Facile fabrication of hierarchical carbon fiber–MoS2 ultrathin nanosheets and its tribological properties†
Beibei Chen*,
Xiaofang Li,
Xiang Li,
Jin Yang,
Weixiang Peng,
Jinze Dong,
Changsheng Li and
Haojie Song
School of Materials Science and Engineering, Institute for Advanced Materials, Jiangsu University, Zhenjiang 212013, PR China. E-mail: chenbb@ujs.edu.cn; Fax: +86 51188783268; Tel: +86 51188783268
First published on 15th June 2016
Abstract
A novel lubricating material of hierarchical carbon fiber–MoS2 ultrathin nanosheets (CF–MoS2) was fabricated by one-step hydrothermal solution reaction method. The corresponding phase composition, morphology and formation mechanism were systematically determined by XRD, FESEM and TEM. And the effects of CF–MoS2 on the tribological properties of epoxy (EP) were investigated comparatively using universal micro-tribotester-2 (UMT-2). Results showed that MoS2 ultrathin nanosheets with the thickness of 5–8 nm and size of 300–500 nm were synthesized successfully. And several MoS2 nanosheets assembled together in flower-like shape and were attached onto CF surface uniformly. Furthermore, the XRD pattern analysis of as-prepared CF–MoS2 confirmed that MoS2 was in the form of hexagonal phase. Besides, the shore hardness of EP matrix was improved obviously by the incorporation of CF–MoS2, suggesting the load-carrying capacity of EP was enhanced. More importantly, EP/CF–MoS2 composite had much better tribological properties than EP and other EP-based composites (EP/CF and EP/MoS2) because of the reinforcement effect of CF and lubricating effect of MoS2. In particular, EP/CF–MoS2 composite still possessed good friction-reducing and anti-wear properties under heavy loads. Namely, hierarchical CF–MoS2 was a promising additive to be used in tribology field.
1 Introduction
Epoxy (EP) is an amorphous and highly cross-linked material when polymerized. The cross-linked microstructure always results in many good properties, i.e., high modulus, failure strength, and low creep, etc.1–3 However, it also leads to an undesirable property whereby the polymer is relatively brittle and with a poor wear resistance under repeated stresses during the friction and wear process, which greatly limits its application.4 So many researchers ameliorate the mechanical and tribological properties of EP by mixing appropriate additives, including reinforcement additives (i.e., CF, CNT, CNF, graphene, and ceramic particles, etc.) and lubricant additives (i.e., graphite, PTFE, and MoS2 etc.).5–9 The former can enhance the hardness, compressive strength to improve the wear resistance of polymer, while the latter can decrease the wear rate by reducing friction coefficient and shear stresses at sliding surfaces.Transition metal dichalcogenides (MS2, M = Ti, W, Mo etc.) have gained widespread interest because of their unique microstructure, physical and chemical properties.10,11 As one member of this family, MoS2 is organized in two layers of sulfur atoms forming a sandwich structure, with a layer of molybdenum atoms in the middle.12,13 Due to the weak van der Waals interaction between the sheets, MoS2 possesses a low friction and thus gives rise to its superior lubricating property.14 Kalin et al. have reported that the addition of MoS2 to the base PAO oil can improve the friction and wear behavior in the boundary-lubrication conditions greatly. The friction coefficient is decreased by more than 2 times, while the wear rate is reduced by as much as 5–9 times.15 Wang et al. have comparatively investigated the effects of MoS2, graphite and PTFE on the tribological properties of phenolic resin based composites, and obtained that the composite with MoS2 shows the lowest friction coefficient and wear rate under the same sliding conditions than that with PTFE or graphite.16 Tang et al. have synthesized flower-like MoS2 microspheres and found that the as-prepared MoS2 can effectively improve the tribological properties of base oil. In particular, the base oil with 2 wt% flower-like MoS2 has much better anti-wear property than that with 2 wt% commercial MoS2.17 In addition, carbon fiber (CF) with exceptional mechanical properties can increase the hardness, compressive strength, and wear resistance of polymer matrix and reduce the adhesion to counterpart. Moreover, CF can carry the main applied load between the contacting surfaces to inhibit the wear of polymer during the process of friction and wear.18–20 Therefore, CF is always chosen to enhance the tribological properties of polymer materials. Indeed, to obtain polymer-based composites with excellent friction and wear performance, two or more kinds of fillers are usually filled into polymer matrix simultaneously, because the synergistic effect between different fillers help to further improve the friction-reducing and wear resistance. In our previous investigations, we have found that CF and PTFE can synergistically improve the wear resistance of PEEK matrix as a result of the enhancing effect of CF and self-lubricating effect of PTFE.21 Wang et al. have improved the tribological properties of nylon 1010 by the incorporation of MoS2 and CF.22 Theiler et al. have found that CF and MoS2 can enhance the friction and wear properties of PEEK in vacuum environment.23 Jia et al. have obtained that the friction coefficient and wear rate of PI under dry sliding or water lubrication are both decreased greatly by filling CF and MoS2.24 Therefore, CF and MoS2 are selected to improve the friction and wear properties of EP in this study.
Recently, various kinds of hybrid structures of MoS2 with other materials, i.e., CF,25 CNT,26 TiO2,27,28 and mesoporous carbon material,29 are prepared to enlarge the applied field of MoS2. Thereinto, carbon-based MoS2 hybrid materials have attracted more attention because carbon materials can enhance the activity of MoS2, and further improve the performance of composites.25,30 Therefore, many researchers have concentrated on constructing hybrid structure of carbon–MoS2 materials. Wang et al. have developed dissolution and high-temperature sintering two-step method to prepare active carbon fiber cloth–MoS2 (ACF–MoS2) composite by growing MoS2 nanosheets on the surface of ACF.25 Zhang et al. have fabricated carbon nanorods or nanotubes–MoS2 by controlling H2S gas sulfidation of the MoOx/polyaniline hybrid.26 Zhang et al. have fabricated reduced graphene oxide–MoS2 (RGO–MoS2) hybrid materials by the method of PVP surfactant-assisted hydrothermal process, and found that RGO–MoS2 enhance the tribological performance of Fe-based composites significantly.31 However, whether CF–MoS2 hybrid material can be prepared and further improve the friction and wear properties of EP matrix, it has not been reported to our knowledge.
In this work, hierarchical CF–MoS2 hybrid material was fabricated by one-step hydrothermal process. This method was facile; neither referred to high-temperature treatment nor other assisted regents. The phase composition and morphology of as-prepared CF–MoS2 were characterized in detail by XRD, FESEM and TEM, and the possible formation mechanism was proposed. More importantly, EP-based composites with hierarchical CF–MoS2 were prepared and their tribological properties were investigated systematically, and the enhancement mechanism of CF–MoS2 was discussed as well.
2 Experimental details
2.1 Preparation of hierarchical CF–MoS2
All the chemical reagents were analytical grade and used without further purification. The preparation procedure of hierarchical CF–MoS2 was as follows: 0.31 g (NH4)6Mo7O24·4H2O and 0.57 g CSN2H4 were dissolved in 10 mL deionized water by magnetic stirring. Then, 1.5 g CF was added into the solution and stirred for 20 min. The obtained solution was transferred into a 50 mL Teflon-lined stainless steel autoclave, and further kept at 220 °C for 24 h. After the reaction, the autoclave was cooled down to room temperature naturally. Finally, the resultant precipitate was collected, and washed with deionized water and ethanol alternatively, and dried for further use.2.2 Preparation of EP/CF–MoS2 composite
To well disperse hierarchical CF–MoS2 in EP-based composite, EP resin was firstly mixed with CF–MoS2, then the mixture was mechanically stirred for 30 min, and the resultant mixture was treated by ultrasonic for 20 min. Afterwards, the curing agent was dropwise added into the mixture in a weight ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (Mcuring agent
100 (Mcuring agent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mepoxy), whereby degassing was carried out with a vacuum pump to eliminate air bubbles. Finally, the resultant mixture was put into a mold to manufacture composite. The CF–MoS2 weight fraction was 5% (when the CF content is 5 wt%, the EP/CF composite has the best friction and wear properties. And the corresponding friction and wear properties have been provided in the ESI.† To well evaluate the reinforcement effect of CF and CF–MoS2, CF–MoS2 content is confirmed 5 wt% accordingly), and the curing process was at room temperature for 24 h. The obtained composite was denoted as EP/CF–MoS2.
Mepoxy), whereby degassing was carried out with a vacuum pump to eliminate air bubbles. Finally, the resultant mixture was put into a mold to manufacture composite. The CF–MoS2 weight fraction was 5% (when the CF content is 5 wt%, the EP/CF composite has the best friction and wear properties. And the corresponding friction and wear properties have been provided in the ESI.† To well evaluate the reinforcement effect of CF and CF–MoS2, CF–MoS2 content is confirmed 5 wt% accordingly), and the curing process was at room temperature for 24 h. The obtained composite was denoted as EP/CF–MoS2.
For comparison, EP, EP/CF, and EP/MoS2 composite were prepared as well according to the method mentioned above. And the shore hardness of EP, EP/CF, EP/MoS2, and EP/CF–MoS2 were shown in Table 1.
| Materials | Shore hardness |
|---|---|
| EP | 80 |
| EP/CF | 85 |
| EP/MoS2 | 79 |
| EP/CF–MoS2 | 84 |
2.3 Characterization
The phase composition of as-prepared hierarchical CF–MoS2 was determined using a D8 advance (Bruker-AXS) X-ray diffractometer with Cu Kα radiation. And the morphology and microstructure were characterized in detail by Field Emission Scanning Electron Microscope (FESEM, JEOLJXA-840A) and Transmission Electron Microscope (TEM, JEM-100CXII).The friction and wear properties of EP-based composites were evaluated by universal micro-tribotester 2 (UMT-2, Center for Tribology Inc., USA). The counterpart was 440 C stainless steel ball. Sliding was performed under dry friction with a period of 30 min, sliding speed of 0.5 m s−1 and 1.0 m s−1, and normal load of 10 N, 15 N, 20 N, and 30 N. The friction coefficient was continuously recorded by an on-line data acquisition system attached to the tester. The width of the wear track was measured using an optical microscope to an accuracy of 0.01 mm. Then, the wear volume loss V of the blocks was calculated from the relationship:
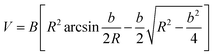 | (1) |
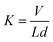 | (2) |
3 Results and discussion
3.1 Microstructure and phase composition of hierarchical CF–MoS2
The morphology of CF and hierarchical CF–MoS2 are shown in Fig. 1. The surface of CF is clean and smooth, except some grooves (Fig. 1A). But, it can be seen from Fig. 1B that many MoS2 nanosheets are attached onto the surface of CF uniformly, and their sizes are about 300–500 nm, which can be determined by high magnification FESEM image (Fig. 1C). As shown in white ring of Fig. 1B, several nanosheets assemble together in flower-like shape. Moreover, Fig. 1D confirms the crystallographic structure of as-prepared hierarchical CF–MoS2. The prominent broad diffraction peak at 2θ = 26°shown in Fig. 1d1 is assigned to graphitic carbon on the surface of CF. As shown in Fig. 1d2, all observed diffraction peaks can be indexed to the pure hexagonal (P63/mmc space group) MoS2 phase, with lattice constants a = 3.16 Å and c = 12.294 Å, which agrees well with the values of standard card of MoS2 (JCPDS no. 37-1492).32 In Fig. 1d3, the peak at 2θ = 26° for graphitic carbon and all typical peaks of MoS2 could be clearly detected. And no peaks from other impurities be cannot found. These results indicate that hierarchical CF–MoS2 with high purity has been fabricated successfully by one-step hydrothermal process. In addition, it also can be seen that all the peaks in the diffraction patterns are broadening slightly, which are mainly caused by the different dimensions of MoS2 nanosheets.33 | ||
| Fig. 1 FESEM morphology and phase composition of CF and hierarchical CF–MoS2: (A) CF; (B and C) CF–MoS2; (D) XRD of CF (d1), MoS2 (d2), and CF–MoS2 (d3). | ||
In order to further reveal the morphology and microstructure of MoS2 nanosheets attached onto CF surface, TEM measurements of as-prepared products are carried out. It can be clearly seen from Fig. 2A that MoS2 nanosheets are transparent and some nanosheets overlap each other. This well corresponds to the fact that MoS2 nanosheets assemble to flower-like shape shown in Fig. 1B. In addition, the selected area electron diffraction (SAED) pattern of MoS2 is presented in the inset image of Fig. 2A, which demonstrates polycrystalline structure. The diffraction rings can be indexed to the reflections of the MoS2 (002), (100), (103), and (110) planes, which are good agreement with the XRD results (Fig. 1d2). More details for MoS2 nanosheets are illustrated in Fig. 2B. It reveals that the thickness is about 5–8 nm, implying MoS2 nanosheets attached onto CF surface are ultrathin. Besides, it can be seen that the MoS2 nanosheets are consisted of several layers, and the distance between two layers is about 0.64 nm, which is in good agreement with the theoretical spacing for (002) planes of hexagonal MoS2.
To well determine the formation mechanism of hierarchical CF–MoS2, the morphology and phase composition of products harvested at different intervals of reaction time are investigated. As shown in Fig. 3A, many sheets with different sizes have been formed and attached onto CF surface for 0.5 h. This implies that CF surface is the nucleation sites of sheet-like products. With the increase of reaction time to 2 h, these sheets suffer to curl and some assemble together (Fig. 3B). Furthermore, when the reaction time prolongs to 12 h, the quantity of curly sheets increases obviously and their sizes become larger as well. Meanwhile, the flower-like products are generated. And Fig. 4A further gives its XRD analysis, it can be seen that there are several peaks at the position of 13.9°, 33.2°, 39.4°, and 58.6°, which are assigned to the peaks of (002), (100), (103), (110) planes of MoS2 hexagonal phase. But some peaks of purities still can be observed, which might be intermediate products. In particular, as the reaction time is 24 h, there are only the characterized peaks of graphitic carbon and MoS2 (Fig. 4B). And the flower-like MoS2 ultrathin sheets are completely formed and uniformly attached onto the surface of CF (Fig. 3D).
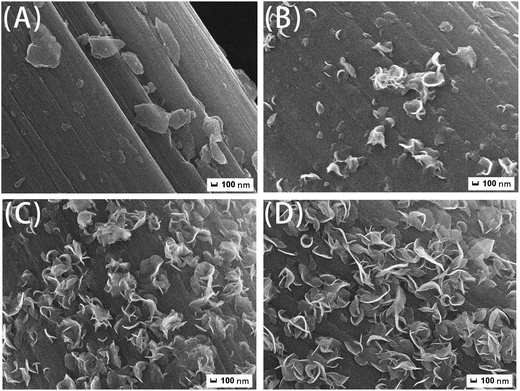 | ||
| Fig. 3 FESEM morphology of products obtained under different reaction times: (A) 0.5 h; (B) 2 h; (C) 12 h; (D) 24 h. | ||
Based on these experimental results mentioned above, Fig. 5 illustrated schematically the formation process of hierarchical CF–MoS2. We believe that the process includes initial nucleation of primary sheets, curliness and assembling of sheets, ripening of sheets etc., which is consistent with some previous reports.34 The chemical reactions might be involved during the whole hydrothermal process:35
| CSN2H4 + 2H2O → NH3 + CO2 + H2S | (3) |
| (NH4)6Mo7O24 → 6NH3 + 7MoO3 + 3H2O | (4) |
| MoO3 + 3H2S + H2O → MoO2 + SO42− + 2H+ | (5) |
| MoO2 + 2H2S → MoS2 + 2H2O | (6) |
In this work, the initial molar ratio of (NH4)6Mo7O24 to CSN2H4 is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, so more H2S would be produced according to the chemical reaction formula (3), and more sheet-like nuclei can be formed onto CF surface on initial stage (Fig. 5A). Subsequently, with the increase of reaction time, these sheets suffer to curl, assemble together driven by reducing the surface energy. Furthermore, the assembled flower-like nanosheets become much more and their sizes increase with the increased reaction time. Finally, they grow to flower-like MoS2 ultrathin nanosheets on CF surface. That is, the hierarchical CF–MoS2 has been prepared.
30, so more H2S would be produced according to the chemical reaction formula (3), and more sheet-like nuclei can be formed onto CF surface on initial stage (Fig. 5A). Subsequently, with the increase of reaction time, these sheets suffer to curl, assemble together driven by reducing the surface energy. Furthermore, the assembled flower-like nanosheets become much more and their sizes increase with the increased reaction time. Finally, they grow to flower-like MoS2 ultrathin nanosheets on CF surface. That is, the hierarchical CF–MoS2 has been prepared.
3.2 Friction and wear properties
The friction coefficient and wear rate of EP, EP/CF, EP/MoS2, and EP/CF–MoS2 sliding against stainless steel ball under dry friction at the load of 10 N and sliding speed of 0.5 m s−1 are exhibited in Fig. 6 and 7, respectively. It can be seen that the friction and wear properties of EP are enhanced by the incorporation of CF or MoS2 or CF–MoS2. Specially, for EP/CF composite, the friction coefficient decreases slightly, but the wear rate decreases nearly 50% in comparison with EP, which mainly attributes to the reinforcement effect of CF. In addition, because MoS2 has good self-lubricating properties determined by its lamellar structure, the friction coefficient of EP decreases from 0.56 to 0.43 by filling MoS2, and the corresponding wear rate decreases by 35%. More importantly, it is noteworthy that the friction coefficient and wear rate of EP/CF–MoS2 composite decrease by 29% and 73% compare to those of pure EP matrix. And they are not only lower than those of EP/CF composite, but also EP/MoS2 composite. That is, EP/CF–MoS2 has the best friction-reducing and anti-wear properties. Generally, CF with high creep resistance, hardness and compressive strength is often used as ideal reinforcement in advanced polymer-based composite.18–20 Xiong have reported that the corporation of CF improves the wear resistance of UHMWPE significantly by enhancing the loading capacity.19 We have also found the hardness and wear resistance of PEEK are increased by filling appropriate CF.20 In this study, it can be seen that the shore hardness of EP/CF and EP/CF–MoS2 composite are much higher than that of pure EP, suggesting the load-carrying capacity of EP is enhanced by filling CF or CF–MoS2 (Table 1). At the initial stage of the friction and wear process, EP matrix is firstly worn off by carrying the load applied onto sliding surface, and CF is exposed onto worn surface gradually. Accordingly, the applied load transfers from EP matrix to CF. Namely, the wear of EP would be inhibited. More importantly, for EP/CF–MoS2 composite, the exposed CF–MoS2 plays the role of reinforcement effect of CF as well as the lubricating effect of MoS2. Many reports have showed that MoS2 is layered structure with weak van der Waals interactions between individual sandwiched S–Mo–S layers, and often used to lubricant additive to ameliorate the friction-reducing and anti-wear properties of polymer, metal and lubricant oil.15,16 That is why the EP/CF–MoS2 composite has the best friction and wear properties in this study. | ||
| Fig. 6 Friction coefficient of EP, EP/CF, EP/MoS2, and EP/CF–MoS2 sliding against stainless steel under dry friction (load: 10 N, sliding speed: 0.5 m s−1, duration: 30 min). | ||
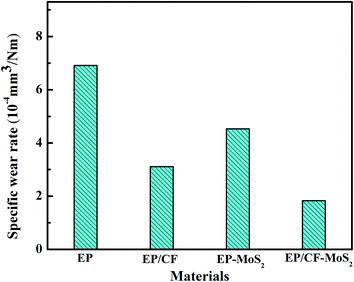 | ||
| Fig. 7 Wear rate of EP, EP/CF, EP/MoS2, and EP/CF–MoS2 sliding against stainless steel under dry friction (load: 10 N, sliding speed: 0.5 m s−1, duration: 30 min). | ||
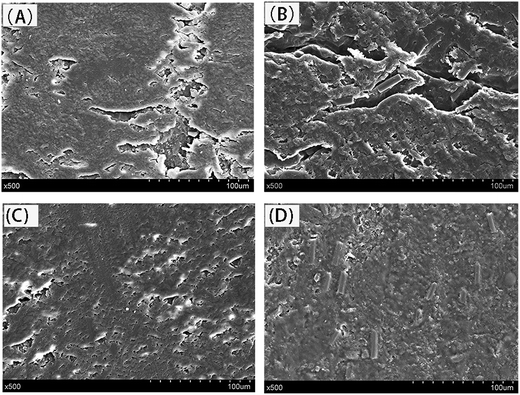
In addition, Fig. 8 exhibits the SEM images of worn surface of EP, EP/CF, EP/MoS2, and EP/CF–MoS2 after sliding against stainless steel under dry friction at the load of 10 N and sliding speed of 0.5 m s−1. It can be clearly observed that the worn surface of pure EP is characterized by serious peeling of sheets and much debris (Fig. 8A), which not only suggests the wear mechanism of EP is fatigue wear, but also agrees well with the result of EP with the highest friction coefficient and wear rate as shown in Fig. 6 and 7. For EP/CF composite, many CFs are exposed on the worn surface and the signs of big EP sheets and debris have been greatly alleviated due to the reinforcement effect of CF. In addition, the worn surface of EP/MoS2 is much smoother than that of EP or EP/CF composite. This well explains EP/MoS2 composite has better friction-reducing property. But some scratches can be seen on the worn surface of EP/MoS2 (Fig. 8C). This might be caused by the fact that the hardness of EP/MoS2 is low, and EP/MoS2 is prone to wear during the friction and wear process. In particular, the worn surface of EP/CF–MoS2 composite is very smooth, and grooves are scarcely can be seen (Fig. 8D), which is well consistent with EP/CF–MoS2 possessing the lowest friction coefficient and wear rate.
In addition, the effects of sliding conditions including applied load and sliding speed on the friction coefficient and wear rate of EP/CF–MoS2 composite are shown in Fig. 9 and 10. It can be found that the friction coefficient and wear rate increase with increasing applied load. The wear rate of EP/CF–MoS2 composite under 30 N is nearly three times that under 10 N. Indeed, the applied load has important influence on the tribological properties of polymer materials.36 Heavy load always leads to much frictional heat and soften polymer materials, which increase the real contact area between counterparts and enhance the adhesion of polymer to counterpart, resulting in high friction force, and finally lead to increased friction coefficient and wear rate. In addition, heavy load leads to the failure of CF–MoS2 in composite because of carrying too much applied load, which enhance abrasion of broken CF–MoS2 as abrasive particles during the friction and wear process. Therefore, the friction coefficient and wear rate of EP/CF–MoS2 increase with the increase in load. And this is consistent with many previous reports.37,38 Besides, it also can be seen that the friction coefficient of EP/CF–MoS2 under high sliding speed is lower than that under low sliding speed, but the wear rate is contrast. This is because there is not enough time to produce more adhesive points at high sliding speed owing to the decreased surface contact time, which lead to reduced friction force. Meanwhile, the increased sliding speed leads to higher contact temperature, which makes the surface of EP/CF–MoS2 would be easily worn off. Nevertheless, the friction coefficient and wear rate of EP/CF–MoS2 composite under 30 N or 1.0 m s−1 are still lower than that of pure EP under the moderate sliding condition (e.g. 10 N or 0.5 m s−1), suggesting hierarchical CF–MoS2 is a good lubricating additive to enhance the tribological properties of polymer matrix.
 | ||
| Fig. 9 Variation of the friction coefficient and wear rate with applied load of EP/CF–MoS2 composite. | ||
4 Conclusions
(1) Hierarchical CF–MoS2 is fabricated via one-step hydrothermal approach, without high-temperature sintering or any assisted surfactants. MoS2 ultrathin nanosheets are with thickness of 5–8 nm, and several nanosheets assemble together in flower-like shape onto CF surface uniformly. And the formation mechanism of CF–MoS2 has been proposed.(2) The friction and wear properties of EP are improved obviously by the incorporation of CF–MoS2. And this is mainly attributed to the reinforcement effect of CF and lubricating effect of MoS2.
(3) EP/CF–MoS2 composite possesses excellent friction and wear properties under heavy load or high sliding speed, suggesting CF–MoS2 is a promising lubricating additive.
Acknowledgements
This research is financial supported by the National Nature Science Foundation of China (51405199 and 51372103), Natural Science Foundation of Jiangsu Province (BK20140551 and BK20140562), Special Financial Grant of Postdoctoral Science Foundation of China (2015T80505), Postdoctoral Science Foundation of Jiangsu Province (1401106C) and the Senior Intellectuals Fund of Jiangsu University (13JDG099).References
- T. H. Hsieh, A. J. Kinloch, K. Masania, A. C. Taylor and S. Sprenger, Polymer, 2010, 51, 6284–6294 CrossRef CAS.
- Y. Zhang and S. S. Huang, RSC Adv., 2016, 6, 26210–26215 RSC.
- O. Jacobs, R. Jaskulka, F. Yang and W. Wu, Wear, 2004, 256, 9–15 CrossRef CAS.
- C. M. Manjunatha, A. C. Taylor, A. J. Kinloch and S. Sprenger, Compos. Sci. Technol., 2010, 70, 193–199 CrossRef CAS.
- X. J. Shen, X. Q. Pei, Y. Liu and S. Y. Fu, Composites, Part B, 2014, 57, 120–125 CrossRef CAS.
- J. Schön, Wear, 2004, 257, 395–407 CrossRef.
- Q. B. Guo, M. Z. Rong, G. L. Jia, K. T. Lau and M. Q. Zhang, Wear, 2009, 266, 658–665 CrossRef CAS.
- X. B. Li, Y. M. Gao, J. D. Xing, Y. Wang and L. Fang, Wear, 2004, 257, 279–283 CrossRef CAS.
- N. Nemati, M. Emamy, S. Yau, J. K. Kim and D. E. Kim, RSC Adv., 2016, 6, 5977–5987 RSC.
- N. Zheng, X. Bu and P. Feng, Nature, 2003, 426, 428–432 CrossRef CAS PubMed.
- Z. Zeng, C. Tan, X. Huang, S. Mao and H. Zhang, Energy Environ. Sci., 2014, 7, 797–804 Search PubMed.
- W. Zhou, X. Zou, S. Najmaei, Z. Liu, Y. Shi, J. Kong, J. Lou, P. M. Ajayan, B. I. Yakobson and J. Idrobo, Nano Lett., 2013, 13(6), 2615–2622 CrossRef CAS PubMed.
- M. Wang, G. Li, H. Xu, Y. Qian and J. Yang, ACS Appl. Mater. Interfaces, 2013, 5(3), 1003–1008 Search PubMed.
- M. Chhowalla and G. A. J. Amaratunga, Nature, 2000, 407, 164–167 CrossRef CAS PubMed.
- M. Kalin, J. Kogovsek and M. Remskar, Wear, 2012, 280–281, 36–45 CrossRef CAS.
- P. Cai, T. M. Wang and Q. H. Wang, Polym. Compos., 2015, 36, 2203–2211 CrossRef CAS.
- G. G. Tang, J. Zhang, C. C. Liu, D. Zhang, Y. Q. Wang, H. Tang and C. S. Li, Ceram. Int., 2014, 40, 11575–44580 CrossRef CAS.
- Q. Zhou, J. X. Huang, J. Q. Wang, Z. G. Yang, S. Liu, Z. F. Wang and S. R. Yang, RSC Adv., 2015, 5, 91802–91812 RSC.
- D. S. Xiong, Mater. Lett., 2005, 59, 175–179 CrossRef CAS.
- B. B. Chen, J. Z. Wang and F. Y. Yan, Tribol. Int., 2012, 52, 170–177 CrossRef CAS.
- B. B. Chen, J. Z. Wang, N. Liu and F. Y. Yan, Tribol. Trans., 2013, 56, 672–680 CrossRef CAS.
- J. X. Wang, M. Y. Gu, S. H. Bai and S. R. Ge, Wear, 2003, 255, 774–779 CrossRef.
- G. Theiler and T. Gradt, Wear, 2010, 269, 278–284 CrossRef.
- J. H. Jia, J. M. Chen, H. D. Zhou, L. T. Hu and L. Chen, Compos. Sci. Technol., 2005, 65, 1139–1147 CrossRef.
- C. Wang, W. Wan, Y. H. Huang, J. T. Chen, H. H. Zhou and X. X. Zhang, Nanoscale, 2014, 6, 5351–5358 RSC.
- C. Zhang, Z. Wang, Z. Guo and X. W. Lou, ACS Appl. Mater. Interfaces, 2012, 4, 3765–3768 Search PubMed.
- W. J. Zhou, Z. Y. Yin, Y. P. Du, X. Huang, Z. Y. Zeng, Z. X. Fan, H. Liu, J. Y. Wang and H. Zhang, Small, 2013, 9, 140–147 CrossRef PubMed.
- X. Xu, Z. Y. Fan, S. J. Ding, D. M. Yu and Y. P. Du, Nanoscale, 2014, 6, 5245–5250 RSC.
- X. Xu, Z. Y. Fan, X. Y. Yu, S. J. Ding, D. M. Yu and X. W. Lou, Adv. Energy Mater., 2014, 4, 1400902 CrossRef.
- W. H. Hu, R. Yu, G. Q. Han, Y. R. Liu, B. Dong, Y. M. Chai, Y. Q. Liu and C. G. Liu, Mater. Lett., 2015, 161, 120–123 CrossRef CAS.
- M. S. Zhang, B. B. Chen, J. Yang, H. M. Zhang, Q. Zhang, H. Tang and C. S. Li, RSC Adv., 2015, 5, 89682–89688 RSC.
- G. G. Tang, J. R. Sun, C. Wei, K. Q. Wu, X. R. Ji, S. S. Liu, H. Tang and C. S. Li, Mater. Lett., 2012, 86, 9–12 CrossRef CAS.
- J. Xie, C. Wu, S. Hu, J. Dai, N. Zhang and J. Feng, Phys. Chem. Chem. Phys., 2012, 14, 4810–4816 RSC.
- G. G. Tang, H. Tang, C. S. Li, W. J. Li and X. R. Ji, Mater. Lett., 2011, 65, 3457–3460 CrossRef CAS.
- C. Feng, J. Ma, H. Li, R. Zeng, Z. P. Guo and H. K. Liu, Mater. Res. Bull., 2009, 44, 1811–1815 CrossRef CAS.
- B. B. Chen, J. Z. Wang, N. Liu and F. Y. Yan, Mater. Des., 2014, 63, 325–332 CrossRef CAS.
- G. Y. Xie, G. X. Sui and R. Yang, Compos. Sci. Technol., 2011, 71(6), 828–835 CrossRef CAS.
- B. B. Chen, J. Z. Wang and F. Y. Yan, Mater. Des., 2012, 36, 366–371 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra11419a |
| This journal is © The Royal Society of Chemistry 2016 |