A family of polypropylene glycol-grafted polyethyleneimines reversibly absorb and release carbon dioxide to blow polyurethanes†
Yuanzhu Long‡
,
Fuhua Sun‡,
Chao Liu and
Xingyi Xie*
College of Polymer Science and Engineering, Sichuan University, Chengdu, Sichuan 610065, China. E-mail: xiexingyi@263.net; xiexingyi@scu.edu.cn; 125682383@qq.com; 714562196@qq.com; 1172562559@qq.com; Fax: +86-28-85405402; Tel: +86-28-85405129
First published on 25th February 2016
Abstract
The polyurethane foam community has encountered increasing pressure to replace the ozone depleting and/or global warming blowing agents, such as chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs). In this study, a series of polypropylene glycol-grafted polyethyleneimines (PPG-PEIs) were synthesised with the grafting rate ranging from 9% to 19%. Their CO2 adducts (PPG-PEI–CO2s) are thermally instable and can release CO2 to blow polyurethanes, whose polymerisation is exothermic. A PPG grafting rate of about 11% (found in sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2) is enough to homogenously disperse the blowing agent into polyurethane raw materials. The CO2 adducts with a PPG grafting rate of about 11–14% are particularly effective to decrease the foam density. The foams blown by 1
9-PPG-PEI–CO2) is enough to homogenously disperse the blowing agent into polyurethane raw materials. The CO2 adducts with a PPG grafting rate of about 11–14% are particularly effective to decrease the foam density. The foams blown by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2 possess a thermal conductivity higher than those of traditional polyurethane foams (0.040 vs. 0.020–0.027 W m−1 K−1), hindering their application in thermal insulation. However, these new blowing agents are advantageous in zero emission of volatile organic compounds (VOCs), as well as their climate friendliness. We believe these blowing agents can be used in thermally insulating foams in cars and aircraft where VOCs are strictly regulated.
9-PPG-PEI–CO2 possess a thermal conductivity higher than those of traditional polyurethane foams (0.040 vs. 0.020–0.027 W m−1 K−1), hindering their application in thermal insulation. However, these new blowing agents are advantageous in zero emission of volatile organic compounds (VOCs), as well as their climate friendliness. We believe these blowing agents can be used in thermally insulating foams in cars and aircraft where VOCs are strictly regulated.
1. Introduction
The worldwide demand for polyurethane foams will increase to 13.5 million tonnes in 2016 accounting for 5% of the world consumption of plastics,1,2 despite the fact that their blowing agents have raised concerns about climate change.3–6 Among them, previously used chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) can severely destroy the stratospheric ozone layer.3–6 The currently acceptable and popular hydrofluorocarbons (HFCs) are greenhouse gases that contribute to global warming.6,7In response to the climate change concerns, volatile hydrocarbons like cyclopentane8 and certain unsaturated fluorinated compounds like 1-chloro-3,3,3-trifluoropropene (HCFO-1233zd)9–11 have been introduced due to their nearly zero ozone depletion potential and very low global warming potential; the latter value is reported as 7 for cyclopentane4 and 5 for HCFO-1233zd,11 in contrast to 950 for HFC-245fa (1,1,1,3,3-pentafluoropropane).7 We recently explored a hydrophobically modified polyethyleneimine which can reversibly absorb and release CO2 to blow polyurethanes.12 Poly(propylene glycol) (PPG) chains were grafted onto branched polyethyleneimine (bPEI) backbones, making the resulting PPG-PEI and its CO2 adduct compatible with PPG polyols which are widely used as raw materials for polyurethane foams. In addition to its climate harmony, this blowing agent releases no volatile organic compounds (VOCs) to atmosphere. This is important in applications like internal car insulation.
In this study, we further optimise the PPG grafting rate of PPG-PEI based blowing agents in order to improve blowing efficiency, while retaining enough raw material compatibility. We believe this optimisation is essential for the real application of this type of climate friendly blowing agents.
2. Materials and methods
2.1. Synthesis of polypropylene glycol-grafted polyethyleneimines (PPG-PEIs) and their CO2 adducts
A series of PPG-PEIs with different grafting rates were synthesised by modifying our previous procedure.12 Briefly, branched PEI (bPEI, Mw = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da by light scattering provided by the supplier) and poly(propylene glycol) monobutyl ether (PPG, Mn = 340 Da) from Sigma-Aldrich served as starting materials. PPG was first transformed into α-glycidyl ether-ω-butyl-poly(propylene glycol) (PPG-EPO) by reaction with epichlorohydrin, as described previously.12 Thereafter, a known amount of PPG-EPO was mixed with 10 g of bPEI in 250 ml of ethanol under stirring. The molar ratio of the epoxy group in PPG-EPO to the amine groups (include primary, secondary and tertiary groups) in bPEI was set as 1
000 Da by light scattering provided by the supplier) and poly(propylene glycol) monobutyl ether (PPG, Mn = 340 Da) from Sigma-Aldrich served as starting materials. PPG was first transformed into α-glycidyl ether-ω-butyl-poly(propylene glycol) (PPG-EPO) by reaction with epichlorohydrin, as described previously.12 Thereafter, a known amount of PPG-EPO was mixed with 10 g of bPEI in 250 ml of ethanol under stirring. The molar ratio of the epoxy group in PPG-EPO to the amine groups (include primary, secondary and tertiary groups) in bPEI was set as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11, 1
11, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 1
9, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 and 1
7 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, respectively. After reaction at 50 °C for 15 h, the ethanol was rotationally evaporated under reduced pressure. Note that previous synthesis was performed at room temperature for 3 d with the molar ratio set as 1
5, respectively. After reaction at 50 °C for 15 h, the ethanol was rotationally evaporated under reduced pressure. Note that previous synthesis was performed at room temperature for 3 d with the molar ratio set as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 only.12 The viscous product was dissolved in ethanol/petroleum ether (1/10, v/v) and precipitated with water. The unreacted PPG and PPG-EPO residues were removed by repeatedly washing with petroleum ether. The purified product was named based on the feed molar ratio of epoxy to amine groups, as shown in Fig. 1A, e.g. 1
5 only.12 The viscous product was dissolved in ethanol/petroleum ether (1/10, v/v) and precipitated with water. The unreacted PPG and PPG-EPO residues were removed by repeatedly washing with petroleum ether. The purified product was named based on the feed molar ratio of epoxy to amine groups, as shown in Fig. 1A, e.g. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI means the feed molar ratio of epoxy to amine groups is 1
9-PPG-PEI means the feed molar ratio of epoxy to amine groups is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 in this sample. To prevent them from taking CO2 and moisture in air, these PPG-PEIs were stored in an N2-filled desiccator before any characterisation.
9 in this sample. To prevent them from taking CO2 and moisture in air, these PPG-PEIs were stored in an N2-filled desiccator before any characterisation.
To synthesise the corresponding CO2 adduct, each PPG-PEI was spread on a piece of release paper and purged with a CO2 flow under mechanical stirring. The PPG-PEI quickly absorbed the CO2 and transformed into a white solid in about 1 min. The solid was ground into powder, put into a steel container and saturated with CO2 at 0.5 MPa for about 1 h. The resulting product was designated by adding “-CO2” after the corresponding 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x-PPG-PEI (x = 5, 7, 9, or 11, Fig. 1A), for example 1
x-PPG-PEI (x = 5, 7, 9, or 11, Fig. 1A), for example 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11-PPG-PEI–CO2, 1
11-PPG-PEI–CO2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2, and so on.
9-PPG-PEI–CO2, and so on.
Pure bPEI absorbs CO2 very slowly (the strong intermolecular H-bonding hindered the permeation of CO2 and such interaction could be reduced by PPG side chains in PPG-PEIs12). Therefore, its CO2 adduct was synthesised by saturation of 10% bPEI in ethanol with CO2 at 0.5 MPa for 2 d to form a white precipitate, followed by a complete ethanol evaporation at 40 °C for 5 d. This control sample was designated PEI–CO2.
2.2. Dispersibility of PPG-PEI–CO2s in the white component of PU foaming system
Formulation I in Table 1 was used to test the dispersibility of each blowing agent with the rest of the white component. To do so, about 32 g of the white component without any blowing agent (i.e., two folds of Formulation I) was distributed into 6 glass vials with 5 g per vial. Five blowing agents (PEI–CO2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x-PPG-PEI–CO2, x = 5, 7, 9, or 11) were separately added into five of the vials, with 0.156 g per vial. The sixth vial served as a blank control. All liquids in the vials were magnetically stirred at 400 rpm for 2 min. After ageing for 3 d, macroscopic photographs were recorded using a digital camera. The aged samples were shaken to homogenise the mixture. Several drops of each mixture were spread on a glass slide and examined under an Olympus BX 43 light microscope (Olympus, Japan).
x-PPG-PEI–CO2, x = 5, 7, 9, or 11) were separately added into five of the vials, with 0.156 g per vial. The sixth vial served as a blank control. All liquids in the vials were magnetically stirred at 400 rpm for 2 min. After ageing for 3 d, macroscopic photographs were recorded using a digital camera. The aged samples were shaken to homogenise the mixture. Several drops of each mixture were spread on a glass slide and examined under an Olympus BX 43 light microscope (Olympus, Japan).
| Raw material | Description | Formulation (g) | |
|---|---|---|---|
| I | II | ||
| a Polymeric 4,4′-diphenylmethane diisocyanate. | |||
| White component | |||
| Polyether 4110 | 4-Arm poly(propylene glycol), OH number 430 mg KOH g−1 | 8.50 | 8.50 |
| Silicone L-3102 | Foam stabiliser | 0.16 | 0.26 |
| Stannous octoate (T-9) | Catalyst | 0.02 | 0.02 |
| Triethylenediamine (A-33) | Catalyst, 33 wt% in ethylene glycol | 0.20 | 0.04 |
| Tris(1-chloro-2-propyl) phosphate (TCPP) | Diluent and plasticiser | 7.15 | 7.30 |
| Propylene glycol | Chain extender | 0 | 0.06 |
| Diethanol amine | Cross-linking agent | 0 | 0.20 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x-PPG-PEI–CO2 (total weight) x-PPG-PEI–CO2 (total weight) |
Blowing agent, x = 5, 7, 9, or 11 | 0.50 (16.53) | 3.00 (19.38) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Black component | |||
| PMDIa | NCO content 31 wt% | 9.60 | 15.00 |
2.3. Preparation of polyurethane foams
To compare the foaming efficiency of all of the blowing agents (PEI–CO2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x-PPG-PEI–CO2, x = 5, 7, 9, or 11), one set of experiments was conducted using the same formulation (Formulation I, Table 1), but with a change in the blowing agent used. Firstly, all of the raw materials constituting the white component (16.53 g, Table 1) were thoroughly mixed in a 250 ml plastic cup by stirring at 1400 rpm for 30 s. Thereafter, 9.6 g of PMDI (black component, Table 1) was added and stirred at the same speed for 15 s. The start of this stirring was set as 0 s. The initially translucent mixture turned white due to the formation of tiny bubbles and rose freely until it became tack free. The whitening time, maximum foam-height time and tack-free time were recorded. Finally, all of the foams were conditioned at room temperature for 4 d before any test.
x-PPG-PEI–CO2, x = 5, 7, 9, or 11), one set of experiments was conducted using the same formulation (Formulation I, Table 1), but with a change in the blowing agent used. Firstly, all of the raw materials constituting the white component (16.53 g, Table 1) were thoroughly mixed in a 250 ml plastic cup by stirring at 1400 rpm for 30 s. Thereafter, 9.6 g of PMDI (black component, Table 1) was added and stirred at the same speed for 15 s. The start of this stirring was set as 0 s. The initially translucent mixture turned white due to the formation of tiny bubbles and rose freely until it became tack free. The whitening time, maximum foam-height time and tack-free time were recorded. Finally, all of the foams were conditioned at room temperature for 4 d before any test.
This set of experiments showed that 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2 was the most efficient blowing agent, resulting in the lowest foam density (about 160 kg m−3). The foaming art of 1
9-PPG-PEI–CO2 was the most efficient blowing agent, resulting in the lowest foam density (about 160 kg m−3). The foaming art of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2 blown polyurethanes was further modified by ageing the thoroughly mixed white component at room temperature for 3 d. After ageing, PMDI was added to start the foaming process described in the previous paragraph.
9-PPG-PEI–CO2 blown polyurethanes was further modified by ageing the thoroughly mixed white component at room temperature for 3 d. After ageing, PMDI was added to start the foaming process described in the previous paragraph.
To further lower the foam density, we conducted another set of experiments using an increased amount of blowing agents (3.00 g vs. 0.5 g) in the foam formulation (Formulation II, Table 1) while keeping the main ingredients like polyether 4110 and TCPP almost the same. Compared with Formulation I (Table 1), more foam stabiliser silicone L-3102 was used because much more bubbles would be generated in the new set of foams. The polymerisation speed was lowered using much less catalyst A-33, to possibly allow a full release of CO2 prior to foam solidification. Two new ingredients, propylene glycol and diethanol amine as the chain extender and cross-linker, respectively, could enhance the mechanical strength of the rising foams to reduce the breakage of growing bubbles. Moreover, they could assume more PMDI per unit mass than polyether 4110 because of their low molecular weights. Thus more PMDI was used in Formulation II. This would generate more heat, helping to release CO2 from the blowing agent. Except for the change in foam formulation, the foaming procedure was the same as described previously for the first set of foams. The whitening time, maximum foam-height time and tack-free time were also recorded.
2.4. Characterisations
The details about all the characterisation methods are described in ESI.† In brief, the chemical structures of PPG-PEIs and PPG-PEI–CO2s were characterised by 1H nuclear magnetic resonance (NMR) and Fourier transform infrared (FTIR) spectroscopy. The molecular weights before and after PPG grafting were measured by gel permeation chromatography (GPC). The CO2 content of each PPG-PEI–CO2 was determined by thermogravimetry (TG) and the enthalpy change (ΔH) due to the CO2 release was measured by differential scanning calorimetry (DSC). Both TG and DSC were performed from 30 to 300 °C at 10 °C min−1 under a nitrogen flow of 100 ml min−1. The cellular morphology of PU foams was examined under a scanning electron microscope.Five replicates (n = 5) were used to test PU foam density and properties unless otherwise specified. The density was calculated based on the precise weight (±0.1 mg) and dimensions (±0.1 mm) of each sample. The compressive stress–strain curve was recorded on an Instron 5507 Universal Testing Machine at a strain rate of 15% per min (i.e. 3 mm min−1) according to ASTM D1621. The thermal conductivity was measured on a Hot Disk TPS 2500-OT thermal analyser. The dimensional stability was measured on cubic samples (about 27 cm3) after annealing at 100 °C and −20 °C for 96 h, respectively, according to ISO 2796. The water uptake was determined by the weight change before and after immersion into distilled water at 23 ± 2 °C for 96 h (ISO 2896), using cubic samples with a volume of about 125 cm3. The water vapour permeability was determined by standard method (GB/T 2411) as well.
2.5. Statistical analysis
The property data from different groups were compared using a two-tailed student t-test where a significance level of p < 0.05 was accepted.3. Results
3.1. Structure and composition of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) x-PPG-PEI and their CO2 adducts
x-PPG-PEI and their CO2 adducts
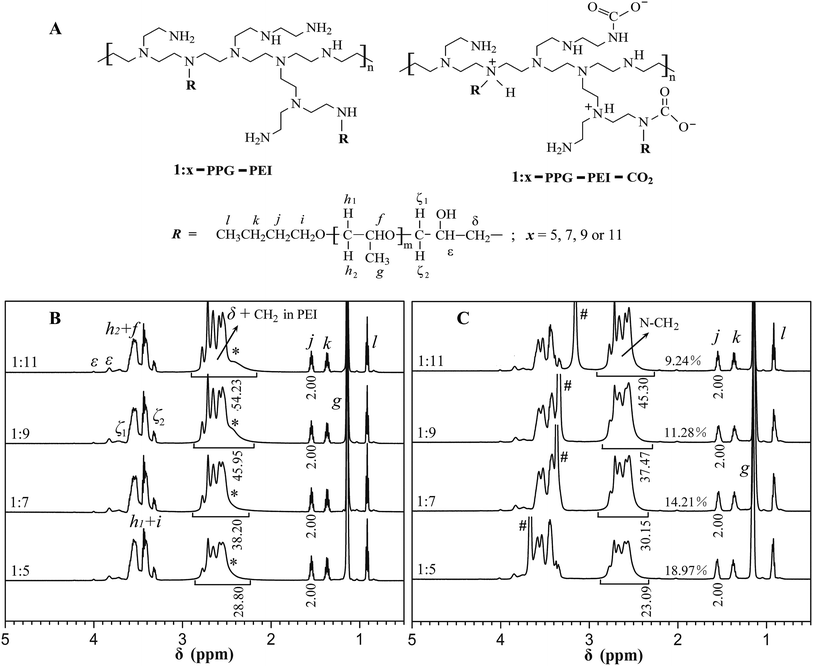
Shown in Fig. 1B, the 1H NMR spectra of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x-PPG-PEI display characteristic proton signals from both PEI backbones (N–CH2, chemical shift at 2.2–2.9 ppm) and PPG side chains (Hf–Hl, Fig. 1A). The presence of new signals associated with the linking groups (Hζ, Hε and Hδ) between the backbones and side chains confirms the success of PPG grafting.12 The active proton (N–H and O–H, Fig. 1A) signal that initially overlapped with the N–CH2 signals was shifted downfield by addition of CD3OD in the solvent CD3Cl (compare peaks indicated by * and # in Fig. 1). The relative area of pure N–CH2 signal (from PEI and Hδ) was used to calculate the PPG grafting rate (see ESI†) which is clearly consistent with corresponding theoretical grafting rate from the feed molar ratio (Table 2). The resultant 1
x-PPG-PEI display characteristic proton signals from both PEI backbones (N–CH2, chemical shift at 2.2–2.9 ppm) and PPG side chains (Hf–Hl, Fig. 1A). The presence of new signals associated with the linking groups (Hζ, Hε and Hδ) between the backbones and side chains confirms the success of PPG grafting.12 The active proton (N–H and O–H, Fig. 1A) signal that initially overlapped with the N–CH2 signals was shifted downfield by addition of CD3OD in the solvent CD3Cl (compare peaks indicated by * and # in Fig. 1). The relative area of pure N–CH2 signal (from PEI and Hδ) was used to calculate the PPG grafting rate (see ESI†) which is clearly consistent with corresponding theoretical grafting rate from the feed molar ratio (Table 2). The resultant 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x-PPG-PEI samples possess high PPG content ranging from 46% to 63.6% (Table 2). The molecular weight obtained from GPC increased as a function of the PPG content in PPG-PEIs, further suggesting that the PPG chains had been grafted onto the PEI backbones.
x-PPG-PEI samples possess high PPG content ranging from 46% to 63.6% (Table 2). The molecular weight obtained from GPC increased as a function of the PPG content in PPG-PEIs, further suggesting that the PPG chains had been grafted onto the PEI backbones.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x-PPG-PEIs and the CO2 adducts
x-PPG-PEIs and the CO2 adducts
| Parameter | Original PEI | Feed molar ratio in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x-PPG-PEI x-PPG-PEI |
|||
|---|---|---|---|---|---|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
||
a From 1H NMR spectra, see ESI.b The calculation of these parameters is shown in ESI. The theoretical CO2 contents are calculated based on the fact that two amino groups or two repeating units capture one CO2 molecule. The normalised ΔH is calculated by excluding the mass of PPG side chains in each 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x-PPG-PEI–CO2, and just based on the mass of the PEI–CO2 backbones. x-PPG-PEI–CO2, and just based on the mass of the PEI–CO2 backbones. |
|||||
| Grafting rate y (%) | |||||
| Theoretical (y = 1/x) | 9.1 | 11.1 | 14.3 | 20.0 | |
| Measureda | 9.2 | 11.3 | 14.2 | 19.0 | |
| PPG content (wt%)b | 46.0 | 50.9 | 56.7 | 63.6 | |
| Mn (104 Da) | 2.42 | 4.30 | 4.62 | 5.19 | 5.87 |
| Mw (104 Da) | 3.01 | 5.57 | 6.08 | 6.97 | 8.20 |
| Mw/Mn | 1.25 | 1.30 | 1.32 | 1.34 | 1.40 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| After conducted with CO2 | |||||
| Theoretical CO2 content (%)b | 33.8 | 21.7 | 20.1 | 18.1 | 15.7 |
| Measured weight loss (%) | 33.0 | 19.4 | 17.6 | 14.3 | 12.5 |
| PPG content (wt%)b | 37.1 | 41.9 | 48.6 | 55.7 | |
| Measured ΔH (J g−1) | 367 | 185 | 177 | 154 | 124 |
| Normalised ΔH (J g−1)b | 294 | 305 | 300 | 280 | |
The FTIR spectra of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x-PPG-PEI also confirm their chemical structure (Fig. 2A). For instance, typical N–H bending from PEI and C–O–C stretching from PPG are identified at 1652 cm−1 and 1114 cm−1, respectively. The intensity of PPG methyl bending at 1375 cm−1 (* indicated, Fig. 2A) increased from 1
x-PPG-PEI also confirm their chemical structure (Fig. 2A). For instance, typical N–H bending from PEI and C–O–C stretching from PPG are identified at 1652 cm−1 and 1114 cm−1, respectively. The intensity of PPG methyl bending at 1375 cm−1 (* indicated, Fig. 2A) increased from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11- to 1
11- to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5-PPG-PEI, consistent with the increase of PPG content across these samples (Table 2).
5-PPG-PEI, consistent with the increase of PPG content across these samples (Table 2).
The CO2 adducted samples displayed the same change trend in PPG methyl band (Fig. 2B). The CO2 adduction generated alkylammonium cations and carbamate anions (>NCOO− and –NHCOO−) (Fig. 1A) in the PEI backbones. This can be confirmed by a series of strong IR absorption bands at about 1630, 1570, 1477 and 1413 cm−1 (Fig. 2B), resulting from N–H bending in the alkylammoniums,13 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching in the carbamates,13 the asymmetrical and symmetrical skeletal stretching of the carbamate anions,13–15 respectively. The changes in IR spectra before and after CO2 adduction agree with the results reported previously.12
O stretching in the carbamates,13 the asymmetrical and symmetrical skeletal stretching of the carbamate anions,13–15 respectively. The changes in IR spectra before and after CO2 adduction agree with the results reported previously.12
Fig. 3 shows TG and DSC curves of the CO2 adducts. Negligible weight loss and zero enthalpy change (ΔH) were observed in the control sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5-PPG-PEI. The weight loss and endothermic process observed in the CO2 adducts can be associated with their CO2 release upon heating, as both showed the same temperature range for each sample (compare Fig. 3A and B). The measured weight loss in PEI–CO2 is very close to its theoretical CO2 content (33.0% vs. 33.8%, Table 2), showing complete CO2 absorption in this sample. Other samples possessed slightly lower weight loss than corresponding theoretical CO2 content (Table 2). Both measured weight loss and measured ΔH decreased from 1
5-PPG-PEI. The weight loss and endothermic process observed in the CO2 adducts can be associated with their CO2 release upon heating, as both showed the same temperature range for each sample (compare Fig. 3A and B). The measured weight loss in PEI–CO2 is very close to its theoretical CO2 content (33.0% vs. 33.8%, Table 2), showing complete CO2 absorption in this sample. Other samples possessed slightly lower weight loss than corresponding theoretical CO2 content (Table 2). Both measured weight loss and measured ΔH decreased from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11- to 1
11- to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5-PPG-PEI–CO2. However, the normalised ΔH's which excluded the weight of PPG side chains were similar across these samples (Table 2).
5-PPG-PEI–CO2. However, the normalised ΔH's which excluded the weight of PPG side chains were similar across these samples (Table 2).
Among all CO2 adducts, PEI–CO2 displayed the widest temperature range of CO2 release spanning from 60 to 180 °C (Fig. 3B). Overall, PPG grafting decreased the upper limit of CO2 release range by about 30 °C. Sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2 demonstrated the lowest initial release temperature (at ∼70 °C) among the PPG-grafted samples.
9-PPG-PEI–CO2 demonstrated the lowest initial release temperature (at ∼70 °C) among the PPG-grafted samples.
3.2. Dispersibility of the CO2 adducts in polyurethane foam raw materials
Fig. 4 shows macroscopic and microscopic photographs of the white components (Formulation I, Table 1) with and without the blowing agents. The control sample without any blowing agent (blank) was clear, showing no phase separation and no suspending particles during the entire experiment. PEI–CO2 displayed the worst dispersibility, as confirmed by the initially turbid and later precipitated appearance. Sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11-PPG-PEI–CO2 demonstrated a subsiding layer for the same period, with a more blurred interface compared with that in PEI–CO2 sample. Microscopically, some large and irregular particles still remained in both PEI–CO2 and 1
11-PPG-PEI–CO2 demonstrated a subsiding layer for the same period, with a more blurred interface compared with that in PEI–CO2 sample. Microscopically, some large and irregular particles still remained in both PEI–CO2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2 samples. Samples with 1
9-PPG-PEI–CO2 samples. Samples with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5-, 1
5-, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7- and 1
7- and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2 retained a translucent appearance for at least 3 d. Likewise, their microscopic images demonstrate a significant decrease in number and size of visible particles. Taking into consideration both macroscopic and microscopic observations, the dispersibility of all samples can be ranked as 1
9-PPG-PEI–CO2 retained a translucent appearance for at least 3 d. Likewise, their microscopic images demonstrate a significant decrease in number and size of visible particles. Taking into consideration both macroscopic and microscopic observations, the dispersibility of all samples can be ranked as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5- ≈ 1
5- ≈ 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9- ≈ 1
9- ≈ 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7- > 1
7- > 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11-PPG-PEI–CO2 > PEI–CO2.
11-PPG-PEI–CO2 > PEI–CO2.
 | ||
| Fig. 4 Evolution of macroscopic and microscopic photographs of the white components (Formulation I, Table 1) with and without the CO2 adducts, after stirring at 400 rpm for 2 min. | ||
3.3. Polyurethane foaming with different blowing agent
Fig. 5 compares density and mechanical strength of polyurethane foams based on Formulation I (Table 1). All foams using the CO2 adducts as blowing agents displayed a lower density than the blank foam blown by water impurity in the raw materials, with the lowest density (159.7 ± 2.2 kg m−3) found in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2 blown foam (Fig. 5A). The compressive strength positively correlated with the foam density, with the lowest strength occurring in 1
9-PPG-PEI–CO2 blown foam (Fig. 5A). The compressive strength positively correlated with the foam density, with the lowest strength occurring in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2 blown sample as well (Fig. 5B). Represented by this sample, white component ageing improved the compressive strength (Fig. 5C), while keeping the density statistically the same. In addition, both mechanical and morphological anisotropy were observed, independent of the white component ageing. The compressive strength at the foam rise direction was higher than that at the vertical direction (Fig. 5C). The vertical cross-sections displayed round pores, in contrast with an elliptical morphology parallel to the foam rise direction (Fig. 5D).
9-PPG-PEI–CO2 blown sample as well (Fig. 5B). Represented by this sample, white component ageing improved the compressive strength (Fig. 5C), while keeping the density statistically the same. In addition, both mechanical and morphological anisotropy were observed, independent of the white component ageing. The compressive strength at the foam rise direction was higher than that at the vertical direction (Fig. 5C). The vertical cross-sections displayed round pores, in contrast with an elliptical morphology parallel to the foam rise direction (Fig. 5D).
 | ||
Fig. 5 Property and morphology of polyurethane foams prepared with Formulation I (Table 1). ***: P < 0.001; **: P < 0.01; *: P < 0.05. (A) The foams blown by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2 possessed the lowest density among all the foams (measured with five samples i.e. n = 5). (B) The compressive yield strength at the foam rise direction (parallel) varied mainly depending on the sample density. (C and D) The anisotropic mechanical strength and anisotropic morphology observed in 1 9-PPG-PEI–CO2 possessed the lowest density among all the foams (measured with five samples i.e. n = 5). (B) The compressive yield strength at the foam rise direction (parallel) varied mainly depending on the sample density. (C and D) The anisotropic mechanical strength and anisotropic morphology observed in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2 blown foams, with the white component aged for 0 or 3 d. The foam rise direction (parallel) is indicated by a white arrowed line. The white component ageing enhanced the mechanical strength (n = 10, (C)), lowered and homogenised the pore size of the foams (D), but kept the densities statistically the same (P > 0.05, n = 10, (C)). Scale bars in (D): 100 μm. 9-PPG-PEI–CO2 blown foams, with the white component aged for 0 or 3 d. The foam rise direction (parallel) is indicated by a white arrowed line. The white component ageing enhanced the mechanical strength (n = 10, (C)), lowered and homogenised the pore size of the foams (D), but kept the densities statistically the same (P > 0.05, n = 10, (C)). Scale bars in (D): 100 μm. | ||
Another set of foams was prepared based on Formulation II (Table 1). Since more blowing agent was used in this set of experiments (3.0 g vs. 0.5 g), the catalyst A-33 content was significantly lowered to decrease the polymerisation speed and to allow a possibly full release of CO2. This polymerisation slowdown was confirmed by the much longer whitening time, foam rising time and track-free time than those in the first set of experiments (Table 3). The blank sample displayed a much lower density than that in previous experiments (148.7 kg m−3 vs. 239.3 kg m−3, Table 4), showing more water present in the raw materials for this set of samples. Addition of the CO2 adducts significantly decreased the foam density and compressive strength as expected (Fig. 6 and Table 4). The density of CO2 adduct-blown samples varied narrowly between 59 and 74 kg cm−3. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2 blown foam possessed a similar density with PEI–CO2 and 1
9-PPG-PEI–CO2 blown foam possessed a similar density with PEI–CO2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7-PPG-PEI–CO2 blown samples (P > 0.05, Fig. 6A). However, its density was statistically lower than those of 1
7-PPG-PEI–CO2 blown samples (P > 0.05, Fig. 6A). However, its density was statistically lower than those of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11- and 1
11- and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5-PPG-PEI–CO2 blown foams.
5-PPG-PEI–CO2 blown foams.
| Blowing agent | Whitening time (s) | Maximum foam-height time (s) | Tack-free time (s) | |||
|---|---|---|---|---|---|---|
| I | II | I | II | I | II | |
| a Without blowing agent used. | ||||||
| Blanka | 26 | 60 | 90 | 259 | 105 | 495 |
| PEI–CO2 | 18 | 44 | 78 | 195 | 94 | 325 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11-PPG-PEI–CO2 11-PPG-PEI–CO2 |
19 | 48 | 80 | 212 | 95 | 354 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2 9-PPG-PEI–CO2 |
22 | 51 | 82 | 230 | 97 | 422 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7-PPG-PEI–CO2 7-PPG-PEI–CO2 |
23 | 53 | 85 | 264 | 98 | 485 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5-PPG-PEI–CO2 5-PPG-PEI–CO2 |
25 | 55 | 85 | 279 | 100 | 504 |
| Blowing agenta | Density (kg m−3) | Foam volume (cm3) | Entrapped CO2b (ml) | Theoretically released CO2c (ml) | Foaming efficiencyd (%) |
|---|---|---|---|---|---|
a No blowing agent was used in blank samples.b Calculated by subtracting blank foam volume from each foam volume.c Calculated based on the moles of CO2 (CO2 content from TG curve) in each blowing agent, the highest temperature of foaming process (about 110 °C and 100 °C for Formulation I and II, respectively) and the standard air pressure (P = 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 325 Pa), using the ideal gas law as PV = nRT.d Obtained from entrapped CO2 volume divided by theoretically released CO2 volume. 325 Pa), using the ideal gas law as PV = nRT.d Obtained from entrapped CO2 volume divided by theoretically released CO2 volume. |
|||||
| 0.5 g, Formulation I | |||||
| Blank | 239.3 ± 2.9 | 109.19 | 0 | ||
| PEI–CO2 | 194.6 ± 2.2 | 134.28 | 25.09 | 117.90 | 21.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11-PPG-PEI–CO2 11-PPG-PEI–CO2 |
174.5 ± 1.9 | 149.74 | 40.55 | 69.31 | 58.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2 9-PPG-PEI–CO2 |
159.6 ± 2.2 | 163.72 | 54.53 | 62.88 | 86.7 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7-PPG-PEI–CO2 7-PPG-PEI–CO2 |
165.0 ± 2.1 | 158.36 | 49.17 | 51.09 | 96.2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5-PPG-PEI–CO2 5-PPG-PEI–CO2 |
175.5 ± 2.6 | 148.89 | 39.70 | 44.66 | 88.9 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| 3.0 g, Formulation II | |||||
| Blank | 148.7 ± 3.1 | 231.20 | 0 | ||
| PEI–CO2 | 59.5 ± 2.1 | 577.82 | 346.62 | 688.94 | 50.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11-PPG-PEI–CO2 11-PPG-PEI–CO2 |
69.2 ± 3.3 | 496.82 | 265.62 | 405.02 | 65.6 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2 9-PPG-PEI–CO2 |
62.4 ± 3.0 | 550.96 | 319.76 | 367.43 | 87.0 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7-PPG-PEI–CO2 7-PPG-PEI–CO2 |
66.0 ± 2.8 | 520.91 | 289.71 | 298.54 | 97.0 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5-PPG-PEI–CO2 5-PPG-PEI–CO2 |
73.3 ± 3.0 | 469.03 | 237.83 | 260.96 | 91.1 |
 | ||
| Fig. 6 Density and compressive strength of polyurethane foams prepared with Formulation II (Table 1). The compression tests (measured with five samples) were conducted at the foam rise direction. ***: P < 0.001; **: P < 0.01. | ||
It is reasonable that the net increase in foam volume relative to the blank sample stands for the CO2 volume trapped within the corresponding foam. Similarly, the percentage of trapped CO2 relative to theoretically released CO2 from each CO2 adduct indicates the foaming efficiency. Shown in Table 4, PEI–CO2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11-PPG-PEI–CO2 demonstrated relatively low foaming efficiencies (21–66%) while 1
11-PPG-PEI–CO2 demonstrated relatively low foaming efficiencies (21–66%) while 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-, 1
9-, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7- and 1
7- and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5-PPG-PEI–CO2 possessed foaming efficiencies of about 87% or over.
5-PPG-PEI–CO2 possessed foaming efficiencies of about 87% or over.
Table 5 compares the properties of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2 blown foams with those required for applications in thermal insulation. The foam properties should be further optimised for these applications; in particular, the thermal conductivity should be lowered.
9-PPG-PEI–CO2 blown foams with those required for applications in thermal insulation. The foam properties should be further optimised for these applications; in particular, the thermal conductivity should be lowered.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2 blown foams from Formulation II, in comparison with the data from Chinese standards
9-PPG-PEI–CO2 blown foams from Formulation II, in comparison with the data from Chinese standards
| Property | Measured | Required properties for thermal insulationa | |||
|---|---|---|---|---|---|
| Building walls | Refrigerators | Underground steel pipes | |||
| a Obtained from Chinese standards GB/T 10800, GB/T 2081 and SY/T 0415, respectively.b The height is parallel to foam rise direction. The minus data indicate shrinkage, and the controlled dimensional changes are given as absolute values. | |||||
| Density (kg m−3) | 62.4 ± 3.0 | ≥30 | 28–35 | 40–60 | |
| Compressive strength (kPa) | 203 ± 16 | ≥100 | ≥100 | ≥100 | |
| Thermal conductivity (W m−1 K−1) | 0.040 ± 0.002 | ≤0.027 | ≤0.022 | ≤0.03 | |
| Dimensional changes (%)b | |||||
| −20 °C for 96 h | Length | −0.96 ± 0.05 | ≤1 | ||
| Width | −1.17 ± 0.02 | ||||
| Height | 1.31 ± 0.13 | ||||
| 100 °C for 96 h | Length | −1.14 ± 0.05 | ≤5 | ≤1.5 | ≤3 |
| Width | −3.71 ± 0.20 | ||||
| Height | 2.11 ± 0.13 | ||||
| Water uptake (v/v%) | 2.3 ± 0.1 | ≤5 | ≤3 | ||
| Water vapour permeability (ng m−1 s−1 Pa−1) | 7.22 ± 1.24 | ≤6.5 | |||
4. Discussion
4.1. CO2 release temperatures and raw material compatibility of the CO2 adducts
We previously confirmed that 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5-PPG-PEI–CO2 can release CO2 to blow polyurethanes.12 To find better blowing agents, we changed the PPG grafting rate of the CO2 adduct whose chemical structure was confirmed by 1H NMR and FTIR spectra (Fig. 1 and 2). The consistency between measured and theoretical values in both PPG grafting rate and CO2 content, and the increase in molecular weight after PPG grafting (Table 2) clearly indicates the success in material synthesis.
5-PPG-PEI–CO2 can release CO2 to blow polyurethanes.12 To find better blowing agents, we changed the PPG grafting rate of the CO2 adduct whose chemical structure was confirmed by 1H NMR and FTIR spectra (Fig. 1 and 2). The consistency between measured and theoretical values in both PPG grafting rate and CO2 content, and the increase in molecular weight after PPG grafting (Table 2) clearly indicates the success in material synthesis.
The weight loss (Fig. 3) of CO2 adducts was an endothermic process caused by the decomposition of alkylammonium carbamates in the main chain that released CO2. The PPG grafting resulted in a decrease in primary amine-derived carbamate anions (–NHCOO−) in corresponding CO2 adduct because PPG grafting can preferentially consume primary amines in original PEI. The –NHCOO− groups decompose at higher temperatures than >NCOO− groups due to extra H-bonds among the former. Therefore, the decomposition temperature range of PEI–CO2 (with more –NHCOO− anions) extended up to 180 °C, about 30 °C higher than the upper limit of other CO2 adducts (Fig. 3). Accordingly, the normalised ΔHs in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x-PPG-PEI–CO2 were lower than the measured ΔH in PEI–CO2 (Table 2).
x-PPG-PEI–CO2 were lower than the measured ΔH in PEI–CO2 (Table 2).
The PPG side chains in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x-PPG-PEI–CO2 tended to expose to the liquid portion of the white component that contains a similar PPG polyol (polyether 4110, Table 1), which drove the dispersion of the CO2 adducts (Fig. 4). Both macroscopic and microscopic observation showed that samples 1
x-PPG-PEI–CO2 tended to expose to the liquid portion of the white component that contains a similar PPG polyol (polyether 4110, Table 1), which drove the dispersion of the CO2 adducts (Fig. 4). Both macroscopic and microscopic observation showed that samples 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-, 1
9-, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7- and 1
7- and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5-PPG-PEI–CO2 are compatible with the liquid white component while 1
5-PPG-PEI–CO2 are compatible with the liquid white component while 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11-PPG-PEI–CO2 and PEI–CO2 are not dispersible. The grafting rate of about 11% in 1
11-PPG-PEI–CO2 and PEI–CO2 are not dispersible. The grafting rate of about 11% in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2 (Table 2) is enough to generate a homogeneous foaming mixture for PU foams.
9-PPG-PEI–CO2 (Table 2) is enough to generate a homogeneous foaming mixture for PU foams.
4.2. Effects of PPG content on the foaming process
As shown in Table 3, the increase in PPG content decreased the foaming speed in both sets of experiments; the whitening time, maximum foam-height time and tack-free time were all roughly delayed with the increase of PPG content from 0% in sample PEI–CO2 to 63.6% in sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5-PPG-PEI–CO2 (Table 2). This might be explained by insight into the foaming process, where the blowing agent released increasing amounts of CO2 and gradually restored its original polyamine structure. The restored free amines quickly reacted with isocyanate groups in the growing polyurethane chains and/or catalysed the chain growth, accelerating the exothermic polymerisation, thus speeding up the CO2 release. Due to the steric hindrance caused by PPG side chains, such an acceleration effect decreased with the increased PPG content. So did the foaming speed.
5-PPG-PEI–CO2 (Table 2). This might be explained by insight into the foaming process, where the blowing agent released increasing amounts of CO2 and gradually restored its original polyamine structure. The restored free amines quickly reacted with isocyanate groups in the growing polyurethane chains and/or catalysed the chain growth, accelerating the exothermic polymerisation, thus speeding up the CO2 release. Due to the steric hindrance caused by PPG side chains, such an acceleration effect decreased with the increased PPG content. So did the foaming speed.
The slowdown in foaming speed allows a more complete release of CO2 before the foam solidification. This partially accounts for the rough increase in foaming efficiency from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11- to 1
11- to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5-PPG-PEI–CO2 (Table 4). The highest upper limit of CO2 release temperatures in PEI–CO2 (Fig. 3) might have prevented it from completely releasing CO2 during the foaming process. The poorest dispersibility of this sample (Fig. 4) resulted in the most inhomogeneous release of CO2; those relatively large bubbles might have broken up before the foam was set. All of these factors led to the lowest foaming efficiency in PEI–CO2 despite its highest CO2 content. For 1
5-PPG-PEI–CO2 (Table 4). The highest upper limit of CO2 release temperatures in PEI–CO2 (Fig. 3) might have prevented it from completely releasing CO2 during the foaming process. The poorest dispersibility of this sample (Fig. 4) resulted in the most inhomogeneous release of CO2; those relatively large bubbles might have broken up before the foam was set. All of these factors led to the lowest foaming efficiency in PEI–CO2 despite its highest CO2 content. For 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11-PPG-PEI–CO2, its poor dispersibility was also responsible for its low foaming efficiency. Samples 1
11-PPG-PEI–CO2, its poor dispersibility was also responsible for its low foaming efficiency. Samples 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-, 1
9-, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7- and 1
7- and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5-PPG-PEI–CO2 could more completely release CO2 during the foaming process because of their similarly low CO2 release temperatures (Fig. 3). Their relatively higher dispersibility (Fig. 4) and thus more homogenous release of CO2 would inhibit the formation of very large bubbles with high potential to break, facilitating the improved foaming efficiency, which was in the range from 86% to 97%.
5-PPG-PEI–CO2 could more completely release CO2 during the foaming process because of their similarly low CO2 release temperatures (Fig. 3). Their relatively higher dispersibility (Fig. 4) and thus more homogenous release of CO2 would inhibit the formation of very large bubbles with high potential to break, facilitating the improved foaming efficiency, which was in the range from 86% to 97%.
The large decrease in foaming speed from Formulation I to Formulation II (Table 3) caused an obvious increase in foaming efficiency in both PEI–CO2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11-PPG-PEI–CO2 (Table 4). On the other hand, samples 1
11-PPG-PEI–CO2 (Table 4). On the other hand, samples 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-, 1
9-, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7- and 1
7- and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5-PPG-PEI–CO2 displayed a stable and high foaming efficiency, regardless of the change in foaming formulation. In this case, the foaming efficiencies of PEI–CO2 and 1
5-PPG-PEI–CO2 displayed a stable and high foaming efficiency, regardless of the change in foaming formulation. In this case, the foaming efficiencies of PEI–CO2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11-PPG-PEI–CO2 were still much lower than those of 1
11-PPG-PEI–CO2 were still much lower than those of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-, 1
9-, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7- and 1
7- and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5-PPG-PEI–CO2 (Table 4). Again, the insufficient and/or inhomogeneous release of CO2 still occurred in both PEI–CO2 and 1
5-PPG-PEI–CO2 (Table 4). Again, the insufficient and/or inhomogeneous release of CO2 still occurred in both PEI–CO2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11-PPG-PEI–CO2 foaming systems.
11-PPG-PEI–CO2 foaming systems.
Overall, a homogeneous CO2 release resulting from the high dispersibility of the CO2 adduct and a match between the polymerisation speed and the CO2 release rate are key factors for a high foaming efficiency. This was the case for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7-PPG-PEI–CO2 foaming system, which displayed a foaming efficiency as high as 96–97% (Table 4). The slightly lower foaming efficiencies (87–91%) found in 1
7-PPG-PEI–CO2 foaming system, which displayed a foaming efficiency as high as 96–97% (Table 4). The slightly lower foaming efficiencies (87–91%) found in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9- and 1
9- and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5-PPG-PEI–CO2 can be improved by finely adjusting the foaming formulation, e.g. using more foam stabiliser to avoid bubble breakage before foam solidification.
5-PPG-PEI–CO2 can be improved by finely adjusting the foaming formulation, e.g. using more foam stabiliser to avoid bubble breakage before foam solidification.
The foam density depends on the CO2 content (Table 2) and the foaming efficiency (Table 4) of each blowing agent. The polyurethanes blown by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2 were among the least dense foams (Fig. 5 and 6). Only PEI–CO2 and 1
9-PPG-PEI–CO2 were among the least dense foams (Fig. 5 and 6). Only PEI–CO2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7-PPG-PEI–CO2 blown polyurethanes based on Formulation II had similar density (P > 0.05, Fig. 6A and Table 4). Currently, both 1
7-PPG-PEI–CO2 blown polyurethanes based on Formulation II had similar density (P > 0.05, Fig. 6A and Table 4). Currently, both 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7- and 1
7- and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5-PPG-PEI–CO2 are acceptable in terms of good dispersibility and high foaming efficiency. Taking into consideration the higher CO2 content and its potential to further improve the foaming efficiency, 1
5-PPG-PEI–CO2 are acceptable in terms of good dispersibility and high foaming efficiency. Taking into consideration the higher CO2 content and its potential to further improve the foaming efficiency, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2 can be the most promising candidate for real applications.
9-PPG-PEI–CO2 can be the most promising candidate for real applications.
The compressive strength is generally related to the density (Fig. 5B and 6B). However, the white component ageing for 3 days promoted the dispersion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2, thereby lowering and homogenising the pore size of the resultant foams (Fig. 5D). This morphological change did not affect the foam density but improved the compressive strength (indicated by *, Fig. 5C). The anisotropic morphology with the major axes of the elliptical pores pointing to the foam rise direction (Fig. 5D) is due to the horizontal confinement of the foam expansion by the container wall. As a result, the compressive strengths at the foam rise direction were larger than across it, both before and after the white component ageing (Fig. 5C). These observations clearly confirm that not only the foam density but also the porous morphology affects the mechanical strength.
9-PPG-PEI–CO2, thereby lowering and homogenising the pore size of the resultant foams (Fig. 5D). This morphological change did not affect the foam density but improved the compressive strength (indicated by *, Fig. 5C). The anisotropic morphology with the major axes of the elliptical pores pointing to the foam rise direction (Fig. 5D) is due to the horizontal confinement of the foam expansion by the container wall. As a result, the compressive strengths at the foam rise direction were larger than across it, both before and after the white component ageing (Fig. 5C). These observations clearly confirm that not only the foam density but also the porous morphology affects the mechanical strength.
4.3. Possible applications and future investigations
In fact, the foams prepared in this study belong to rigid polyurethane foams (whose yield strain < 10%, Fig. 5B and 6B) with closed individual pores (Fig. 5D). Their potential applications are as structural materials or as thermal insulation materials. As shown in Table 5, most of the current properties of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2 blown foams approximate to those required for insulating underground steel pipes. For the other two applications (building wall and refrigerator insulation), the foam density should be further lowered; this might be achieved by using more blowing agent in the formulation. Accordingly, the resultant compressive strength would be lowered to some extent, and it is not difficult to satisfy the required value of ≥100 kPa as the current strength is about 200 kPa (Table 5).
9-PPG-PEI–CO2 blown foams approximate to those required for insulating underground steel pipes. For the other two applications (building wall and refrigerator insulation), the foam density should be further lowered; this might be achieved by using more blowing agent in the formulation. Accordingly, the resultant compressive strength would be lowered to some extent, and it is not difficult to satisfy the required value of ≥100 kPa as the current strength is about 200 kPa (Table 5).
The dimensional change of the current foam is suitable for building wall insulation, but not acceptable for insulations of refrigerators and underground steel pipes (Table 5). The roughly higher shrinkage perpendicular to foam rise direction than expansion parallel to it suggests a reduced pressure in the individual foam pores resulting from a fast outward diffusion of CO2 and a slow inward diffusion of air.16 This diffusion mismatch has been reported by Tseng et al.17 in a 25 mm thick polyurethane foam blown by CO2 and CFC 11 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, by v/v) where CO2 completely diffused out in about 3 days, while the volume of air infiltrated for about 1 month (almost no diffusion of CFC 11 was observed in the same period). The anisotropic dimension change is related to the anisotropic mechanical strength (Fig. 5C) showing that the foams are more easily compressed horizontally (i.e. perpendicular to foam rise direction). The dimensional change can be reduced by ageing the foams for a longer time (e.g. for one month) and/or by improving the mechanical strength via optimisation in foaming art (e.g. ageing the white component to homogenise the pore size, Fig. 5C and D).
1, by v/v) where CO2 completely diffused out in about 3 days, while the volume of air infiltrated for about 1 month (almost no diffusion of CFC 11 was observed in the same period). The anisotropic dimension change is related to the anisotropic mechanical strength (Fig. 5C) showing that the foams are more easily compressed horizontally (i.e. perpendicular to foam rise direction). The dimensional change can be reduced by ageing the foams for a longer time (e.g. for one month) and/or by improving the mechanical strength via optimisation in foaming art (e.g. ageing the white component to homogenise the pore size, Fig. 5C and D).
A major obstacle for application in thermal insulations is the relatively high current thermal conductivity (Table 5). According to previously proposed models,17–19 the thermal conductivity (K) of a foam material is calculated by:
| K = Kg + 2Ksρf/3ρs + 16σT3/3(42.038ρf + 121.55) | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2 blown polyurethane (62.4 kg m−3, Table 5) and other parameters into eqn (1), one obtains the thermal conductivity of fresh foams (the trapped gas was CO2) as 0.0286 W m−1 K−1. However, with the replacement of CO2 by air due to diffusion, the thermal conductivity finally increases to the value of an air-filled foam which is calculated as 0.0377 W m−1 K−1. The measured value (0.040 ± 0.002 W m−1 K−1) is consistent with this calculated value, showing the trapped CO2 had been completely replaced by air when measuring the thermal conductivity.
9-PPG-PEI–CO2 blown polyurethane (62.4 kg m−3, Table 5) and other parameters into eqn (1), one obtains the thermal conductivity of fresh foams (the trapped gas was CO2) as 0.0286 W m−1 K−1. However, with the replacement of CO2 by air due to diffusion, the thermal conductivity finally increases to the value of an air-filled foam which is calculated as 0.0377 W m−1 K−1. The measured value (0.040 ± 0.002 W m−1 K−1) is consistent with this calculated value, showing the trapped CO2 had been completely replaced by air when measuring the thermal conductivity.
Eqn (1) shows that the thermal conductivity is density-dependent; the minimum value reaches 0.0358 W m−1 K−1 at about 33 kg m−3 for the air-equilibrated foams, decreasing by only 0.002 W m−1 K−1 compared with the calculated value at 62 kg m−3. Lowering density is not an effective way to decrease the thermal conductivity. The minimum value is even higher than the upper limit of the required thermal conductivities in standards (Table 5). Therefore, the current foam is not suitable for the applications listed in Table 5, just taking into account the thermal insulation property.
Traditional blowing agents (CFCs, HCFCs and HFCs) on the one hand possess very low thermal conductivity (e.g. HCFC 141b: 0.01 W m−1 K−1; CFC 11: 0.0082 W m−1 K−1)20 and on the other hand diffuse very slowly through polyurethane foams (the diffusion coefficients of CO2, N2 and O2 are two to three orders of magnitude higher than those of HCFCs and CFCs).21 Therefore, the out-diffusion of traditional blowing agents can last for years,22,23 helping to preserve the low thermal conductivity of the foams. For instance, a 1 cm-thick polyurethane foam blown by HCFC 141b still retains a thermal conductivity below 0.026 W m−1 K−1 after a 2 year ageing at 32.2 °C.22
The thermal conductivity (0.040 W m−1 K−1) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-PPG-PEI–CO2 blown polyurethane foams is comparable to those of expanded polystyrene (0.029–0.041 W m−1 K−1), but higher than those of traditional polyurethane foams (0.020–0.027 W m−1 K−1).24 The benefits due to zero emission of climate changing substances in the new blowing agents and the benefits due to the higher thermal resistance in traditional blowing agents should be comprehensively compared in terms of climatic impact, for example using life cycle analysis,7,25 which considers all impacts during the manufacturing processes and the overall life cycle of the corresponding foam product.
9-PPG-PEI–CO2 blown polyurethane foams is comparable to those of expanded polystyrene (0.029–0.041 W m−1 K−1), but higher than those of traditional polyurethane foams (0.020–0.027 W m−1 K−1).24 The benefits due to zero emission of climate changing substances in the new blowing agents and the benefits due to the higher thermal resistance in traditional blowing agents should be comprehensively compared in terms of climatic impact, for example using life cycle analysis,7,25 which considers all impacts during the manufacturing processes and the overall life cycle of the corresponding foam product.
The long-term release of blowing agents from traditional polyurethane foams can limit their application as thermal insulation materials in a closed or semi-closed environment (e.g. in cars, trains and aircrafts) where the local concentration of VOCs is strictly regulated.26,27 In this case, the new polyurethane foam in this study is a good alternative.
5. Conclusion
A series of CO2 adducts from polypropylene glycol-grafted polyethyleneimines were synthesised as climate-friendly blowing agents for polyurethanes. A grafting rate of 11% or higher facilitated a homogenous disperse of the CO2 adduct in the raw materials of polyurethane foams. Foams blown by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9- and 1
9- and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7-PPG-PEI–CO2 (with a PPG grafting rate of 11.3% and 14.2%, respectively) displayed the lowest foam density when the same amount of each blowing agent was used. The current thermal conductivity of the resultant foams is higher than those of traditional polyurethane foams (0.040 vs. 0.020–0.027 W m−1 K−1). Besides their climate neutrality, these new blowing agents do not release any VOCs, making them good candidates as thermal insulation materials in closed and semi-closed environments like in cars and aircrafts.
7-PPG-PEI–CO2 (with a PPG grafting rate of 11.3% and 14.2%, respectively) displayed the lowest foam density when the same amount of each blowing agent was used. The current thermal conductivity of the resultant foams is higher than those of traditional polyurethane foams (0.040 vs. 0.020–0.027 W m−1 K−1). Besides their climate neutrality, these new blowing agents do not release any VOCs, making them good candidates as thermal insulation materials in closed and semi-closed environments like in cars and aircrafts.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 51173111). Ms Hui Wang from the Analytical Centre, Sichuan University, China, helped to observe the SEM images, which is highly appreciated.References
- A. Cornille, S. Dworakowska, D. Bogdal, B. Boutevin and S. Caillol, Eur. Polym. J., 2015, 66, 129–138 CrossRef CAS.
- H. Singh and A. K. Jain, J. Appl. Polym. Sci., 2008, 111, 1115–1143 Search PubMed.
- V. Naik, A. K. Jain, K. O. Patten and D. J. Wuebbles, J. Geophys. Res., 2000, 105, 6903–6914 CrossRef CAS.
- L. D. D. Harvey, Build. Environ., 2007, 42, 2860–2879 CrossRef.
- K.-H. Kim, Z.-H. Shon, H. T. Nguyen and E.-C. Jeon, Atmos. Environ., 2011, 45, 1369–1382 CrossRef CAS.
- G. J. Velders, S. O. Andersen, J. S. Daniel, D. W. Fahey and M. McFarland, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 4814–4819 CrossRef CAS PubMed.
- A. McCulloch, J. Cell. Plast., 2009, 46, 57–72 CrossRef.
- W. Bazzo, A. Cappella and S. Talbot, J. Cell. Plast., 1996, 32, 46–61 CrossRef CAS.
- F. Molés, J. Navarro-Esbrí, B. Peris, A. Mota-Babiloni, Á. Barragán-Cervera and K. Kontomaris, Appl. Therm. Eng., 2014, 71, 204–212 CrossRef.
- Y. K. Ling and D. J. Williams, US Pat., 9,000,061 B2, 2015.
- R. J. Hulse, R. S. Basu, R. R. Singh and R. H. P. Thomas, J. Chem. Eng. Data, 2012, 57, 3581–3586 CrossRef CAS.
- Y. Long, L. Zheng, Y. Gu, H. Lin and X. Xie, Polymer, 2014, 55, 6494–6503 CrossRef CAS.
- N. Hiyoshi, K. Yogo and T. Yashima, Microporous Mesoporous Mater., 2005, 84, 357–365 CrossRef CAS.
- X. Wang, V. Schwartz, J. C. Clark, X. Ma, S. H. Overbury, X. Xu and C. Song, J. Phys. Chem. C, 2009, 113, 7260–7268 CrossRef CAS.
- G. Qi, Y. Wang, L. Estevez, X. Duan, N. Anako, A.-H. A. Park, W. Li, C. W. Jones and E. P. Giannelis, Energy Environ. Sci., 2011, 4, 444–452 Search PubMed.
- C. Cecchini, R. Zannetti and A. Stefani, J. Cell. Plast., 1999, 35, 514–530 CrossRef CAS.
- C. Tseng, M. Yamaguchit and T. Ohmorit, Cryogenics, 1997, 37, 305–312 CrossRef CAS.
- A. G. Leacht, J. Phys. D: Appl. Phys., 1993, 26, 733–739 CrossRef.
- W. H. Tao, H. C. Hsu, C. C. Chang, C. L. Hsu and Y. S. Lin, J. Cell. Plast., 2001, 37, 310–332 CrossRef CAS.
- H. Macchi-Tejeda, H. Opatovà and J. Guilpart, Int. J. Refrig., 2007, 30, 338–344 CrossRef CAS.
- M. C. Page and L. R. Glicksman, J. Cell. Plast., 1992, 28, 268–283 CrossRef CAS.
- K. E. Wilkes, W. A. Gabbard, F. J. Weaver and J. R. Booth, J. Cell. Plast., 2001, 37, 400–428 CrossRef CAS.
- K. E. Wilkes, D. W. Yarbrough, W. A. Gabbard and G. E. Nelson, J. Cell. Plast., 2002, 38, 317–339 CrossRef CAS.
- A. M. Papadopoulos, Energ. Build., 2005, 37, 77–86 CrossRef.
- R. W. Johnson, Int. J. Refrig., 2004, 27, 794–799 CrossRef CAS.
- K. Brodzik, J. Faber, D. Łomankiewicz and A. Gołda-Kopek, J. Environ. Sci., 2014, 26, 1052–1061 CrossRef CAS.
- C. Wang, X. D. Yang, J. Guan, K. Gao and Z. Li, Build. Environ., 2014, 78, 89–94 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Details on characterization methods, calculations about grafting rate of PPG-PEIs, and calculations about the theoretical CO2 content of each CO2 adduct. See DOI: 10.1039/c6ra00422a |
| ‡ Both authors equally contributed to the paper. |
| This journal is © The Royal Society of Chemistry 2016 |