than ethylene/ethane (Table 2). Larger bond angles of the C–H and C–C in olefins implies that their cross-section diameters are larger than that of the paraffins.13,14,35,47 That is why the kinetic diameter of olefins is greater than that of the paraffins (Table 3). But for two main reasons, olefins permeation was higher than that of the paraffins. The first reason is related to the smaller molecular length of the olefins to paraffin molecules. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond length is shorter than the C–C length, thereby making the diffusion (penetration) of molecules in the polymer chain easier. The second reason is related to electrons in pi bonds that cause momentary polarization of olefins and change the physical and chemical properties of olefins.6,15,35,42,48
C bond length is shorter than the C–C length, thereby making the diffusion (penetration) of molecules in the polymer chain easier. The second reason is related to electrons in pi bonds that cause momentary polarization of olefins and change the physical and chemical properties of olefins.6,15,35,42,48
Table 2 Permeability and ideal selectivities of studied gases through PI and nanocomposite membranes at 30 °C and 3 bar
| Membrane samples |
Selectivity |
| PC2H4/PC2H6 |
PC3H6/PC3H8 |
| PI |
3.25 |
9.00 |
| PI/S5 |
3.68 |
10.80 |
| PI/S10 |
4.14 |
12.70 |
| PI/S15 |
5.43 |
13.20 |
| PI/S20 |
6.13 |
18.03 |
Table 3 Physical properties of studied gases14,33
| Gas |
Condensability |
Molecular size |
| Tb (K) |
ε/k (K) |
σLJ (nm) |
σkt (nm) |
| Ethane |
184.5 |
230 |
0.442 |
0.38 |
| Ethylene |
169.5 |
205 |
0.423 |
0.39 |
| Propane |
231.1 |
254 |
0.506 |
0.43 |
| Propylene |
225.5 |
303 |
0.465 |
0.45 |
As reported in Fig. 8 in membrane containing 20% silica nanoparticles, the permeation of propylene and ethylene gases was increased from 0.099 barrer and 0.267 barrer in the pure membrane to 0.160 barrer and 0.411 barrer respectively. However, the results of the permeation of the propane and ethane gases showed a different trend. The increase in the number of particles in the polymeric matrix caused a slight reduction in the permeation of these gases, especially in the case of ethane. The permeation of ethane and propane in pure membrane was decreased from 0.082 barrer and 0.011 barrer to 0.067 barrer and 0.009 barrer in a membrane containing 20% silica nanoparticles respectively. The reasons for these changes in the gas permeation can be found by checking the effect of adding nanoparticles to the diffusion and solubility coefficient of gases. These reasons are provided in Sections 3.2.2 and 3.2.3. The increase in olefins permeation and the small changes in the paraffin permeation caused an increase in the selectivity of C2H4/C2H6 and C3H6/C3H8, which was due to the addition of silica nanoparticles. Therefore, the selectivity of C3H6/C3H8 was increased from 9.05 to 18.03, and the C2H4/C2H6 selectivity was increased from 3.25 to 6.13 (Table 2). The results also showed about 100% increase in C3H6/C3H8 selectivity and a 90% increase in C2H4/C2H6 selectivity by adding 20% silica nanoparticles to the pure polyimide membrane.
3.2.2. Effect of the silica nanoparticles on gas diffusivity. The diffusion coefficient of gases studied in pure and nanocomposite membranes has been reported in Table 4. The findings revealed that the order of the diffusion coefficients of the gases in all the membranes were as follows:
| DC2H4 > DC2H6 > DC3H6 > DC3H8 |
Table 4 Diffusion coefficients and diffusivity selectivity of studied gases through PI and PI–silica nanocomposite membranes at 30 °C and 3 bar
| Membrane samples |
Diffusivity coefficient (D × 1010 cm2 s−1) |
Diffusivity selectivity |
| C2H4 |
C2H6 |
C3H6 |
C3H8 |
DC2H4/DC2H6 |
DC3H6/DC3H8 |
| PI |
3.29 |
1.61 |
0.430 |
0.084 |
2.04 |
5.09 |
| PI/S5 |
2.82 |
1.36 |
0.334 |
0.055 |
2.07 |
6.07 |
| PI/S10 |
2.76 |
1.21 |
0.312 |
0.047 |
2.28 |
6.64 |
| PI/S15 |
2.27 |
1.10 |
0.276 |
0.043 |
2.06 |
6.36 |
| PI/S20 |
2.17 |
1.10 |
0.207 |
0.034 |
1.97 |
6.10 |
The higher diffusion coefficient of olefins, in the polymeric membrane indicated a smaller olefin molecular size in comparison with the paraffin with the same number of carbons. On the other hand, with the same number of carbons, the kinetic diameter of the paraffin was smaller than that of the olefins (Table 3). The kinetic diameter is calculated from the minimum equilibrium cross-sectional diameter but is not a good criterion to show the effective size of the hydrocarbon molecules in determining the molecular diffusion coefficient of the olefins and paraffins through the flexible polymer pores. For various reasons, as expressed in detail in the references, using the Lennard-Jones (σLJ) diameter rather than the kinetic diameter can yield more precise information on the size of the molecules with a large number of carbon atoms and therefore, it is used as the effective size of the C2–C4 gases. In Fig. 9, the logarithmic changes in the diffusion coefficients and the Lennard-Jones diameter of the various gases are shown. As can be seen, the linearity of this relationship, also referred to in other studies,14,35,51 represents the Lennard-Jones diameter as a criterion for the effective size of target gases passing through the polymeric membrane. The results in Table 4 also show that the diffusion coefficients of the gases were reduced by increasing the percentage of nanoparticles in the nanocomposite membranes. This amount of reduction in the diffusion coefficients from the nanocomposites with 15% nanoparticles to nanocomposites with 20% nanoparticles was negligible. Compared with that in the pure polymer, the reduction in the diffusion coefficients of the ethane, ethylene, propane and propylene gases in nanocomposites with 20% nanoparticles, was 32, 34, 59 and 52 percent respectively. These findings indicated that silica nanoparticles acted as rigid barriers against the diffusion of gases in polymer. Silica nanoparticles, served both as a filler by limiting the mobility of polymer chains and individually as a physical barrier in the path of the gas molecules. It can be concluded that the interaction between the silica nanoparticles and the polymeric chains was proper and the free volume and smooth path facilitating the penetration of the gas molecules were not created in nanocomposites with less than 15% nanoparticles. The findings from SEM analysis also indicated that below 15 wt% silica, agglomeration of silica particles was insignificant and negligible.
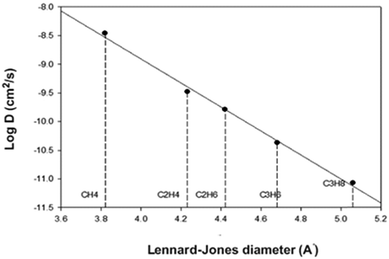 |
| | Fig. 9 Correlation of diffusion coefficients to Lenard-Jones diameter of gases in PI membranes. | |
3.2.3. Effect of silica nanoparticles on gas solubility. The solubility coefficient of the gases studied in pure and nanocomposite membranes is reported in Table 5. The findings indicated that the order of the solubility coefficients of gases in all the membranes were as follows:
| SC3H6 > SC3H8 > SC2H4 > SC2H6 |
Table 5 Solubility coefficients and solubility selectivity of studied gases through PI and PI–silica nanocomposite membranes at 30 °C and 3 bar
| Membrane samples |
Solubility coefficient (cm3 (STP) cm−3 cm−1 Hg) |
Solubility selectivity |
| C2H4 |
C2H6 |
C3H6 |
C3H8 |
SC2H4/SC2H6 |
SC3H6/SC3H8 |
| PI |
0.08 |
0.05 |
0.23 |
0.13 |
1.59 |
1.77 |
| PI/S5 |
0.10 |
0.06 |
0.32 |
0.18 |
1.76 |
1.78 |
| PI/S10 |
0.11 |
0.06 |
0.40 |
0.21 |
1.83 |
1.90 |
| PI/S15 |
0.16 |
0.06 |
0.47 |
0.23 |
2.67 |
2.04 |
| PI/S20 |
0.19 |
0.06 |
0.77 |
0.26 |
3.10 |
2.93 |
As for noble gas, on the ground of lack of particular interactions between polymer and the molecules of gas, the solubility of such gases as argon and neon in polymeric membranes, depends on the condensability of the gases. Thus, the solubility of the gases increases by means of increasing the size of the molecule, the critical temperature, and the boiling temperature. For olefins, due to the flat structure, polarity of these gases, and the presence of electron cloud in the pi bond in such as ethylene and propylene, the molecules could interact with the polymer. Therefore, despite the lower critical temperature of olefins in comparison with their corresponding paraffin, their solubility was greater. As known, sorption of gases in glassy polymers occur by dual mode sorption mechanism. Based on this mechanism, gases sorb in holes and active sites as Langmuir mode and then sorption promote by sorption of gases through polymer chains via Henry mode sorption. So, in glassy polyimide membrane there is some free volumes trapped in polymer chains which are suitable positions for sorption of all olefin and paraffin gases. But, on the ground that there are some polar sites which are only suitable for trapping polar olefin gases, the sorption of olefins are higher than paraffin in polyimide membrane. By studying the changes in the solubility coefficients of the membranes prepared after adding silica nanoparticles, it was found that the solubility coefficients of olefins significantly increased due to the presence of electron cloud on the double bond, the formation of momentary polar molecules and the appropriate interaction with hydroxyl polar functional group on the surface of the inorganic phase. In other words, the residual OH groups on the silica domain increase the polarity of the membrane matrix. So, the polar olefin molecules can interact more to the membrane media which increase the solubility of these gases in nanocomposite membranes. As mentioned earlier, the increase in the permeability of condensable CH4 and CO2 gases for hybrid membranes is considerably higher than that of non-condensable paraffin gases. As discussed, by incorporation of silica particles as mentioned above, polar sites like amine and OH groups which are interred by silica nano particles prepare extra active sites for interaction of olefins in membrane media. So, the sorption of olefins increase more than paraffins in MMMs.28,29,32,35,49 Moreover, APTMOS acts as a spacer and prevent aminopropyl groups of APTMOS from agglomerating. This phenomenon increases the homogeneous distribution of silica nanoparticles in the polymer.50 Although by creating physical barriers and tortuous path, the silica nanoparticles, decrease the diffusion of the gas molecules, they increase the solution of gas molecules in the polymer chain.6,14,26,29,35 For this reason, despite the reduction in the diffusion coefficient of all the target gases, due to the presence of silica nanoparticles, and the significant increase in the solubility coefficients of olefins, the permeation of these gases was enhanced. Investigation of the rate of the increase in the solubility coefficients of the gases studied revealed that the solubility coefficients of ethane, ethylene, propane and propylene gases were enhanced by 20, 137, 103, and 150 percent respectively with an addition of 20 percent silica nanoparticles. The lower increment in paraffin's solubility in nano-composites in comparison to olefins is related to their non-polar nature. Also, the lowest increment in solubility of ethane may be is due to its smaller size which increase the mobility of this gas in polymer and reduces its affinity to membrane matrix which decrease the sorption of this gas in sorption sites. Comparing the selectivity of both solubility and diffusivity of the membranes reported in Tables 4 and 5, indicated the dominance of the diffusivity selectivity in the separation of the gases studied through the pure glassy polyimide. The results indicated that addition of silica nanoparticles has a significant effect on increased solubility selectivity of membranes. However, the effect on the diffusivity selectivity was very low. Therefore, it can be said that the ideal selectivity increased with an increase in silica particles in the polymer (Table 2), probably associated more with increased solubility selectivity. Checking the solubility selectivity indicated an increase by 67 and 90 percent in the solubility selectivity of propylene/propane and ethylene/ethane respectively. However, no significant change was observed in diffusivity selectivity. In nanocomposite membranes containing 15 and 20% silica, diffusion selectivity showed a significant decrease. This was due to the holes and free spaces created at the interface of the agglomerated particles and the polymer matrix.
3.2.4. Effect of feed pressure on gas separation. The effect of feed pressure on the permeability, diffusion and solubility coefficients of gases can be seen in the pure polyimide Matrimid® membrane reported in Tables 7 and 8. The findings reported in Tables 6 and 7 showed that the permeability coefficient of ethane and ethylene gases decreased as the pressure increased, whereas a reduced permeability coefficient of up to 4 to 6 bar of feed pressure was observed in propane and propylene gases respectively. The passing of gases through the glassy polymers is described by the dual sorption model. According to this model, as described in detail in the references, with increased feed pressure, the permeation of gases decreased due to the saturation of the Langmuir sites.20,35,52 As a result, the permeability behavior of ethylene and ethane gases was found to be consistent with that of this model. However, in accordance with this model a bar feed pressure of 4 to 6 was observed for propane and propylene gases respectively. At higher feed pressures, permeation of these gases increased indicating the occurrence of the undesirable plasticization phenomenon.53 The feed pressure at which permeability shows an upturn is referred to as the plasticization pressure. Plasticization occurs due to the sorption of gases with high condensability in the polymer matrix and swelling of the polymer matrix increasing the amount of free volumes in polymer matrix. This phenomenon leads to a dramatic increase in the chain mobility and an increase in the membrane permeation.16,20,21 The findings reported in Tables 6 and 7 indicated that increased feed pressure results in increased diffusion coefficient of the gases. The increase in the diffusion coefficient was because of the important role of Henry's sites at high pressures. According to theory of partial immobilization introduced by Koros and Paul,54 gases sorbed in Henry's sites have greater mobility than the gases sorbed in Langmuir sites. Thus, at higher pressures, the diffusion coefficient is higher. Regarding more condensable gases, i.e. propane and propylene, at pressures higher than that required for plasticization, the increase in diffusion coefficient occurred with greater intensity. The reason was the excessive absorption of the gases in Henry's sites of polymer and the weakening of the forces between the molecules of polymer–polymer leading to easier diffusion of the gas molecules. Also, the findings from Table 6 indicated that with increased feed pressure, the solubility coefficient of ethane and ethylene gases is reduced in polymer. Based on the dual sorption model, at low pressures, Langmuir sites become saturated and at higher pressures, gas sorption occurs at Henry's sites, requiring a higher driving force. Therefore, with increased feed pressure, the solubility coefficient of the gases can decreases. For propane and propylene gases, this reduction of solubility coefficient was observed up to the plasticization pressure. Increased solubility coefficients at pressures higher than that for the plasticization pressure was due to the polymer chains swelling and reduced essential driving force for sorption in Henry's sites.35,55
Table 6 Effect of feed pressure on permeability, solubility and diffusion coefficients of ethane and ethylene in PI membrane at 30 °C
| Feed pressure (bar) |
Permeability (barrer) |
Diffusivity coefficient (D × 1010 cm2 s−1) |
Solubility coefficient (cm3 (STP) cm−3 cm−1 Hg) |
| C2H4 |
C2H6 |
C2H4 |
C2H6 |
C2H4 |
C2H6 |
| 1 |
0.305 |
0.090 |
3.21 |
1.58 |
0.095 |
0.057 |
| 3 |
0.267 |
0.082 |
3.29 |
1.61 |
0.081 |
0.051 |
| 5 |
0.251 |
0.080 |
3.54 |
1.78 |
0.071 |
0.045 |
| 8 |
0.228 |
0.062 |
5.71 |
3.01 |
0.04 |
0.02 |
Table 7 Effect of feed pressure on permeability, solubility and diffusion coefficients of propane and propylene in PI membrane at 30 °C
| Feed pressure (bar) |
Permeability (barrer) |
Diffusivity coefficient (D × 1010 cm2 s−1) |
Solubility coefficient (cm3 (STP) cm−3 cm−1 Hg) |
| C3H6 |
C3H8 |
C3H6 |
C3H8 |
C3H6 |
C3H8 |
| 1 |
0.122 |
0.013 |
0.393 |
0.072 |
0.31 |
0.18 |
| 3 |
0.099 |
0.011 |
0.430 |
0.084 |
0.23 |
0.13 |
| 4 |
0.084 |
0.010 |
0.442 |
0.091 |
0.19 |
0.11 |
| 5 |
0.087 |
0.010 |
0.457 |
0.125 |
0.19 |
0.08 |
| 6 |
0.090 |
0.009 |
0.473 |
0.160 |
0.19 |
0.06 |
| 7 |
0.100 |
0.011 |
0.500 |
0.180 |
0.20 |
0.06 |
| 8 |
0.117 |
0.014 |
0.505 |
0.196 |
0.23 |
0.07 |
Fig. 10 shows the effect of feed pressure on the permeation of propylene gas in pure membrane and the prepared nanocomposite membranes. As can be seen, with the addition of 5 wt% and 10 wt% of nanoparticles, the plasticization pressure of propylene was delayed from 4 to 5 and 6 bar in the pure membrane respectively. In the case of propane and propylene, the plasticizing effect was evaluated by measuring the ideal selectivity of oxygen/nitrogen, before and after the membrane's contact with propylene and propane. For example, permeability coefficient of propane in pure Matrimid® remained slightly unchanged as the pressure increases (up to 6 bar) and detection of plasticization was impossible except by measuring the ideal selectivity of oxygen/nitrogen. However, at 15 and 20 wt% silica, plasticization pressure did not improve. These findings suggested that due to limited polymer chain mobility, addition of inorganic silica nanoparticles up to the maximum content of 10 wt%, increased the polymer resistance to the plasticization phenomenon.35,56 But with a higher proportion of silica nanoparticles, agglomeration of particles and the free spaces around them led to the creation of suitable sites for the sorption of the more condensable gas, i.e., propylene. In our previous work the similar results obtained in separation of more condensable hydrocarbons from methane through polyurethane–silica nanocomposite membranes.57 This agglomeration and free space, as confirmed in the SEM images of nanocomposites containing 15% and 20% silica, resulted in the reduction of the plasticization pressure of propylene gas. Fig. 11 shows the effect of feed pressure on the permeation of propane gas in the prepared nanocomposite membranes. The findings revealed that none of the nanocomposite membranes was plasticized up to the testing pressure of 8 bar. However, pure polyimide membrane had a plasticization pressure of 6 bar. As a result, adding nanoparticles had a positive effect on the reduced undesirable plasticization phenomenon due to restricted motion of the polymer chains resulted from the chemical interactions established between chain polymer and nanoparticles. Further, since propane is less condensable than propylene, the presence of free spaces around agglomerated particles had no effect on plasticization. Fig. 12 and 13 show the changes in diffusion coefficient for propylene and propane gases as a function of feed pressure in nanocomposite membranes with different proportions of silica. As reported in these figures, for the above reasons, with increased feed pressure, the diffusion coefficient of the gases increased. Fig. 14 and 15 show the effect of feed pressure on the solubility coefficient of propylene and propane gases in nanocomposite membranes with different proportions of silica. As referred to above, it can be seen that increased feed pressure prior to plasticization pressure, led to decreased solubility coefficient. For the propylene gas, following the occurrence of the plasticization phenomenon, the solubility coefficient increased. The results of changes in the permeation coefficient of ethylene and ethane gases with feed pressure, as expressed in Table 6, showed no plasticization of pure polyimide membrane. Due to lack of plasticization, the effect of propane on nanocomposite membranes, it can be concluded that ethylene gas with less condensability could not plasticize the nanocomposite membranes. This was proved by investigating the effect of the feed pressure on the permeation coefficient of ethylene gas in nanocomposite membrane with 20% silica with no reference to the results. The changes of ideal selectivity in pure and nanocomposite membranes for propylene/propane gases at different feed pressures and at a temperature of 30 °C are reported in Table 8. The results indicated the low effect of feed pressure on the ideal selectivity of membranes prior to the plasticization pressure. But at pressures higher than the plasticization pressure, due to the sudden increase in the permeation coefficient of propylene, the ideal selectivity increased. At pressures higher than the plasticization pressure, the ideal selectivity was not a suitable and accurate criterion for the separation of the gas mixtures, indicating great differences in real selectivity. In Table 8, ideal selectivities calculated under plasticization condition are marked with asterisks.
 |
| | Fig. 10 Effect of feed pressure on permeation of propylene in prepared membranes at 30 °C. | |
 |
| | Fig. 11 Effect of feed pressure on permeation of propane in prepared membranes at 30 °C. | |
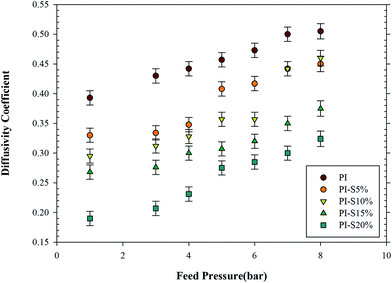 |
| | Fig. 12 Effect of feed pressure on diffusivity coefficient (D × 1010 cm2 s−1) of propylene in prepared membranes at 30 °C. | |
 |
| | Fig. 13 Effect of feed pressure on diffusivity coefficient (D × 1010 cm2 s−1) of propane in prepared membrane at 30 °C. | |
 |
| | Fig. 14 Effect of feed pressure on solubility coefficient (cm3 (STP) cm−3 cm−1 Hg) of propylene in prepared membranes at 30 °C. | |
 |
| | Fig. 15 Effect of feed pressure on solubility coefficient (cm3 (STP) cm−3 cm−1 Hg) of propane in prepared membranes at 30 °C. | |
Table 8 Effect of feed pressure on the ideal selectivity of propylene/propane at prepared membranes ata 30 °C
| Feed pressure (bar) |
Ideal selectivity |
| PI |
PI/S5 |
PI/S10 |
PI/S15 |
PI/S20 |
| *The ideal selectivities calculated in plasticization conditions. |
| 1 |
9.4 |
11.4 |
12.6 |
12.4 |
18.0 |
| 3 |
9.0 |
10.8 |
12.7 |
13.2 |
18.0 |
| 4 |
8.3 |
10.4 |
11.6 |
12.6 |
22.7* |
| 5 |
8.7* |
10.3 |
11.7 |
16.0* |
27.9* |
| 6 |
9.4* |
10.6* |
11.6 |
20.8* |
28.8* |
| 7 |
9.2* |
13.0* |
14.3* |
23.3* |
28.7* |
| 8 |
8.5* |
15.8* |
17.3* |
25* |
29.4* |
3.3. Performance of polyimide Matrimid®/silica membranes in olefin/paraffin separation
Finally, to compare the performance of nanocomposite membranes prepared in this study with other existing membranes for olefin–paraffin separation, the upper-bound diagram available in the ref. 15 and 58 was used. These diagrams indicated the location of the membrane on the selectivity curve based on olefin permeation. Since permeability and selectivity change in opposite directions, and usually, as one increases the other decreases, the membrane cannot be easily chosen as an appropriate option for industrial applications. For this reason, the upper-bound curves are good criteria for the possibility of a commercialized membrane. In Fig. 16 and 17, the performance of the membranes in comparison with that of an experimental upper-bound curve prepared for this study for separation of propylene/propane and ethylene/ethane pairs are shown. These diagrams indicate the desirability of the effectiveness of silica particles to improve the separation properties of polyimide membrane.
 |
| | Fig. 16 C2H4/C2H6 experimental upper bound based on pure gas permeation data. Symbols: (◆) 3 bar feed pressure and 30 °C (this study), (▲) 3.4 bar and 35 °C.48 | |
 |
| | Fig. 17 C3H6/C3H8 experimental upper bound based on pure gas permeation data. Symbols: (◆) 3 bar feed pressure and 30 °C (this study), (▲) 2 bar and 26 °C,21 (×) 2 bar and 35 °C.15 | |
4. Conclusion
In this study, the effect of incorporation of silica particles on the olefins/paraffin separation properties and plasticization resistance of Matrimid® membrane was studied. The results of the permeation of target gases indicated a significant increase in olefins permeation (as a result of considerable increase in the C3H6 and C2H4 solubility) and a small change in paraffin permeation an increase in the selectivity of C2H4/C2H6 and C3H6/C3H8, due to incorporation of silica particles in the polymer matrix. The results thus obtained indicated that addition of inorganic silica nanoparticles up to a maximum of 10% wt increased the polymer resistance to plasticization. Finally, by comparing the performance of nanocomposite membranes prepared in this study with that of the upper-bound diagram, the desirability of silica particles effectiveness in improving the separation properties of polyimide membrane was confirmed.
References
- R. Faiz and K. Li, Chem. Eng. Sci., 2012, 73, 261–284 CrossRef CAS.
- R. B. Eldridge, Ind. Eng. Chem. Res., 1993, 32, 2208–2212 CrossRef.
- D. Sanders and B. D. Freeman, Energy-efficient polymeric gas separation membranes for a sustainable future: a review, Polymer, 2013, 54, 4729–4761 CrossRef CAS.
- M. G. Buonomenna, RSC Adv., 2013, 3, 5694–5740 RSC.
- A. Shimazu, K. Ikeda and H. Hachisuka, Method of selectively separating unsaturated hydrocarbon, US Pat., 5,749,943, 1998 Search PubMed.
- C. Staudt-Bickel and W. J. Koros, J. Membr. Sci., 2000, 170, 205–214 CrossRef CAS.
- W. Koros, D. Paul and G. Huvard, Polymer, 1979, 20, 956–960 CrossRef CAS.
- R. D. Noble, J. Membr. Sci., 1990, 50, 207–214 CrossRef CAS.
- T. K. Ghosh, H. D. Lin and A. L. Hines, Ind. Eng. Chem. Res., 1993, 32, 2390–2399 CrossRef CAS.
- R. Kumar, T. Golden, T. White and A. Rokicki, Sep. Sci. Technol., 1992, 27, 2157–2170 CrossRef CAS.
- H. T. Kwon and H.-K. Jeong, Chem. Eng. Sci., 2015, 124, 20–26 CrossRef CAS.
- A. Ito and S. T. Hwang, J. Appl. Polym. Sci., 1989, 38, 483–490 CrossRef CAS.
- S. Sridhar and A. Khan, J. Membr. Sci., 1999, 159, 209–219 CrossRef CAS.
- K. Tanaka, A. Taguchi, J. Hao, H. Kita and K. Okamoto, J. Membr. Sci., 1996, 121, 197–207 CrossRef CAS.
- R. L. Burns and W. J. Koros, J. Membr. Sci., 2003, 211, 299–309 CrossRef CAS.
- Y. Shen and A. C. Lua, Chem. Eng. Sci., 2012, 188, 199–209 CrossRef CAS.
- M. Das and W. J. Koros, J. Membr. Sci., 2010, 365, 399–408 CrossRef CAS.
- G. Dong, H. Li and V. Chen, J. Membr. Sci., 2011, 369, 206–220 CrossRef CAS.
- A. F. Ismail and W. Lorna, Sep. Purif. Technol., 2002, 27, 173–194 CrossRef CAS.
- T. Visser and M. Wessling, J. Membr. Sci., 2008, 312, 84–96 CrossRef CAS.
- J. Krol, M. Boerrigter and G. Koops, J. Membr. Sci., 2001, 184, 275–286 CrossRef CAS.
- H. Eguchi, D. J. Kim and W. J. Koros, Polymer, 2015, 58, 121–129 CrossRef CAS.
- M. Askari and T.-S. Chung, J. Membr. Sci., 2013, 444, 173–183 CrossRef CAS.
- M. Askari, Y. Xiao, P. Li and T.-S. Chung, J. Membr. Sci., 2012, 390, 141–151 CrossRef.
- M. Buonomenna, W. Yave and G. Golemme, RSC Adv., 2012, 2, 10745–10773 RSC.
- S. A. Sabzevari, M. Sadeghi and A. Mehrabani-Zeinabad, Macromol. Chem. Phys., 2013, 214, 2367–2376 CrossRef CAS.
- S. Japip, H. Wang, Y. Xiao and T. S. Chung, J. Membr. Sci., 2014, 467, 162–174 CrossRef CAS.
- M. Sadeghi, G. Khanbabaei, A. H. S. Dehaghani, M. Sadeghi, M. A. Aravand, M. Akbarzade and S. Khatti, J. Membr. Sci., 2008, 322, 423–428 CrossRef CAS.
- M. Sadeghi, M. M. Talakesh, B. Ghalei and M. Shafiei, J. Membr. Sci., 2013, 427, 21–29 CrossRef CAS.
- A. Sharif, H. Koolivand, G. Khanbabaie, M. Hemmati, J. Aalaie, M. R. Kashani and A. Gheshlaghi, J. Polym. Res., 2012, 19, 1–8 CrossRef CAS.
- M. Mohagheghian, M. Sadeghi, M. P. Chenar and M. Naghsh, Korean J. Chem. Eng., 2014, 31, 2041–2050 CrossRef CAS.
- M. Sadeghi, M. A. Semsarzadeh and H. Moadel, J. Membr. Sci., 2009, 331, 21–30 CrossRef CAS.
- J. Ahn, W.-J. Chung, I. Pinnau and M. D. Guiver, J. Membr. Sci., 2008, 314, 123–133 CrossRef CAS.
- C. Joly, M. Smaihi, L. Porcar and R. Noble, Chem. Mater., 1999, 11, 2331–2338 CrossRef CAS.
- M. Naghsh, M. Sadeghi, A. Moheb, M. P. Chenar and M. Mohagheghian, J. Membr. Sci., 2012, 423, 97–106 CrossRef.
- D. Pye, H. Hoehn and M. Panar, J. Appl. Polym. Sci., 1976, 20, 1921–1931 CrossRef CAS.
- S. Rafiq, Z. Man, A. Maulud, N. Muhammad and S. Maitra, Sep. Purif. Technol., 2012, 90, 162–172 CrossRef CAS.
- A. Bos, High pressure CO2/CH4 separation with glassy polymer membranes: aspects of CO2-induced plasticization, University of Twente, 1996 Search PubMed.
- F. Zhou and W. J. Koros, Polymer, 2006, 47, 280–288 CrossRef CAS.
- M.-H. Tsai, Y.-C. Huang, I.-H. Tseng, H.-P. Yu, Y.-K. Lin and S.-L. Huang, Thin Solid Films, 2011, 519, 5238–5242 CrossRef CAS.
- Y. Xiao, T.-S. Chung, M. L. Chng, S. Tamai and A. Yamaguchi, J. Phys. Chem. B, 2005, 109, 18741–18748 CrossRef CAS PubMed.
- B. Zornoza, C. Téllez and J. Coronas, J. Membr. Sci., 2011, 368, 100–109 CrossRef CAS.
- O. Bakhtiari, S. Mosleh, T. Khosravi and T. Mohammadi, J. Membr. Sci. Technol., 2011, 1, 101, DOI:10.4172/2155-9589.1000101.
- M. Rahmani, A. Kazemi, F. Talebnia and G. Khanbabaei, Polym. Sci., Ser. B, 2014, 56, 650–656 CrossRef CAS.
- S. Qiu, Preparation and characterization of Matrimid/P84 blend films, M.Sc Thesis, Kansas State University, 2015 Search PubMed.
- X.-l. Liu, Y. Han, G. Gao, Z.-y. Li and F.-q. Liu, Chinese Journal of Polymer Science, 2008, 26, 255–262 CrossRef CAS.
- D. Droste and A. Dibenedetto, J. Appl. Polym. Sci., 1969, 13, 2149–2168 CrossRef CAS.
- S. S. Chan, R. Wang, T.-S. Chung and Y. Liu, J. Membr. Sci., 2002, 210, 55–64 CrossRef CAS.
- S. Kim and E. Marand, Microporous Mesoporous Mater., 2008, 114, 129–136 CrossRef CAS.
- M. Laghaei, M. Sadeghi, B. Ghalei and M. Dinari, Progress in Organic Coatings, 2016, 90, 163–170 CrossRef CAS.
- S. Semenova, J. Membr. Sci., 2004, 231, 189–207 CrossRef CAS.
- K. Okamoto, K. Noborio, J. Hao, K. Tanaka and H. Kita, J. Membr. Sci., 1997, 134, 171–179 CrossRef CAS.
- W. F. Yong, K. H. A. Kwek, K.-S. Liao and T.-S. Chung, Polymer, 2015, 77, 377–386 CrossRef CAS.
- D. Paul and W. Koros, J. Polym. Sci., Polym. Phys. Ed., 1976, 14, 675–685 CrossRef CAS.
- W. J. Koros, Sorption and transport in glassy polymers, in Chemical Engineering, The University of Texas at Austin, Austin, 1977 Search PubMed.
- N. N. Li, A. G. Fane, W. W. Ho and T. Matsuura, Advanced membrane technology and applications, John Wiley & Sons, 2011 Search PubMed.
- M. Sadeghi, M. A. Semsarzadeh, M. Barikani and M. P. Chenar, J. Membr. Sci., 2011, 376, 188–195 CrossRef CAS.
- M. Rungta, C. Zhang, W. J. Koros and L. Xu, AIChE J., 2013, 59, 3475–3489 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra00553e |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 40
000 and 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. Fig. 3 shows the cross-section of the membranes with a magnification of 600 which represents a defect-free and uniform cross-section of the membranes. For a more accurate investigation of the distribution and dispersion of the particles in the polymer matrix, a higher magnifications of up to 20
000. Fig. 3 shows the cross-section of the membranes with a magnification of 600 which represents a defect-free and uniform cross-section of the membranes. For a more accurate investigation of the distribution and dispersion of the particles in the polymer matrix, a higher magnifications of up to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 40
000 and 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 was taken from the samples. As can be seen in Fig. 4 and 5, the silica particles with nanometric dimensions of smaller than 50 nm were distributed in the polymer matrix. The appropriate distribution of nanometric particles indicated establishment of a good particle–polymeric matrix compatibility which may be due to the occurrence of hydrogen bonding between the particles and the polymer. In nanocomposite membranes containing 15 and particularly 20 wt% silica, some groups of agglomerated particles could be observed, which, unlike the nanometric distributed particles, did not have an appropriate interaction with the polymer matrix. This lack of interaction led to the creation of voids at the agglomerated particle–polymeric matrix interface (Fig. 5). Therefore, it can be concluded that by adding particles up to 10 wt% only the nanometric distribution of the particles with a good polymer–particle interaction occurred, while with higher silica loading, agglomerated particles appeared in addition to the nanometric distributed particles.
000 was taken from the samples. As can be seen in Fig. 4 and 5, the silica particles with nanometric dimensions of smaller than 50 nm were distributed in the polymer matrix. The appropriate distribution of nanometric particles indicated establishment of a good particle–polymeric matrix compatibility which may be due to the occurrence of hydrogen bonding between the particles and the polymer. In nanocomposite membranes containing 15 and particularly 20 wt% silica, some groups of agglomerated particles could be observed, which, unlike the nanometric distributed particles, did not have an appropriate interaction with the polymer matrix. This lack of interaction led to the creation of voids at the agglomerated particle–polymeric matrix interface (Fig. 5). Therefore, it can be concluded that by adding particles up to 10 wt% only the nanometric distribution of the particles with a good polymer–particle interaction occurred, while with higher silica loading, agglomerated particles appeared in addition to the nanometric distributed particles.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O symmetric stretch [imide I]
O symmetric stretch [imide I]![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O anti-symmetric stretch [imide I]
O anti-symmetric stretch [imide I]![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch of benzophenone carbonyl
O stretch of benzophenone carbonyl![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic stretching
C aromatic stretching
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O symmetric stretch band.
O symmetric stretch band.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond length is shorter than the C–C length, thereby making the diffusion (penetration) of molecules in the polymer chain easier. The second reason is related to electrons in pi bonds that cause momentary polarization of olefins and change the physical and chemical properties of olefins.6,15,35,42,48
C bond length is shorter than the C–C length, thereby making the diffusion (penetration) of molecules in the polymer chain easier. The second reason is related to electrons in pi bonds that cause momentary polarization of olefins and change the physical and chemical properties of olefins.6,15,35,42,48














