Polypyrrole-decorated 2D carbon nanosheet electrodes for supercapacitors with high areal capacitance†
Jayesh Cherusseria and
Kamal K. Kar*ab
aAdvanced Nanoengineering Materials Laboratory, Materials Science Programme, Indian Institute of Technology Kanpur, Kanpur, Uttar Pradesh-208016, India. E-mail: kamalkk@iitk.ac.in; Fax: +91-512-2597408; Tel: +91-512-2597687
bDepartment of Mechanical Engineering, Indian Institute of Technology Kanpur, Kanpur, Uttar Pradesh-208016, India
First published on 16th June 2016
Abstract
Nanocarbon electrodes such as carbon nanotubes, graphene, graphene oxide, etc. are costly materials and hence it is necessary to develop new carbonaceous materials for application as electrodes for low cost supercapacitors (SCs) with high performance. Highly capacitive cost-free electrodes are synthesized with hierarchically mesoporous 2D exfoliated graphite nanosheet (EGN)/polypyrrole (PPY) (EGN/PPY) nanocomposites for supercapacitors (SCs). Initially, EGN is synthesized by a microwave irradiation technique from natural flake graphite with the help of perchloric acid–sulphuric acid–potassium dichromate–acetic acid anhydride based graphite intercalation system and EGN/PPY nanocomposites are prepared by in situ oxidative chemical polymerization thereafter. The EGN exhibits a Brunauer–Emmett–Teller surface area of 620 m2 g−1 with a mesoporous structure (average pore diameter ∼ 11 nm). The electron probe-microanalysis reveals that the PPY is uniformly coated over the surface of EGN. The surface topographies of the nanocomposites are examined by scanning electron microscopy and transmission electron microscopy. The structure and chemical bonding of the nanocomposites are determined by X-ray diffraction, Raman spectroscopy and Fourier-transform infrared spectroscopy. XPS analysis shows that various chemical species are attached on the surface of the EGN/PPY nanocomposites. The electrochemical properties of the symmetric type EGN/PPY SC cells are examined by electrochemical impedance spectroscopy, cyclic voltammetry and galvanostatic charge/discharge measurements. The galvanostatic charge/discharge measurement shows that the EGN/PPY SC cell exhibits a maximum area specific capacitance of 376.9 mF cm−2 with an area specific energy density of 0.052 mW h cm−2 at a current density of 0.25 mA cm−2 and it has a good cycle life too.
1. Introduction
Graphite is composed of layer planes of hexagonal networks of carbon atoms and these planes are considerably flat and have well-ordered structure. The basal planes are parallel, situated equidistant to one another and are linked together to form crystallites. Based on the number of layers of these well-oriented crystallites, graphite can be classified as exfoliated graphite (EG), multilayer graphene or single layer graphene. All these carbon nanostructures are examples of 2D carbon nanomaterials. Among the 2D nanocarbons, graphene has attracted much scientific attention due to its exceptional properties.1 Graphene based 2D nanostructures are potential candidates for applications in sensors,2 thin film transistors,3 batteries,4 solar cells,5 etc. but the major demerit associated with this fantastic material is its huge production cost. Hence the formation of 2D carbon nanomaterials with large surface area and electrical conductivity along with low cost is a necessity at present.Supercapacitors (SCs) are high power devices, which are different from the conventional capacitors in terms of the energy storage mechanism possessed by it. Electrochemical double layer capacitors and pseudocapacitors are the two different type of SCs in which the charges are stored in the electrochemical double layer capacitors by means of electrochemical double layers formed at the electrode/electrolyte interface whereas the mechanism of charge storage is by means of pseudo-faradaic reactions for the pseudocapacitors. Electric double layer capacitors exhibit comparatively low capacitance when compared to that of pseudocapacitors due to the enhanced redox-activity of the pseudocapacitive materials. In general, SCs utilize large surface area materials with good electronic conductivity. In this context, carbon nanomaterials are widely used as electrode materials in the case of electrochemical double layer capacitors. SC electrodes comprising of carbon nanofibers, carbon nanotubes (CNTs), graphene oxide, graphene etc. are widely used.6–10 The performance of SC is purely depending on the type, porosity, specific surface area, electronic conductivity, electrochemical activity, etc. of the electrode nanostructure. The major drawback of CNTs and graphene in SC electrode application is their huge cost. Hence the development of highly capacitive electrode materials of low cost is mandatory for the development of low cost SCs. In this context, EG is one of the best choice as it is a low cost 2D carbon nanomaterial with all the desired features for the SC electrode as described above. The number of layers of graphitic sheets in EG is nearly equal to the number present in the case of a multilayer graphene if the graphitic sheets in EG are not stacked together. Common method of synthesizing EG is temperature assisted procedure.11–14 Microwave irradiation is a simple and rapid process of preparing EG with high exfoliated volume in bulk quantities.15–17 Although carbon nanomaterials exhibit large surface area, good electronic conductivity, etc. but they exhibit low capacitance due to their electrochemical double layer charge storage. Hence nanocomposite electrodes consisting of carbon nanomaterials and pseudocapacitive materials (such as, transition metal oxides and electronically conducting polymers) have achieved much importance due to their high capacitance and good power delivery capability.18–24 The reason behind the enhancement in the supercapacitive performance of the nanocomposite electrode is that it stores energy by means of both faradaic and non-faradaic reactions. Development of lightweight SCs is mandatory for their application in portable devices. A large fraction of the weight of SC is taken by the current collector. Most of the SCs are massive due to the usage of heavy current collectors such as, metallic plates (e.g., Au, Cu, Ni, etc.), alloy (e.g., steel), etc. Hence by using current collectors with low specific gravity, one can fabricate a lightweight SC. Electronically conducting polymers are widely used for manufacturing the lightweight nanocomposite electrodes for SCs. Electronically conducting polymers such as, polyaniline, polypyrrole (PPY), poly(3,4-ethylenedioxythiophene), etc. exhibit good redox-activity and hence are promising candidates for boosting the performance of SC electrodes.25–29 Among the electronically conducting polymers, PPY has achieved much attention due to their good electronic conductivity, easy processability, good electrochemical stability, etc. Carbon nanomaterial/PPY nanocomposite electrodes exhibit good electrochemical properties due to their enhanced redox-activity and are suitable candidates for fabricating lightweight electrodes for SCs.30–40 Although PPY is used to synthesize nanocomposite with CNTs, graphene oxide, graphene, etc. but the use of EG for the same is not explored. As EG is a low cost material and it can be synthesized in bulk quantities by simple room temperature procedure, hence in the present study, we have used natural flake graphite (NFG) to synthesize EG nanosheet (EGN) by microwave irradiation. Further, the potential of EGN/PPY nanocomposite electrodes for application in lightweight SCs is examined. The EGN/PPY SCs exhibit high area specific capacitance due to their mesoporous electrode architecture coupled with large specific surface area.
2. Experimental section
2.1 Materials
NFG (carbon content > 99.9%, particle size ∼ 520 μm, conductivity ∼ 104 S cm−1) is obtained from Sigma Aldrich, India. Polyacrylonitrile based carbon fiber (CF) (specific gravity ∼1.74 g cm−3, tensile strength ∼1900–2750 MPa and diameter ∼8 μm) was received from M/S Fortafil Industries Inc., U.K. Perchloric acid (60%, HClO4), potassium dichromate (99%, K2Cr2O7) and sulphuric acid (98%, H2SO4) were purchased from M/S Qualigens Fine chemicals, India. Acetic acid anhydride (97%, (CH3CO)2O) was purchased from M/S Loba Chemie, India. Acetonitrile (CH3CN, 99.8%) was purchased from Merck Specialties Pvt. Ltd., India. Pyrrole (C4H5N) was received from Sigma Aldrich, India. Lithium perchlorate anhydrous (LiClO4, 99%) was received from Alfa Aesar, India.2.2 Synthesis of EGN
The EGN was prepared from NFG by microwave irradiation technique by using a domestic microwave oven (Samsung CE103VD). Initially, NFG was mixed with a graphite intercalation compound, which consisted of HClO4 (10.5 g), H2SO4 (5 g), K2Cr2O7 (3.7 g) and (CH3CO)2O (4.5 g). During the intercalation process, the molecules were intercalated ((CH3CO)2O) in between the boundaries of NFG with the help of oxidants (K2Cr2O7, HClO4 and H2SO4). After proper mixing of EGN with GIC with the help of a glass rod, it was transferred into a beaker and directly placed inside the microwave oven. The irradiation power was set at 600 W for a short period of 60 s. The as synthesized EGN were washed several times with deionized water followed by ethanol and was dried at 80 °C in a vacuum oven for a period of 24 hours thereafter.2.3 Syntheses of EGN/PPY nanocomposites
Prior to use, the pyrrole monomer was vacuum distilled as per standard procedures. A set of EGN/PPY nanocomposite samples were prepared by in situ oxidative chemical polymerization by varying the EGN to pyrrole mass ratio, with the help of ferric chloride (FeCl3) as an oxidant. The mass ratios of EGN to pyrrole (in wt%) selected were 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, 20
90, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, 30
80, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, 40
70, 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, 50
60, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 60
50, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, 70
40, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, 80
30, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and 90
20 and 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and the resulting nanocomposite samples were denominated as EGN0.1PPY0.9, EGN0.2PPY0.8, EGN0.3PPY0.7, EGN0.4PPY0.6, EGN0.5PPY0.5, EGN0.6PPY0.4, EGN0.7PPY0.3, EGN0.8PPY0.2 and EGN0.9PPY0.1, respectively. For the reference, pure PPY was also synthesized in a similar procedure without adding the EGN. In all the above cases, the molar ratio of pyrrole to FeCl3 was fixed to 1. In a typical procedure, EGN was initially dispersed in deionized water taken in a round bottom flask and ultrasonicated for a period of 30 min thereafter. In another procedure, FeCl3 was dispersed in deionized water and was stirred for 20 min with the help of a magnetic stirrer. After the sonication and stirring procedures, both the mixtures were added together in a round bottom flask and again ultrasonicated for a period of 10 min. Further, pyrrole monomer dispersed in deionized water was added dropwise to this mixture with the help of a burette fitted with a stop-cork and the mixture was kept for polymerization at 25 °C for 6 hours at vigorous stirring. After the polymerization, the samples were collected from the round bottom flask and washed several times with deionized water followed by ethanol and were dried in a vacuum oven for 24 hours thereafter. The overall process of synthesizing EGN/PPY nanocomposite from NFG is schematically shown in Fig. 1.
10 and the resulting nanocomposite samples were denominated as EGN0.1PPY0.9, EGN0.2PPY0.8, EGN0.3PPY0.7, EGN0.4PPY0.6, EGN0.5PPY0.5, EGN0.6PPY0.4, EGN0.7PPY0.3, EGN0.8PPY0.2 and EGN0.9PPY0.1, respectively. For the reference, pure PPY was also synthesized in a similar procedure without adding the EGN. In all the above cases, the molar ratio of pyrrole to FeCl3 was fixed to 1. In a typical procedure, EGN was initially dispersed in deionized water taken in a round bottom flask and ultrasonicated for a period of 30 min thereafter. In another procedure, FeCl3 was dispersed in deionized water and was stirred for 20 min with the help of a magnetic stirrer. After the sonication and stirring procedures, both the mixtures were added together in a round bottom flask and again ultrasonicated for a period of 10 min. Further, pyrrole monomer dispersed in deionized water was added dropwise to this mixture with the help of a burette fitted with a stop-cork and the mixture was kept for polymerization at 25 °C for 6 hours at vigorous stirring. After the polymerization, the samples were collected from the round bottom flask and washed several times with deionized water followed by ethanol and were dried in a vacuum oven for 24 hours thereafter. The overall process of synthesizing EGN/PPY nanocomposite from NFG is schematically shown in Fig. 1.
2.4 Preparation of EGN/PPY SC cells
Symmetric type SC cells were fabricated with EGN/PPY nanocomposite electrodes. Initially, SC electrode was fabricated by spray-coating the EGN/PPY nanocomposite on CF substrate. The SC cell was fabricated by sandwiching electrolyte soaked Whatman® filter paper between two EGN/PPY nanocomposite electrodes and the entire device was sealed with the help of biaxially oriented polypropylene films. A 5 M LiClO4 in acetonitrile was used as the electrolyte. The uncoated portion of the CF was used as current collector for the SC cell and no separate current collector is used.2.5 Material characterizations
The microstructure and surface topography of the EGN and EGN/PPY nanocomposites were studied using a scanning electron microscope (SEM; Carl Zeiss EVO MA 15) and transmission electron microscope (TEM; FEI Tecnai G2 12 Twin TEM 120 kV). Electron probe micro-analysis (EPMA) (JEOL JXA-8230) was carried out to understand the spatial distribution of PPY over the EGN/PPY nanocomposites. The thicknesses of the SC electrodes and the SC cell were measured by using a thickness gauge (S. C. Dey & Co., India). Raman spectroscopy was employed to determine the structure of the EGN/PPY nanocomposites with the help of LabRam Micro Raman Spectrometer (Jobin-Yvon HR 800 UV) by using a He–Ne (632.7 nm) laser excitation source. Fourier transform infrared spectroscopy (FTIR) experiments were performed by using infrared spectrometer (Perkin Elmer Spectrum 1) at the frequency range (4000–400) cm−1 in transmission mode spectrum. X-ray photoelectron spectroscopy (XPS; PHI 5000 Versa Probe II, FEI Inc.) was used to probe the chemical states of EGN/PPY nanocomposites. The high-resolution XPS spectra corresponding to C 1s, O 1s and N 1s peaks of EGN/PPY nanocomposites were analyzed by using the XPS Peak version 4.1 program, where Shirley-typed background and Gaussian–Lorentzian distributions are used to fit the baselines and the XPS peaks, respectively. The N2 sorption surface area measurement of EGN was performed with a surface area analyzer (Quantachrome Instruments Version 3.01, U.S.A.).2.6 Electrochemical measurements
Cyclic voltammetry (CV), electrochemical impedance spectroscopy and galvanostatic charge/discharge measurements were used to investigate the electrochemical performances of the EGN/PPY SC cells. The CV and galvanostatic charge/discharge measurements were carried out with the help of an electrochemical testing station (CHI 608D, CH Instruments, U.S.A.). The CV measurements were performed within a potential window of 0 to 1 V at different scan rates. The A.C. impedance spectroscopic measurement was carried out with an impedance analyzer (CHI 660C, CH Instruments, U.S.A.) at a frequency range of 105 to 0.02 Hz. All the calculations related to the SC cell testing are discussed in the ESI†.3. Results and discussion
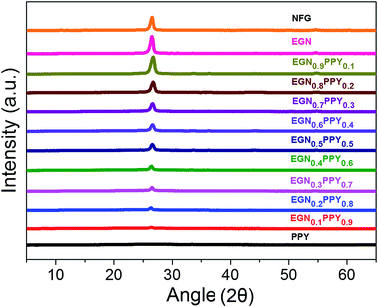
EGN with a large exfoliated volume of 594 ml g−1 is synthesized by microwave irradiation technique. In order to examine the surface morphology of the EGN, SEM analysis is performed. Fig. 2 represents the SEM images of EGN synthesized by microwave irradiation technique. From this figure, it is clear that the graphitic sheets are exfoliated along the c-axis by the microwave power. It is a versatile method of synthesizing bulk quantities of EG in a short span of time. Each EGN consists of few layers of graphene sheets stacked together by weak van der Waals forces. In order to examine the surface area of EGN, N2 sorption Brunauer–Emmett–Teller (BET) surface area measurement is carried out. The EGN exhibits a BET surface area of 620 m2 g−1. The N2 sorption isotherms of EGN is shown in Fig. 3a, which indicate that the type of sorption process is of type V in nature, which demonstrates the capillary condensation of gas within the opened pores of EGN. The total pore volume of EGN is 1.7 cm3 g−1 for pores smaller than 2490 Å (diameter) at P/Po = 0.99. The average pore diameter of EGN is calculated from the Barrett–Joyner–Halenda (BJH) pore-size distribution curve, as shown in Fig. 3b. An average pore diameter of EGNs is found to be 11 nm, which confirms that EGN exhibits a mesoporous structure. EGN with a mesoporous nanostructure coupled with large specific surface area is mandate for enhancing the effective surface area of the redox-active PPY in the EGN/PPY nanocomposite. Electrode with mesoporous structure is much preferred for SC application since such electrodes are capable for attaining maximum capacitances.23 Since EGN exhibits a mesoporous structure, the addition of thin layers of PPY can leads to an enhancement in the electrochemical performance of the electrode due to the enhancement in the effective surface area of the redox-active PPY. With this assumption, we have deposited PPY over the large surface area bearing EGN so as to synthesize EGN/PPY nanocomposites.Fig. 4a–i shows the SEM images of the EGN/PPY nanocomposites. From Fig. 4a–i, it can be seen that nanometer thick EGN is coated with PPY at varying thickness. In the case of EGN0.1PPY0.9 nanocomposite (Fig. 4a), high amount of PPY was deposited on the surface of EGN. The entire surface of EGN was covered by different PPY nanostructures such as, nanoparticles, dendrites and thin films. As the concentration of PPY increases, the presence of dendritic nanostructures of PPY are dominated in the EGN/PPY nanocomposites, this is due to the agglomeration of PPY nanoparticles on the surface of the PPY thin films, which were initially deposited on the surface of EGN. The dendritic growth of PPY is found to reduce from the EGN0.5PPY0.5 (Fig. 4e) and the succeeding nanocomposites (Fig. 4f–i) as the concentration of PPY is reduced within the nanocomposite. As the mass loading of EGN is beyond 60% in the EGN/PPY nanocomposite, PPY become insufficient to deposit on the surface of EGN and as a result, the nanocomposites with a stacked morphology is observed. This is possibly due to the re-stacking of individual EGNs together, which can be clearly seen from Fig. 4f–i. Hence it can be said that the PPY act as an agglomeration inhibiting agent in the EGN/PPY nanocomposite apart from its major role of charge storage. Fig. 5a–i shows the TEM images of various EGN/PPY nanocomposites and their corresponding selected area diffraction (SAED) pattern is given in the inset. From the SAED pattern, it is clear that the crystallinity of the EGN/PPY nanocomposite reduces upon the PPY incorporation. For e.g., in the case of EGN0.9PPY0.1 nanocomposite (Fig. 5i), it exhibit some order of crystallinity and completely vanished in the case of EGN0.1PPY0.9 nanocomposite (Fig. 5a). Although the thickness of the PPY coating can't be determined from these TEM images but the amorphous nature of EGN/PPY nanocomposite at an increasing PPY concentration can be easily analyzed. The crystallinity of the EGN/PPY nanocomposites can also be viewed from the XRD spectra, as shown in Fig. 6. A broadened 2θ diffraction peak at 20–30° represents the amorphous nature of PPY. The characteristic 2θ diffraction peak of C (002) at 26.6° and C (004) at 54.6° are present in the case of NFG and EGN with high intensity.41–43 As the concentration of EGN increases, the peak at 26.6° starts to appear in the spectra of EGN/PPY nanocomposites and a very intense and sharp peak can be seen in the case of EGN0.9PPY0.1 nanocomposite. Also, the 2θ diffraction peak at 54.6° is prominent in the case of EGN/PPY nanocomposites with large content of EGN.
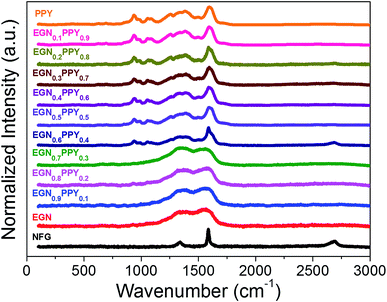
Raman spectroscopy is an effective tool to determine the structure of carbon nanomaterials and their composites. Fig. 7 shows the fingerprint Raman spectra of pure PPY, NFG, EGN and EGN/PPY nanocomposites. In the spectra of NFG and EGN, the G-band located at 1581 cm−1 represents the ordered in-plane sp2 carbon atoms and the D-band located at 1337 cm−1 with a small shoulder band at 1621 cm−1 (D′-band) indicate the disorder of edge carbons. An intense 2D-band at 2664 cm−1 can also be seen in their spectra, which originates from a two-phonon double resonance. It is to be noted here that these peaks are very sharp in the case of NFG and is broadened after the microwave exfoliation and it is due to the reduction in the particle size. In other words, it can be said that the broadening of peaks is due to the decreased thickness of the graphitic nanosheets and hence the formation of EGN. The characteristic spectra of PPY consisting of peaks located at 934 and 1086 cm−1 indicate the bipolaron structure and the peaks at 968 and 1055 cm−1 indicate the polaron structure.44 The skeletal band of PPY is appeared at 1500 cm−1 with low intensity and peak located at 1595 cm−1 represents the backbone stretching mode of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds. Apart from these, other peaks located at 1080 and 1380 cm−1 are corresponding to C–H in-plane stretching and C–H ring stretching, respectively.45,46 The EGN/PPY nanocomposites exhibit the characteristic features of both EGN and PPY components in their spectra. A broadening in the peaks can be seen after the incorporation of the PPY on the EGN, suggesting that the nanosheet size decreases due to the phonon confinement.47,48 While increasing the amount of PPY in the EGN/PPY nanocomposites, the characteristic peaks of PPY are found to be very sharp. From Fig. 7, it is clear that the interaction between EGN and PPY in the nanocomposites is very good, which indicates the successful formation of EGN/PPY nanocomposites.
C bonds. Apart from these, other peaks located at 1080 and 1380 cm−1 are corresponding to C–H in-plane stretching and C–H ring stretching, respectively.45,46 The EGN/PPY nanocomposites exhibit the characteristic features of both EGN and PPY components in their spectra. A broadening in the peaks can be seen after the incorporation of the PPY on the EGN, suggesting that the nanosheet size decreases due to the phonon confinement.47,48 While increasing the amount of PPY in the EGN/PPY nanocomposites, the characteristic peaks of PPY are found to be very sharp. From Fig. 7, it is clear that the interaction between EGN and PPY in the nanocomposites is very good, which indicates the successful formation of EGN/PPY nanocomposites.
The chemical structures of pure PPY, NFG, EGN and EGN/PPY nanocomposites are analyzed by FTIR and the corresponding spectra of all the samples are depicted in Fig. S1, ESI.† In the FTIR spectra of pure PPY, NFG and EGN, the band at ∼3433 cm−1 as a broad and intense signal can be assigned to O–H stretches due to the vibrations of intercalated water molecules. However, similar peaks are observed in the spectra of EGN/PPY nanocomposites also. In the spectra of EGN, peaks located at ∼1616, 1242, 1040, and 1126 cm−1 are correspond to the skeletal vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C from un-oxidized sp2 CC bonds, C–OH stretching, C–O stretching and C–O–C symmetric stretching, respectively.49,50 From the spectra of EGN, it is clear that various oxygen-containing functional groups are attached to the surface of EGN. Apart from these peaks, the spectra of EGN/PPY nanocomposites contain peaks positioned at ∼760, 1282, 1039, 1392 and 1560 cm−1 represent C–H vibration outside the plane, C–N stretching vibration and N–H stretching vibrations of PPY respectively.51,52 The intensity of these peaks are found to increase with an increase in the PPY concentration and these peaks are found very prominent in the case of EGN0.2PPY0.8 and EGN0.1PPY0.9 nanocomposites. And the peaks at 1616 and 1242 cm−1 are found to have less intensity for the nanocomposites with a minimum PPY content of 50% and it is due to the absence of oxygen groups on the PPY layers.
C from un-oxidized sp2 CC bonds, C–OH stretching, C–O stretching and C–O–C symmetric stretching, respectively.49,50 From the spectra of EGN, it is clear that various oxygen-containing functional groups are attached to the surface of EGN. Apart from these peaks, the spectra of EGN/PPY nanocomposites contain peaks positioned at ∼760, 1282, 1039, 1392 and 1560 cm−1 represent C–H vibration outside the plane, C–N stretching vibration and N–H stretching vibrations of PPY respectively.51,52 The intensity of these peaks are found to increase with an increase in the PPY concentration and these peaks are found very prominent in the case of EGN0.2PPY0.8 and EGN0.1PPY0.9 nanocomposites. And the peaks at 1616 and 1242 cm−1 are found to have less intensity for the nanocomposites with a minimum PPY content of 50% and it is due to the absence of oxygen groups on the PPY layers.
Symmetric type SC cells have been fabricated with EGN/PPY nanocomposites. The electrochemical impedance spectra, represented by Nyquist plot of EGN and EGN/PPY SC cells is shown in Fig. 8a. Nyquist plot of EGN and EGN/PPY SC cells consist of three segments: (i) a semicircle in the high frequency region, (ii) a Warburg line with a slope of ∼45° in the mid-frequency region and (iii) a nearly vertical straight line in the lower frequency region. The first region of Nyquist plot indicates the resistive nature and the second region represents the combined resistive and capacitive behaviour of the SC cell. From Fig. 8a, it can be observed that the EGN and EGN/PPY SC cells exhibit low bulk electrolyte resistance, Rb. A low Rb indicates high ionic conductivity of the SC electrodes. An ionic conductivity of 0.1 S cm−1 is obtained for pristine EGN electrode and the value is found to increase upon the PPY incorporation. The variation in the conductivity with respect to an increase in the PPY concentration in the EGN/PPY nanocomposites is shown in Fig. 8b. From Fig. 8b, it is clear that the conductivity is initially increased till a PPY concentration of 50 wt% in the nanocomposite and is found to decrease thereafter. The high conductivities indicate enhanced ion-diffusion through the porous network of EGN/PPY nanocomposite electrodes. At very low frequencies, the nearly vertical straight line represents good capacitive behaviour of EGN/PPY SC cells. In order to understand the typical I–E characteristics of the EGN/PPY SC cells, CV study is performed and the corresponding CV curves of EGN and EGN/PPY SC cells scanned at a constant rate of 300 mV s−1 are shown in Fig. 8c. The CV curve of EGN SC cell possesses a nearly rectangular shape whereas those utilizing EGN/PPY nanocomposite electrodes have shown slightly slopped rectangular-like shapes within a potential window of 0 to 1 V. The slightly slopped rectangular CV curves are very common to the SCs utilizing carbon nanomaterial/PPY nanocomposite electrodes.53–58 It can be inferred from Fig. 8c that the area under the curve of EGN SC cell is lesser than that of the EGN/PPY SC cells, which indicates that the nanocomposite electrodes exhibit enhanced electrochemical activities due to the increased redox activity exhibited by the PPY. Among the various EGN/PPY SC cells, the CV curve of EGN0.5PPY0.5 SC exhibits the largest encircled area and hence it can be said that this particular SC cell has displayed superior electrochemical behaviour when compared to the others. This may be due to the unique porous nanostructure of the EGN0.5PPY0.5 nanocomposite electrode in which an enhanced pseudo-faradaic reaction can be expected. The area under the CV curve correlates to the effective surface area of the SC electrode and CV curve with large encircled area will have the largest effective surface area. In this context, the EGN0.5PPY0.5 nanocomposite electrode exhibit large area under the CV curve and hence enhanced pseudo-faradaic reactions due to the accessibility of large effective surface area of the electrode can be expected. Although the BET surface area of EGN0.5PPY0.5 nanocomposite is 248 m2 g−1 but it exhibits a mesoporous structure with an average pore diameter of 6 nm, can be verified from Fig. S2, ESI.† It is understood that due to the PPY incorporation, some of the pores will be blocked by the PPY chains during the formation of EGN/PPY nanocomposite and hence the porous structure of the nanocomposite changes apparently. As the surface area of the electrode is directly related to the capacitance of EDLCs, a reduction in the surface area can results in a diminished performance.59 But the scenario is different for SCs utilizing pseudocapacitive electrode materials (for instance, PPY), the performance of SC is depending mainly on the pseudo-faradaic activity of the electrodes.60,61 Hence even if the EGN0.5PPY0.5 nanocomposite exhibits low surface area, due to the enhanced redox-activity of the nanocomposite electrode, high capacitance can be expected. The galvanostatic charge/discharge measurement of EGN and EGN/PPY SC cells are carried out at a constant current density of 0.25 mA cm−2 and the corresponding discharge curves are shown in Fig. 8d. From Fig. 8d, it can be observed that the rate performance of EGN SC cell is lower than that of the EGN/PPY SC cells and it is due to the improved pseudo-faradaic charge storage exhibit by the EGN/PPY nanocomposite electrodes. Fig. 9a represents the area specific capacitance obtained for the EGN and various EGN/PPY SC cells, calculated from the galvanostatic charge/discharge curves. The EGN SC exhibits an area specific capacitance of 3.5 × 10−4 mW h cm−2 and higher values can be seen in the case of EGN/PPY SC cells. It is already discussed that the conductivity of the EGN/PPY nanocomposites are high when compared to that of the pristine EGN electrode and this high conductivity is responsible for this enhanced performance. Here, mainly PPY contributes to the net capacitance of the EGN/PPY SC cell as compared to that of the EGN due to the high redox-activity of PPY component in the nanocomposite. A maximum area specific capacitance of 376.9 mF cm−2 is obtained for the EGN0.5PPY0.5 SC cell. The area specific capacitance obtained for the EGN0.5PPY0.5 SC cell is the highest when compared to that of the SCs in the literature.62–72 When the concentration of PPY in the nanocomposite electrode is increased, an increase in the areal capacitance till a PPY concentration of 50 wt% is observed. A further increase in the PPY concentration is found to diminish the performance of SC cell and this can be due to the excess deposition of PPY over the EGN in order to form the dendritic nanostructures of PPY. In such dendritic nanostructured electrodes, the electrolyte wetting is not proper towards the interior of the electrode and hence the redox-activity of the PPY is significantly reduced and as a result, the area specific capacitance is decreased. Here, the large surface area bearing EGN serve as the template for the nucleation of PPY nano-architectures. The volumetric capacitance of the EGN and EGN/PPY SC cells is depicted in Fig. 9b. A maximum volumetric capacitance of 3149 mF cm−3 is obtained for the EGN0.5PPY0.5 SC cell. In the case of EGN0.5PPY0.5 SC cell, a maximum area specific energy density of 0.052 mW h cm−2 (Fig. 9c) and a volumetric energy density of 0.437 mW h cm−3 (Fig. 9d) are obtained. The variation in the volume specific capacitance and volume specific energy density of EGN/PPY SC cells utilizing electrodes with varying EGN (or, PPY) concentration are shown in Fig. 9e and f, respectively.
Hierarchically mesoporous 2D electrode architecture is thus proved maximizing the electrochemical performance of the SC cell. Among the EGN/PPY SC cells, EGN0.5PPY0.5 SC cell exhibits enhanced supercapacitive performance and it may be due to the optimal loading of the PPY in the EGN/PPY nanocomposite electrode and the thickness of the coated films on the EGN surface. Fig. 10 shows the high resolution SEM images of EGN0.5PPY0.5 nanocomposite. From Fig. 10, it is evident that nanometer thick EGN/PPY nanocomposite films are formed with an average thickness of ∼200 nm. The mesoporous EGN0.5PPY0.5 nanocomposite electrode has undergone enhanced charge transfer reactions with the electrolyte ions and thereby an enhanced charge storage is achieved. XPS analysis is performed to determine the surface functional moieties present on the EGN0.5PPY0.5 nanocomposite, which may also contribute the net capacitance. Fig. 11 represents the XPS spectra of pristine EGN and EGN0.5PPY0.5 nanocomposite. Fig. 11a shows the C 1s de-convolution spectra of EGN. The main two components of C 1s peaks in Fig. 11a are corresponding to the sp2-hybridized carbons (at 282.8 eV) and the sp3-hybridized carbons (at 283.7 eV) and a peak at 286.2 eV with low intensity is corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or O–C
O or O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.73–76 Apart from these peaks, a major peak at 284.9 eV can be seen in the C 1s de-convolution spectra of EGN0.5PPY0.5 nanocomposite, as shown in Fig. 11c and this peak is corresponds to C–N or C
O.73–76 Apart from these peaks, a major peak at 284.9 eV can be seen in the C 1s de-convolution spectra of EGN0.5PPY0.5 nanocomposite, as shown in Fig. 11c and this peak is corresponds to C–N or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, which confirms the presence of PPY on the EGN surface.76 The O 1s de-convolution spectra of pristine EGN and EGN0.5PPY0.5 nanocomposite are shown in Fig. 11b and d, respectively. In both these spectra, the presence of various oxygen-containing surface functional groups can be observed. In Fig. 11b, the peaks at 529.9, 531.2, 532.4 and 534.8 eV are corresponding to C
N, which confirms the presence of PPY on the EGN surface.76 The O 1s de-convolution spectra of pristine EGN and EGN0.5PPY0.5 nanocomposite are shown in Fig. 11b and d, respectively. In both these spectra, the presence of various oxygen-containing surface functional groups can be observed. In Fig. 11b, the peaks at 529.9, 531.2, 532.4 and 534.8 eV are corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O–C, C–O and chemisorbed oxygen, respectively. But in the case of EGN0.5PPY0.5 nanocomposite, the peaks are slightly shifted towards the lower energy such as, 528.8, 530.3 and 531.7 eV for C
O, C–O–C, C–O and chemisorbed oxygen, respectively. But in the case of EGN0.5PPY0.5 nanocomposite, the peaks are slightly shifted towards the lower energy such as, 528.8, 530.3 and 531.7 eV for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O–C and C–O, respectively.75,77,78 The peak corresponding to chemisorbed oxygen is absent in the spectra of EGN0.5PPY0.5 nanocomposite due to the deposition of PPY over the surface of EGN. From Fig. 11d, it is evident that the intensity of oxygen containing functional groups is decreased significantly after the PPY deposition. It is important to note that the oxygen-containing surface functional groups on nanocarbon motifs also contribute to the electrochemical energy storage by means of charge-transfer reactions exhibited by such moieties. In the N 1s de-convolution spectra (Fig. 11e) of EGN0.5PPY0.5 nanocomposite, the peaks at 398, 398.6 and 399.6 eV are corresponding to –NH–, C–N and C–N+, respectively. The distribution of PPY over the EGN surface in the EGN0.5PPY0.5 nanocomposite is examined by EPMA. The SEM image of EGN0.5PPY0.5 nanocomposite used for the EPMA is depicted in Fig. 12a and the EPMA 2D map of elements such as carbon, oxygen and nitrogen of the nanocomposite are shown in Fig. 12b–d, respectively. From Fig. 12d, it is clear that the PPY is distributed uniformly over the surface of EGN.
O, C–O–C and C–O, respectively.75,77,78 The peak corresponding to chemisorbed oxygen is absent in the spectra of EGN0.5PPY0.5 nanocomposite due to the deposition of PPY over the surface of EGN. From Fig. 11d, it is evident that the intensity of oxygen containing functional groups is decreased significantly after the PPY deposition. It is important to note that the oxygen-containing surface functional groups on nanocarbon motifs also contribute to the electrochemical energy storage by means of charge-transfer reactions exhibited by such moieties. In the N 1s de-convolution spectra (Fig. 11e) of EGN0.5PPY0.5 nanocomposite, the peaks at 398, 398.6 and 399.6 eV are corresponding to –NH–, C–N and C–N+, respectively. The distribution of PPY over the EGN surface in the EGN0.5PPY0.5 nanocomposite is examined by EPMA. The SEM image of EGN0.5PPY0.5 nanocomposite used for the EPMA is depicted in Fig. 12a and the EPMA 2D map of elements such as carbon, oxygen and nitrogen of the nanocomposite are shown in Fig. 12b–d, respectively. From Fig. 12d, it is clear that the PPY is distributed uniformly over the surface of EGN.
 | ||
| Fig. 11 XPS spectra of EGN and EGN0.5PPY0.5 nanocomposite: C 1s (a) and O 1s (b) de-convolution spectra of EGN; C 1s (c), O 1s (d) and N 1s (e) de-convolution spectra of EGN0.5PPY0.5 nanocomposite. | ||
 | ||
| Fig. 12 EPMA 2D mapping of EGN0.5PPY0.5 nanocomposite: (a) SEM secondary electron image, (b) C-map, (c) O-map and (d) N-map [scale bar = 50 μm]. | ||
In order to study the electrochemical properties of EGN0.5PPY0.5 SC cell in detail, two-electrode cell CV and galvanostatic charge/discharge measurements have been performed. Fig. 13a represents the CV curves of EGN0.5PPY0.5 SC cell at different scan rates. The CV curves possess slightly slopped rectangular-like shapes within the potential window of 0 to 1 V and such curves indicate a high rate performance and efficient electronic and ionic transports within the EGN0.5PPY0.5 nanocomposite electrodes.79 The rate performance of EGN0.5PPY0.5 SC cell is evaluated by galvanostatic charge/discharge measurement at different current densities. The current densities opted for this measurement are 0.25, 0.5, 1, 1.5, 2.5, 4, 5 and 10 mA cm−2. The galvanostatic discharge curves of EGN0.5PPY0.5 SC cell at different current densities are shown in Fig. 13b. A periodic decrement in the rate performance can be observed from the discharge curves and this behaviour indicates the high reversibility of the EGN0.5PPY0.5 nanocomposite electrodes. The area specific capacitance of the EGN0.5PPY0.5 SC cell is calculated at different current densities and is shown in Fig. 13c. The SC cell exhibits high area specific capacitance at lower current densities and lower values at high current densities, which is very common in the case of SCs.80 This behaviour of the SC cell is due to the high rate of discharging at higher current densities and significantly low rate of discharging at lower current densities. An area specific capacitance of 301.6 mF cm−2 is obtained for the EGN0.5PPY0.5 SC cell at a comparatively higher current density of 5 mA cm−2. The reason behind the high area specific capacitance of EGN0.5PPY0.5 SC cell is the unique way of preparing the SC electrode with 2D nanocarbon architecture decorated with pseudocapacitive PPY in which the effective surface area of the redox-active PPY is fairly utilized for maximizing the charge-transfer reactions. The volumetric and volume specific capacitances of EGN0.5PPY0.5 SC cell obtained at different current densities are plotted in Fig. 13d and e, respectively. Similar to the area specific capacitance, the volumetric capacitance and volume specific capacitance are found to decrease at higher current densities. The variation in the area specific energy density with respect to an increase in the current density is shown in Fig. 13f. An area specific energy density of 0.042 mW h cm−2 is obtained at a comparatively high current density of 10 mA cm−2. A similar variation can be observed in the case of volume specific energy density also (Fig. 13g).
In order to examine the electrochemical cycling stability of the EGN0.5PPY0.5 SC cell, galvanostatic charge/discharge measurement has been carried out for 5000 continuous charge/discharge cycles at a constant current density of 15 mA cm−2 at 30 °C. The cell capacitance (Ccell) retention at different cycle numbers is depicted in Fig. 11h. From the galvanostatic charge/discharge electrochemical cycling study, it is clear that the EGN0.5PPY0.5 SC cell exhibits good cycle life. As discussed earlier, we have used CF current collector for the EGN0.5PPY0.5 SC cell and hence the weight of the SC cell can also be reduced significantly due to the low specific gravity of CF. It is important to note here that the specific gravities of the metallic current collectors are very high to that of CF, for instance, the weight of the SC cell can be reduced 10 times when compared to those utilizing gold current collectors. Since the present EGN0.5PPY0.5 SC cell exhibits high area specific capacitance and good cycle life, it is a promising candidate among the various high-performance lightweight SCs.
4. Conclusions
Lightweight and cost-free SCs have been fabricated with EGN/PPY nanocomposite electrodes and CF current collector. The EGN synthesized from NFG by microwave irradiation method exhibits a BET surface area of 620 m2 g−1 with an average pore diameter of 11 nm. Large surface area bearing 2D nanocarbon motif acted as the template for the polymerization of PPY, by this way mesoporous EGN/PPY electrodes have been synthesized. The SEM and TEM analyses have helped to understand the microstructure and surface morphology of the EGN/PPY nanocomposites. EPMA reveals that the distribution of PPY over the EGN surface is uniform and the effective formation of the nanocomposites was confirmed by XRD, FTIR and Raman spectroscopy. The various oxygen containing surface functional groups have been determined by XPS analysis. Galvanostatic charge/discharge measurement show that the EGN/PPY SC cell exhibits a maximum area specific capacitance of 376.9 mF cm−2 with an area specific energy density of 0.052 mW h cm−2 at a current density of 0.25 mA cm−2. The EGN/PPY SC cell is found to have a good cycle life too. The present EGN/PPY SC cell is a potential candidate among the SCs utilizing carbon nanomaterial/PPY nanocomposite electrodes in terms of high area specific capacitance, lightweight and good cycle life.Acknowledgements
The authors acknowledge the financial support provided by the Indian Institute of Technology Kanpur, India for carrying out this research work. The authors acknowledge Dr Malay K. Das, Department of Mechanical Engineering, Indian Institute of Technology Kanpur, India for providing the experimental facility for the SC testing and Dr Y. N. Mohapatra, Department of Physics, Indian Institute of Technology Kanpur, India for providing the A.C. impedance measurement facility for this work.Notes and references
- X. Zhang, B. R. S. Rajaraman, H. Liu and S. Ramakrishna, RSC Adv., 2014, 4, 28987 RSC
.
- Y. Liu, X. Liu, M. Li, Y. Liu, Z. Guo, Z. Xue and X. Lu, RSC Adv., 2015, 5, 100268 RSC
.
- R. Farghadan and A. Saffarzadeh, RSC Adv., 2015, 5, 87411 RSC
.
- J. Zai and X. Qian, RSC Adv., 2015, 5, 8814 RSC
.
- T. Jiao, D. Wei, J. Liu, W. Sun, S. Jia, W. Zhang, Y. Feng, H. Shi and C. Du, RSC Adv., 2015, 5, 73202 RSC
.
- J. Cherusseri and K. K. Kar, RSC Adv., 2015, 5, 34335 RSC
.
- J. Cherusseri and K. K. Kar, J. Mater. Chem. A, 2015, 3, 21586 RSC
.
- J. Cherusseri, R. Sharma and K. K. Kar, Carbon, 2016, 105, 113 CrossRef CAS
.
- J. Liu, B. Wang, F. Mirri, M. Pasquali and N. Motta, RSC Adv., 2015, 5, 84836 RSC
.
- L. Yuan, B. Yao, B. Hu, K. Huo, W. Chen and J. Zhou, Energy Environ. Sci., 2013, 6, 470 Search PubMed
.
- X. Zhang, H. Zhang, C. Li, K. Wang, X. Sun and Y. Ma, RSC Adv., 2014, 4, 45862 RSC
.
- H. Shioyama, J. Mater. Sci. Lett., 2001, 20, 499 CrossRef CAS
.
- M. Mazurkiewicz, US Pat., 54995, 2002
.
- H. Wu, C. Lu, W. Zhang and X. Zhang, Mater. Des., 2013, 52, 621 CrossRef CAS
.
- X. Y. Pang, M. K. Song, Y. Tian and M. W. Duan, J. Chil. Chem. Soc., 2012, 57, 1318 CrossRef CAS
.
- N. Sykam, R. K. Gautam and K. K. Kar, Polym. Eng. Sci., 2015, 55, 917 Search PubMed
.
- N. Sykam and K. K. Kar, Mater. Lett., 2014, 137, 150 CrossRef
.
- J. Cherusseri and K. K. Kar, Phys. Chem. Chem. Phys., 2016, 18, 8587 RSC
.
- J. Cherusseri and K. K. Kar, J. Mater. Chem. A, 2016 10.1039/c6ta02690g
.
- Y. Xie, Y. Liu, Y. Zhao, Y. H. Tsang, S. P. Lau, H. Huang and Y. Chai, J. Mater. Chem. A, 2014, 2, 9142 RSC
.
- D. Li, Y. Li, Y. Feng, W. Hu and W. Feng, J. Mater. Chem. A, 2015, 3, 2135 RSC
.
- R. Oraon, A. D. Adhikari, S. K. Tiwari, T. S. Sahu and G. C. Nayak, Appl. Clay Sci., 2015, 118, 231 CrossRef CAS
.
- H. Wang, Y. Zhang, W. Sun, H. T. Tan, J. B. Franklin, Y. Guo, H. Fan, M. Ulaganathan, X. L. Wu, Z. Z. Luo, S. Madhavi and Q. Yan, J. Power Sources, 2016, 307, 17 CrossRef CAS
.
- M. Liu, J. Chang, Y. Bai and J. Sun, RSC Adv., 2015, 5, 91389 RSC
.
- P. Tammela, Z. Wang, S. Frykstrand, P. Zhang, I. M. Sintorn, L. Nyholm and M. Strømme, RSC Adv., 2015, 5, 16405 RSC
.
- R. Mukkabla, M. Deepa and A. K. Srivastava, RSC Adv., 2015, 5, 99164 RSC
.
- W. K. Chee, H. N. Lim, N. M. Huang and I. Harrison, RSC Adv., 2015, 5, 68014 RSC
.
- J. T. Zhang, P. Chen, B. H. L. Oh and M. B. Chan-Park, Nanoscale, 2013, 5, 9860 RSC
.
- K. Chi, Z. Y. Zhang, J. B. Xi, Y. A. Huang, F. Xiao, S. Wang and Y. Q. Liu, ACS Appl. Mater. Interfaces, 2014, 6, 16312 Search PubMed
.
- Y. Xie and H. Du, RSC Adv., 2015, 5, 89689 RSC
.
- Y. Gao, K. Ding, X. Xu, Y. Wang and D. Yu, RSC Adv., 2014, 4, 27130 RSC
.
- Y. Ge, C. Wang, K. Shu, C. Zhao, X. Jia, S. Gambhir and G. G. Wallace, RSC Adv., 2015, 5, 102643 RSC
.
- S. Y. Liew, D. A. Walsh and W. Thielemans, RSC Adv., 2013, 3, 9158 RSC
.
- R. Xu, J. Wei, F. Guo, X. Cui, T. Zhang, H. Zhu, K. Wang and D. Wu, RSC Adv., 2015, 5, 22015 RSC
.
- A. A. Ensafi, N. Ahmadi and B. Rezaei, RSC Adv., 2015, 5, 91448 RSC
.
- G. Han, Y. Liu, E. Kan, J. Tang, L. Zhang, H. Wang and W. Tang, RSC Adv., 2014, 4, 9898 RSC
.
- S. Chabi, C. Peng, Z. Yang, Y. Xia and Y. Zhu, RSC Adv., 2015, 5, 3999 RSC
.
- H. Zhou, T. Ni, X. Qing, X. Yue, G. Li and Y. Lu, RSC Adv., 2014, 4, 4134 RSC
.
- H. Du, Y. Xie, C. Xia, W. Wang and F. Tian, New J. Chem., 2014, 38, 1284 RSC
.
- H. Fu, Z. J. Du, W. Zou, H. Q. Li and C. Zhang, J. Mater. Chem. A, 2013, 1, 14943 RSC
.
- G. Wang, J. Yang, J. Park, X. Gou, B. Wang, H. Liu and J. Yao, J. Phys. Chem. C, 2008, 112, 8192 CrossRef CAS
.
- Y. Matsuo, S. Higashika, K. Kimura, Y. Miyamoto, T. Fukutsuka and Y. J. Sugie, J. Mater. Chem., 2002, 12, 1592 RSC
.
- L. M. Viculis, J. J. Mack, O. M. Mayer, H. T. Hahn and R. B. Kaner, J. Mater. Chem., 2005, 15, 974 RSC
.
- J. Ducket, R. Legras and S. D. Champagne, Synth. Met., 1998, 98, 113 CrossRef
.
- Y. C. Liu, B. J. Hwang, W. J. Jian and R. Santhanam, Thin Solid Films, 2000, 85, 374 Search PubMed
.
- Y. C. Liu and B. J. Hwang, Synth. Met., 2000, 113, 203 CrossRef CAS
.
- D. Bersani, P. P. Lottici and X. Z. Ding, Appl.
Phys. Lett., 1998, 72, 73 CrossRef CAS
.
- D. Zhang, X. Zhang, Y. Chen, P. Yu, C. Wang and Y. Ma, J. Power Sources, 2011, 196, 5990 CrossRef CAS
.
- T. Szabo, O. Berkesi and I. Dekany, Carbon, 2005, 43, 3186 CrossRef CAS
.
- C. Hontoria-Lucas, A. J. Lopez-Peinado, J. D. Lopez-Gonzalez, M. L. Rojas-Cervantes and R. M. Martin-Aranda, Carbon, 1995, 33, 1585 CrossRef CAS
.
- S. Grover, S. Shekhar, R. K. Sharma and G. Singh, Electrochim. Acta, 2014, 116, 137 CrossRef CAS
.
- H. Mi, X. Zhang, Y. Xu and F. Xiao, Appl. Surf. Sci., 2010, 256, 2284 CrossRef CAS
.
- J. Wang, Y. Xu, X. Chen and X. Sun, Compos. Sci. Technol., 2007, 67, 2981 CrossRef CAS
.
- F. Liu, G. Han, Y. Chang, D. Fu, Y. Li and M. Li, J. Appl. Polym. Sci., 2014, 131, 41023 Search PubMed
.
- S. R. Sivakkumar, J. M. Ko, D. Y. Kim, B. C. Kim and G. G. Wallace, Electrochim. Acta, 2007, 52, 7377 CrossRef CAS
.
- P. Li, Y. Yang, E. Shi, Q. Shen, Y. Shang, S. Wu, J. Wei, K. Wang, H. Zhu, Q. Yuan, A. Cao and D. Wu, ACS Appl. Mater. Interfaces, 2014, 6, 5228 Search PubMed
.
- X. Sun, Y. Xu, J. Wang and S. Mao, Int. J. Electrochem. Sci., 2012, 7, 3205 CAS
.
- H. Lee, H. Kim, M. S. Cho, J. Choi and Y. Lee, Electrochim. Acta, 2011, 56, 7460 CrossRef CAS
.
- D. Yu, K. Goh, H. Wang, L. Wei, W. Jiang, Q. Zhang, L. Dai and Y. Chen, Nat. Nanotechnol., 2014, 9, 555 CrossRef CAS PubMed
.
- K. Shi and I. Zhitomirsky, J. Mater. Chem. A, 2013, 1, 11614 RSC
.
- C. Peng, J. Jin and G. Z. Chen, Electrochim. Acta, 2007, 53, 525 CrossRef CAS
.
- Z. Weng, Y. Su, D. W. Wang, F. Li, J. H. Du and H. M. Cheng, Adv. Energy Mater., 2011, 1, 917 CrossRef CAS
.
- G. Y. Zheng, L. B. Hu, H. Wu, X. Xie and Y. Cui, Energy Environ. Sci., 2011, 4, 3368 Search PubMed
.
- Y. L. Chen, L. H. Du, P. H. Yang, P. Sun, X. Yu and W. J. Mai, J. Power Sources, 2015, 287, 68 CrossRef CAS
.
- X. B. Zang, X. Li, M. Zhu, X. M. Li, Z. Zhen, Y. J. He, K. L. Wang, J. Q. Wei, F. Y. Kang and H. W. Zhu, Nanoscale, 2015, 7, 7318 RSC
.
- B. Yao, L. Y. Yuan, X. Xiao, J. Zhang, Y. Y. Qi, J. Zhou, J. Zhou, B. Hu and W. Chen, Nano Energy, 2013, 2, 1071 CrossRef CAS
.
- B. Anothumakkool, R. Soni, S. N. Bhange and S. Kurungot, Energy Environ. Sci., 2015, 8, 1339 Search PubMed
.
- J. T. Zhang, P. Chen, B. H. L. Oh and M. B. Chan-Park, Nanoscale, 2013, 5, 9860 RSC
.
- X. Wang, K. Z. Gao, Z. Q. Shao, X. Q. Peng, X. Wu and F. J. Wang, J. Power Sources, 2014, 249, 148 CrossRef CAS
.
- Q. F. Wang, X. F. Wang, B. Liu, G. Yu, X. J. Hou, D. Chen and G. Z. Shen, J. Mater. Chem. A, 2013, 1, 2468 RSC
.
- C. Huang, N. P. Young and P. S. Granta, J. Mater. Chem. A, 2014, 2, 11022 RSC
.
- R. Z. Li, X. Ren, F. Zhang, C. Du and J. P. Liu, Chem. Commun., 2012, 48, 5010 RSC
.
- P. C. Wong, Y. S. Li and K. A. R. Mitchell, Appl. Surf. Sci., 1995, 84, 245 CrossRef CAS
.
- R. W. Paynter, Surf. Interface Anal., 1998, 26, 674 CrossRef CAS
.
- T. Ishizaki, S. Chiba, Y. Kaneko and G. Panomsuwan, J. Mater. Chem. A, 2014, 2, 10589 RSC
.
- P. Hojati-Talemi and G. P. Simon, J. Phys. Chem. C, 2010, 114, 13962 CrossRef CAS
.
- M. Seredych, D. Hulicova-Jurcakova, G. Q. Lu and T. J. Bandosz, Carbon, 2008, 46, 1475 CrossRef CAS
.
- J. R. Pels, F. Kapteijn, J. A. Moulijn, Q. Zhu and K. M. Thomas, Carbon, 1995, 33, 1641 CrossRef CAS
.
- S. Biswas and L. T. Drzal, Chem. Mater., 2010, 22, 5667 CrossRef CAS
.
- X. Lu, M. Yu, G. Wang, Y. Tong and Y. Li, Energy Environ. Sci., 2014, 7, 2160 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra01402j |
| This journal is © The Royal Society of Chemistry 2016 |