Cu@Sn nanostructures based on light-weight current collectors for superior reversible lithium ion storage
Ruoxu Lin,
Shichao Zhang*,
Yanbiao Ren,
Xiaomeng Wu,
Hua Fang and
Xin Wei
School of Materials Science and Engineering, Beijing University of Aeronautics and Astronautics, Beijing 100191, China. E-mail: csc@buaa.edu.cn
First published on 5th February 2016
Abstract
A binder-free method is applied to avoid the huge irreversible capacity of Sn-based composite anodes in this paper. We report two types of copper-based current collector: (i) a light and flexible current collector, which is fabricated from copper nanowires (CuNWs), and (ii) Cu foam with copper nanowires grown on it. The charge capacity of the thin CuNW sheet based Sn–Cu composite anode remains above 760 mA h g−1 after 60 cycles with a relatively stable coulombic efficiency fluctuating around 97%. The Cu foam based composite anode also shows a good capacity retention of 79.8% after the same test, compared with the Cu foil based anode. According to the good rate performance and the light weight of the composite electrode, the CuNW sheet based current collector may be a promising material in energy fields in the future.
Introduction
The increasing demand for higher energy density and power density of batteries in electronic devices has attracted researchers to develop new materials for lithium-ion batteries. In commercial lithium-ion batteries, carbonaceous materials are generally used as anode materials.1 However, since their theoretical capacity is only 372 mA h g−1, it is important to develop new anode materials with higher capacities. Sn and Li can form Li4.4Sn in lithiation process with theoretical capacity of 994 mA h g−1, which has attracted researchers' interest.2 Metallic tin delivers a theoretical specific capacity of 991 mA h g−1 or 7313 mA h cm−3 as Li4.4Sn, much larger than 372 mA h g−1 or 833 mA h cm−3 of commercial graphite as LiC6.3 In addition, tin has a higher operating potential than graphite and does not undergo solvent intercalation, consequently being less reactive and much safer than graphite.4 Unfortunately, a dramatic volume change (about 300%) commonly occurs in Sn during lithiation/delithiation, which causes not only severe pulverization and subsequent electrical disconnection from the current collector but also aggregation of Sn nanoparticles and continual formation of a very thick solid electrolyte interphase (SEI) on the Sn surfaces upon cycling, thereby leading to rapid capacity decay and poor cyclability.5–7To solve this problem, some strategies have been proposed: (1) dispersing Sn particles in a carbon matrix to alleviate volume effect of Sn, since carbon is elastic and conductive;8–12 (2) applying nano-sized Sn particles, thus shortening diffusion length and accommodating large mechanical strain associated with structure and volume changes;13,14 and (3) dispersing Sn into inactive components to form the active/inactive system and strengthen the interfacial binding force, such as Sn–Cu, Sn–Ni, Sn–Co, etc., in which highly conductive framework are formed and severe pulverization can be lessened.15,16 Although nanoscale Sn particles and tin alloys improved the cyclability of batteries to some extent, they are still far from commercialization. Recently, graphene has drawn special attention and is preferable to replace other types of carbon materials, due to its outstanding electrical conductivity, superior mechanical flexibility, and high thermal/chemical stability.17,18 Hence, many Sn–graphene hybrids with various structures such as directly decorated Sn–graphene,19–23 Sn@C–graphene,24,25 and sandwich-like graphene-supported hybrids26–30 have been developed. Although improved electrochemical performances have been achieved in these hybrid anodes, their rate performances and cycling stability at high charge/discharge rates are not superior to or even worse than that of the other Sn–carbon hybrid anodes. This may be attributed to severe structural defects introduced during exfoliation and reduction processes, leading to poor electron conductivity and structural stability. Meanwhile, the additive in composite electrodes such as binders could also deteriorate the cycle performance of graphene based anodes. Simultaneously, binder-free techniques such as electrodeposition, electroless plating, and sputtering have been reported to prepare Cu6Sn5/Sn anode materials on copper substrates.31–33 Using foam-type substrates, several groups obtained Sn–Cu active materials with improved electrochemical performances.34–37 However, although it showed better performances than Sn–Cu film anodes, most of Sn was in fact not well connected to Cu substrates, and the specific capacity was very low.
In this work, copper foam is used as substrate/current collector, on the surface of which copper nanowires grow. The collector with hierarchical structure has micro-sized pores, making a better transfer of lithium ions. To reduce the weight of current collector for a preferable energy density of composite electrode in commercial application, we also firstly report a light and flexible current collector, which is fabricated by copper nanowires (CuNWs). The CuNWs sheets used in this paper act as collectors and substrates simultaneously. Copper foil is selected to make comparison. Sn is electrodeposited directly on copper nanowires or copper foil. The morphology of nanowires favors Li+ insertion/extraction process of active materials and the composite anodes based on copper foam and copper nanowires perform a stable capacity retention and improves the energy density compared with conventional Sn–Cu composite anodes. Eventually, the improved electrochemical behavior of the electrodes and the probable mechanism are discussed.
Experimental
Preparation of Cu foam and CuNWs based current collector and composite Sn–Cu electrodes
Synthesis: the copper foam was fabricated by electrodeposition of copper onto the polyurethane foam template. And then the template was burnt out. The copper foam became brittle and was partially oxidized in this process and therefore, a heat treatment at 800 °C for 1 hour under hydrogen/nitrogen (vol% = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) mixed gas protection was applied. Cu foam was placed in water bath contained 0.1 M ammonium persulphate and 2.5 M sodium hydroxide for 2 hours. The sample was washed by deionized water and died in vacuum for 5 hours after the surface of copper foam turning to blue. To prepare copper nanowires from copper hydroxide, the as-prepared foam was further dipped in 50 mL of NaBH4 (0.5 M) aqueous solution with continuous stirring for about 2 hours and the reduction process of Cu(OH)2 finished when the color changed from blue to puce.
3) mixed gas protection was applied. Cu foam was placed in water bath contained 0.1 M ammonium persulphate and 2.5 M sodium hydroxide for 2 hours. The sample was washed by deionized water and died in vacuum for 5 hours after the surface of copper foam turning to blue. To prepare copper nanowires from copper hydroxide, the as-prepared foam was further dipped in 50 mL of NaBH4 (0.5 M) aqueous solution with continuous stirring for about 2 hours and the reduction process of Cu(OH)2 finished when the color changed from blue to puce.
Synthesis method of copper nanowires was optimized as follows: 500 mL of NaOH (12 M) and ethylenediamine (EDA; 99 wt%) were mixed in a glass reactor, then the glass reactor was ultrasoniced to form a uniform ethylenediamine suspension. 1.0 mL of Cu(NO3)2 (0.10 M) aqueous solution and hydrazine (0.25 mL; 35 wt%) were added sequentially, followed by an ultrasonic mixing of all reagents. The reactor was then placed in a water bath with temperature control at 60 °C for 3 hours. Finally, CuNWs products were obtained. For further treatment, copper nanowires in alkaline solution were washed with diluted hydrochloric acid solution for three times and then washed with deionized water. With vacuum filtration, the films fabricated by copper nanowires were easily peeled off from filter membranes. The CuNWs sheets were heated at 80 °C for 12 hours and then were sandwiched between two PTFE plate with electrodes and membrane to accommodate a electrodeposition route called 'tight-contact' deposition described in our previous work to enhance the electrical connection between copper nanowires.38 Copper foil was first ultrasoniced in acetone to remove the organic contaminants on the surface. After drying process, the foil was washed with diluted sulfuric acid solution for three times and then washed with deionized water. Sequentially, the pretreated foil was dried in vacuum at 80 °C for 12 hours for the further Sn coating process.
Sn coating was prepared by electrodeposition, using prepared copper foam, copper nanowires sheet and copper foil as the working electrodes (cathode), and platinum foil as the counter electrode. The electrolyte was comprised of 8.05 g SnSO4 and 19.58 mL H2SO4 (98 wt%) dissolved in 250 mL deionized water. The current density was 10 mA cm−2 to perform the electrodeposition process at 30 °C. The loading mass of tin on copper foam, copper sheet and copper foil were 1.74, 1.63 and 1.89 mg cm−2, respectively.
Characterization and electrochemical tests
Characterizations: coin-type test cells with three different copper current collector based anodes mentioned above were assembled in an argon-filled glove box (MB-10-G with TP170b/mono, MBRAUN) with lithium sheets as counter electrodes. Electrolyte was 1 M LiPF6 in a mixed solution of EC and DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume ratio) and membrane separator (Celgard® 2300) was applied. Cells were aged for 2 hours at room temperature before test, and measurements were carried out in a battery test system (NEWARE BTS-610, Neware Technology Co., Ltd., China). Electrochemical impedance spectra (EIS) were recorded on a Chenhua® 660D electrochemical workstation at room temperature, in the frequency range from 100 kHz to 0.01 Hz under ac simulus with 10 mV of amplitude and no applied voltage bias. Cyclic voltammogram method was tested by Chenhua® 1100D workstation and structural characterization and surface analysis were made using scanning electron microscopy (SEM, Hitachi S-4800). Nitrogen sorption isotherms and BET surface area were measured at 77 K with an ASAP 2020 physisorption analyzer. The phase of the samples was analyzed by X-ray diffraction (XRD) (Rigaku D/Max-2400) with Cu Kα radiation at a scanning rate of 6° min−1.
1 in volume ratio) and membrane separator (Celgard® 2300) was applied. Cells were aged for 2 hours at room temperature before test, and measurements were carried out in a battery test system (NEWARE BTS-610, Neware Technology Co., Ltd., China). Electrochemical impedance spectra (EIS) were recorded on a Chenhua® 660D electrochemical workstation at room temperature, in the frequency range from 100 kHz to 0.01 Hz under ac simulus with 10 mV of amplitude and no applied voltage bias. Cyclic voltammogram method was tested by Chenhua® 1100D workstation and structural characterization and surface analysis were made using scanning electron microscopy (SEM, Hitachi S-4800). Nitrogen sorption isotherms and BET surface area were measured at 77 K with an ASAP 2020 physisorption analyzer. The phase of the samples was analyzed by X-ray diffraction (XRD) (Rigaku D/Max-2400) with Cu Kα radiation at a scanning rate of 6° min−1.
Results and discussion
Characterization of Cu foam based current collector and composite Sn–Cu anode
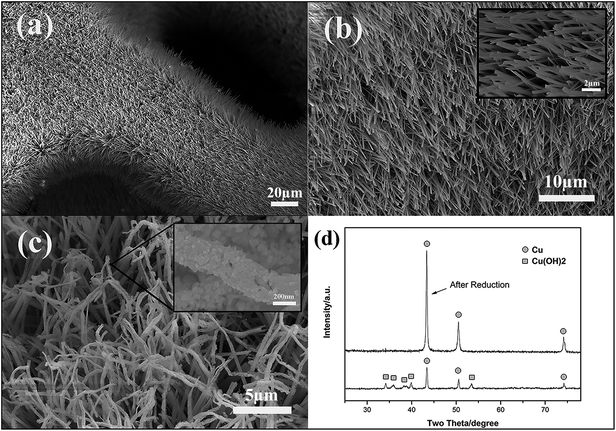
The copper foam is composed of many coarse branches and macro-sized pores. The 3D structure makes lithium ions move freely during discharge/charge process and alleviates the volume effect of active materials to some extent. However, tin is deposited as thin films on the surface of these branches actually, which easily crack after several cycles severely. To make a better distribution of Sn on the surface of the copper foam, a hierarchical structure is designed. The synthesis procedure of Cu nanowires grow on copper branches is described in Fig. 1(a). It can be seen that firstly Cu(OH)2 nanowires array grow orderly in alkaline solution. The SEM images in Fig. 2(a) and (b) also shows that the nanowires stand vertically along the branches of the copper foam. Sequentially, copper hydroxide nanowires is reduced in NaBH4 aqueous solution and it should be noticed that there is a trend of granulation of the surface of the nanowires due to the H2 bubbles generating during the reduction process. The rough surface showed in Fig. 2(c) provides more space for loading Sn and can simultaneously alleviate the volume expansion of active materials in the case of lithiation. XRD pattern of the Cu(OH)2 nanowires before and after reduction is showed in Fig. 2(d). It indicates that the hydroxide is completely reduced with one-hours treatment, which assures the good conductivity of the current collector. As shown in Fig. 1(b), the BET surface area plots exhibits typical lines in the P/P0 range of 0.02–0.18, suggesting the evident change of the surface area of the 3D material before and after the growth of copper nanowires. The hierarchical structure is further verified and the calculated results are 0.24 ± 0.01 m2 g−1 and 8.7 ± 0.06 m2 g−1, respectively. | ||
| Fig. 1 (a) Schematic image of the process of copper nanowires growing on the surface of the copper foam. (b) BET surface area plots of copper foam before and after the growth of copper nanowires. | ||
Fig. 3(a) shows the XRD pattern of Cu@Sn nanowires on the surface of the copper foam, indicating the presence of two basic phases: the main deposited product β-Sn and Cu substrate. The peak at 30.2° refers to (101) plane of Cu6Sn5 phase with minor quantity. In acid electrodeposition solution, copper dissolves while tin is plating on current collector and finally a layer of Cu6Sn5 is formed along the interface. The formation of Cu6Sn5 layer enhances the bonding force between active materials and current collector, which prevents the shedding of Sn from the current collector and leads to good cycling performance.39 TEM image in Fig. 3(b) clearly shows that the Sn layer has a thickness in the range of 70 to 80 nm and also has a tendency of granulation on the surface, providing more space to accommodate the expansion of Sn during cycling. EDX line scanning of Cu@Sn composite are measured and shown in Fig. 3(c). The red line stands for the Sn element and the green line represents the Cu element. The EDX line scan results demonstrate that Sn covers CuNWs uniformly as shell and Cu element mainly exists in the internal bulk as core. The cycling performance will be discussed later in this work.
 | ||
| Fig. 3 (a) XRD patterns of Cu@Sn nanowires on the surface of copper foam. ((b) and (c)) TEM image and EDX line scan of Cu@Sn nanowires. | ||
Characterization of CuNWs based current collector and composite Sn–Cu anode
In practical use, the energy density of the electrode/battery should be considered and calculated including the important part referring to the weight of the current collector. Although the copper foam mentioned above has been already widely used in different fields, the relatively high weight still prevent it from being applied in micro-electronics area. Therefore, we introduce a light and flexible current collector, which is fabricated by copper nanowires (CuNWs) for composite anode of lithium-ion battery shown in Fig. 4. The copper nanowires network used in this paper both act as collector and substrate. The data of mass per unit area of three samples are measured and the results are 4.2 ± 0.2 mg cm2, 48.5 ± 0.2 mg cm2 and 11.4 ± 0.2 mg cm2 for CuNWs sheet, copper foam and copper foil, respectively, from which we can see that the weight of nanowires sheet is almost a tenth of the weight of copper foam. It is worth noting that the sheet is lighter than the commercial copper foil of a thickness of ∼15 μm, indicating that the new type of collector can be a candidate for commercial use. | ||
| Fig. 4 Data of mass per unit area of copper nanowires sheet, copper foam, and copper foil, relatively, and SEM images of the morphology of the three samples. | ||
The flexible copper sheet is fabricated by copper nanowires which simply stacks together by vacuum filtration. Therefore, the contact point may lose electric connection if the thin film is stretched or bent by external force, or it can be affected by the volume expansion of active materials. A copper electrodeposition method was brought up to enhance the electric contact between single copper nanowires shown in Fig. 5(a). It depicts in Fig. 5(b) that the two nanowires weld together when copper acts as welding flux, and the inset shows that the deposited Cu is only partially oxidized due to the strong surface energy of nanostructure inevitably. The schematic representation gives a concept that electrons move freely after the enhancement of electric contact, and the conductivity is surely improved in the case of this advantages. In our previous work, a four-point probe test was used to determine the effort to reduce the sheet resistance (Rs) of thin films. The copper sheet is called 'pseudofilm' for its porous structure. So that the thickness is measured and read by SEM image to simplify the calculations. The two types of CuNWs sheets were then measured and the calculated results shows that the Rs of the CuNWs sheet by enhanced treatment is far less than it of the sheet without enhancement (∼4 Ohm sq.−1 to ∼300 Ohm sq.−1).38 According to it, the electrodeposition process made good progress to reduce the resistance of CuNWs sheets, with which the conventional substrates could be replaced to fabricate Sn based electrodes. SEM image in Fig. 5(c) shows the morphology of the copper nanowires coated by Sn and it is proved by the inset XRD pattern. It can also be found that the surface of the nanowires is coarse by electrodeposition method, which makes an beneficial effect on lithiation/delithiation behaviour.
Electrochemical performance
The three binder-free samples were assembled into coin-type cells with lithium sheets as the counter-electrode to investigate their electrical property. Cyclic voltammogram of first two cycles (scanning rate of 80 μV s−1) of Cu foam based composite anode are shown in Fig. 6(a1). During the first lithiation process, there is a broad cathodic peak at around 1.5 V, and disappears in the second cycle. This cathodic peak ranged from 1.6 V to 1.25 V can be assigned to the formation of solid electrolyte interface (SEI) and the decomposition of electrolyte on Sn surface. The distinct cathodic current peaks at 0.67, 0.54 and 0.34 V during discharge and the distinct anodic peaks at 0.42, 0.66, 0.73, and 0.81 V during charge correspond to lithium alloying and dealloying with Sn, respectively, in accord with previous reports on Sn based anodes.40 Since the second cycle, the first large peak of discharge potential shifts from ca. 0.67 V to ca. 0.62 V. This voltage fluctuation may be due to the inactive features of Sn against the electrochemical reactions. Fig. 6(a2) shows the voltage profiles of the composite anode after 60 cycles in the voltage range of 0.01–2.5 V at a current density of 0.49 A g−1 (rate = 0.5C). The initial charge and discharge capacities are approximately 882.6 and 1167.3 mA h g−1, respectively, resulting in an initial coulombic efficiency of ∼75.6%. The initial irreversible capacity loss of the active materials could be associated with the inevitable formation of SEI and decomposition of electrolyte41 in good agreement with the above CV results shown in Fig. 6(a1). It is important to note that both charge and discharge profiles exhibit little change between the first two cycles, further demonstrating that the binder-free electrode are very stable during cycling. Fig. 6(b1) and (b2) show that the curves of CV and voltage profile vs. specific capacity are very similar to the data shown in Fig. 6(a1) and 6(a2), which means that the Cu@Sn nanowires perform stable in both copper foam and copper nanowires based composite electrodes. There is only one different behavior between the two electrodes that the charge capacity of Cu foam based anode attenuates from the 1st to the 60th cycle, wherein that of CuNWs sheet based anode has an increase from 1st to 10th cycle, and then attenuates from the 20th to the 60th cycle, which could be assigned to the more compact structure of CuNWs sheet based electrode preventing Sn from shedding off from the collector in the first several cycles. The curves of CV of the first two scanning process of the Cu foil based electrode do not overlap well, which is shown in Fig. 6(c1), and the charge/discharge curves of the 2D Sn coating on the Cu foil shown in Fig. 6(c2) suggest a poor efficiency during the first cycle with 805.2 mA h g−1 initial charge capacity and 70.5% columbic efficiency, which is assigned to the 2-dimensional compact microstructure of the Sn film severely hindering the lithiation and delithiation process. According to the unfavorable structure, there is an obvious degradation of discharge/charge capacity of the Cu foil based anode after 60 cycles.Comparative study was carried out to distinguish the variation of cycling performance among the samples of Cu foam based, CuNWs sheet based and Cu foil based electrodes. The three types of composite anodes were tested at a charge/discharge rate of 0.5C. As is shown in Fig. 7(a), the charge capacity of CuNWs sheet based anode has a long plateau from the 6th cycle up to the 25th cycle test with a relatively stable coulombic efficiency fluctuating around 97%. It should be noticed that Sn on copper foil has the highest irreversible capacity of 347.1 mA h g−1 compared to the other two Sn based anodes, whereas it has a severe sloping region dropping to 345.3 mA h g−1 with a low capacity retention of 42.6%, which is shown as the inset in Fig. 7(a). The trend of the charge capacity of Cu foam based anode is mentioned above with a capacity loss of 0.3% per cycle and the capacity after 60 cycles (689.7 mA h g−1) is lower than that of CuNWs sheet based anode (777.8 mA h g−1). The rate performance of the three samples at the current densities from 0.20 A g−1 (0.2C) to 4.9 A g−1 (5C) were examined, as shown in Fig. 7(b). Slow rate of 0.2C was operated in the first seven cycles to enhance the stability of composite electrode and all of the three types of electrodes show good charge capacity. And then, at high rates of 2C and 5C, competitive charge capacities of the CuNWs sheet based anode of 695.1 and 527.8 mA h g−1 can be maintained, respectively. Meanwhile, the Cu foam based anode shows an acceptable results of 595.3 and 485.7 mA h g−1, which are a bit lower than that of CuNWs sheet based anode. The main cause is ascribed to the relatively loose structure the Cu foam based electrode has. However, Sn on copper foil only delivers unsatisfactory capacity of 270.5 mA h g−1 after cycles at the rate of 5C. It is noticed that a preferable reversible capacity of CuNWs sheet based anode of 790.6 mA h g−1 could be recovered after 35 cycles upon reducing the current rate to 0.49 A g−1 (0.5C), indicating an excellent rate performance that the rate capacity is maintainable even after the repetitive cycles at relatively high rates. To confirm the better results from rate capacity tests, the electrochemical impedance spectra of the three samples after 60 cycles were investigated. An equivalent circuit model base on the EIS result is built up as shown in the inset of Fig. 7(c). The intercept of the high-frequency semicircle on the Z′ axis can be attributed to the resistance of electrolyte (Rs). RSEI and Rct are the resistance of the SEI layer and the charge transfer, respectively. The slope line at low frequency is related to Warburg impedance (Zw), corresponding to the diffusion of lithium ions into the bulk electrode. The fitting values of kinetic parameters of composite electrodes after different cycles are listed in Table 1. The Rct of CuNWs sheet based anode is 71.8 Ω, and is much lower than that of Cu foil based anode (242 Ω), implying much faster charge transfer at the electrode/electrolyte interface. The Rct of Cu foam based anode shows a relatively low result of 100.1 Ω compared to flat composite anode at the same time. According to Fig. 7(c), it can be found clearly that the diameter of the semicircle for the two 3D electrodes in the high–medium-frequency region is significantly smaller than that of the 2D composite, which illustrates the superior rate performance of the 3D hybrid anode as well as implies that the advanced structure could effectively enhance the electrical conductivity and reduce the contact and charge-transfer resistances in the Cu@Sn electrodes.
| Rs (Ω) | RSEI (Ω) | Rct (Ω) | CPE1 (F) | CPE2 (F) | |
|---|---|---|---|---|---|
| 1 | 6.8 | 15.61 | 242 | 5.13 × 10−6 | 6.89 × 10−6 |
| 2 | 4.0 | 10.63 | 100.1 | 6.65 × 10−6 | 1.52 × 10−5 |
| 3 | 3.8 | 7.78 | 71.8 | 3.19 × 10−6 | 2.27 × 10−5 |
Morphology analysis
Coin-type half cells with three types of anodes were disassembled to investigate the surface morphology after assigned cycles. In Fig. 8(a) and (e) we can observe SEM images of CuNWs and Cu foil based composite electrodes before electrochemical process. Fig. 8(c) shows the Cu@Sn nanowires on the surface of the copper foam after Sn electrodeposition. It can be distinguished that the vertical array turns into unordered structure due to the deposition process, which is assigned to the mass loading of tin unexpectedly. After 60 galvanostatic cycles, nanowires in Fig. 8(b) and (d) become thicker and rougher. The small particles visible on the surface of the copper nanowires are bigger than that before cycling, caused by the inevitable little pulverization of Sn, which is also the origin of capacity loss in initial cycles. It is noted that the morphology of nanowires and three-dimensional structure were maintained, contributing to stable cycling performance after initial cycles. However, there is a huge pulverization and crack observed in Fig. 8(f) and the flat structure of the 2D Sn coating is not retained after 60 electrochemical cycles. Consequently, the super rate and cycling performances result from the enhanced copper nanowires network providing more active sites and shortening the transfer distance for lithiation and delithiation, and the small size of the active material on nanowires alleviating pulverization and aggregation.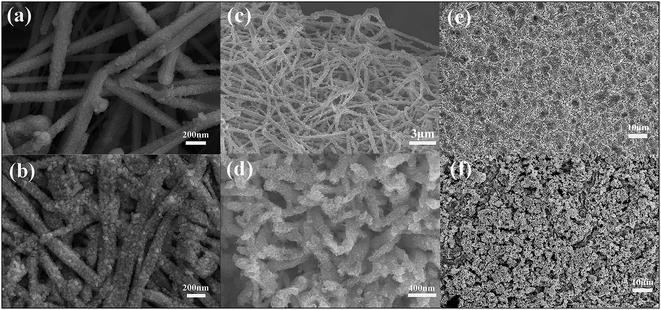 | ||
| Fig. 8 SEM images of surface morphology of CuNWS sheet, Cu foam and Cu foil based electrode ((a), (c) and (e)) before cycling, ((b), (d) and (f)) after 60 cycles. | ||
The loading mass of tin on copper foam, copper sheet and copper foil are 1.74, 1.63 and 1.89 mg cm−2, respectively. According to the data, the areal capacity can be calculated and the results are 1.40, 1.33 and 0.76 mA h cm−2 in average at the current density of 0.49 A g−1. Fig. 4 gives the data of mass per unit area of the three samples. If the specific capacity is calculated considering the weight of active materials and current collectors, the three samples will perform the capacities of 27.8, 228.1 and 57.2 mA h g−1, respectively. Therefore, it needs to be pointed out that the CuNWs sheet based current collector may be a promising material for micro-electronics devices in the future.
Conclusions
In summary, copper nanowires grow on copper foam to make a hierarchical structure, which has micro-sized pores and nano-sized metal wires, making a better transfer of ions. We also report a light and flexible current collector, which is fabricated by copper nanowires (CuNWs). The charge capacity of the thin CuNWs sheet based Sn–Cu composite anode remains above 760 mA h g−1 after 60 cycles' test with a relatively stable coulombic efficiency fluctuating around 97%. The Cu foam based composite anode also shows a good capacity retention of 79.8% after the same test, compared with Cu foil based anode. According to the good rate performance and the light weight of the composite electrode, the CuNWs sheet based current collector may be a promising material in energy fields in the future.Conflict of interest
The authors declare no competing financial interest.Acknowledgements
This study was supported by the National Basic Research Program of China (973 Program) (2013CB934001), National Natural Science Foundation of China (51074011 and 51274017), and National 863 Program (2007AA03Z231 and 2011AA11A257).References
- H. Zheng, K. Jiang, T. Abe and Z. Ogumi, Carbon, 2006, 44, 203–210 CrossRef CAS.
- M. Winter and J. O. Besenhard, Electrochim. Acta, 1999, 45, 31–50 CrossRef CAS.
- H. Zhao, C. Jiang, X. He, J. Ren and C. Wan, Electrochim. Acta, 2007, 52, 7820–7826 CrossRef CAS.
- M. Winter, J. O. Besenhard, M. E. Spahr and P. Novak, Adv. Mater., 1998, 10, 725–763 CrossRef CAS.
- R. Hu, M. Zhu, H. Wang, J. Liu, O. Liuzhang and J. Zou, Acta Mater., 2012, 60, 4695–4703 CrossRef CAS.
- K. Kravchyk, L. Protesescu, M. I. Bodnarchuk, F. Krumeich, M. Yarema, M. Walter, C. Guntlin and M. V. Kovalenko, J. Am. Chem. Soc., 2013, 135, 4199–4202 CrossRef CAS PubMed.
- B. Wang, B. Luo, X. Li and L. Zhi, Mater. Today, 2012, 15, 544–552 CrossRef CAS.
- W.-M. Zhang, J.-S. Hu, Y.-G. Guo, S.-F. Zheng, L.-S. Zhong, W.-G. Song and L.-J. Wan, Adv. Mater., 2008, 20, 1160–1165 CrossRef CAS.
- Y. Yu, L. Gu, C. Wang, A. Dhanabalan, P. A. van Aken and J. Maier, Angew. Chem., 2009, 48, 6485–6489 CrossRef CAS PubMed.
- G. Wang, B. Wang, X. Wang, J. Park, S. Dou, H. Ahn and K. Kim, J. Mater. Chem., 2009, 19, 8378 RSC.
- J. Jiang, Y. Y. Li, J. P. Liu, X. T. Huang, C. Z. Yuan and X. W. Lou, Adv. Mater., 2012, 24, 5166–5180 CrossRef CAS PubMed.
- J. S. Chen and X. W. Lou, Small, 2013, 9, 1877–1893 CrossRef CAS PubMed.
- Y. Yu, Q. Yang, D. Teng, X. Yang and S. Ryu, Electrochem. Commun., 2010, 12, 1187–1190 CrossRef CAS.
- G. Cui, Y. S. Hu, L. Zhi, D. Wu, I. Lieberwirth, J. Maier and K. Mullen, Small, 2007, 3, 2066–2069 CrossRef CAS PubMed.
- W. Pu, X. He, J. Ren, C. Wan and C. Jiang, Electrochim. Acta, 2005, 50, 4140–4145 CrossRef CAS.
- H. Guo, S. Zhao, H. Zhao and Y. Chen, Electrochim. Acta, 2009, 54, 4040–4044 CrossRef CAS.
- G.-H. Lee, R. C. Cooper, S. J. An, S. Lee, A. van der Zande, N. Petrone, A. G. Hammerberg, C. Lee, B. Crawford and W. Oliver, Science, 2013, 340, 1073–1076 CrossRef CAS PubMed.
- Y. Xue, B. Wu, L. Jiang, Y. Guo, L. Huang, J. Chen, J. Tan, D. Geng, B. Luo, W. Hu, G. Yu and Y. Liu, J. Am. Chem. Soc., 2012, 134, 11060–11063 CrossRef CAS PubMed.
- Y. Wang, M. Wu, Z. Jiao and J. Y. Lee, Chem. Mater., 2009, 21, 3210–3215 CrossRef CAS.
- X. Hou, H. Jiang, Y. Hu, Y. Li, J. Huo and C. Li, ACS Appl. Mater. Interfaces, 2013, 5, 6672–6677 CAS.
- K. T. Lee, Y. S. Jung and S. M. Oh, J. Am. Chem. Soc., 2003, 125, 5652–5653 CrossRef CAS PubMed.
- S.-D. Seo, G.-H. Lee, A.-H. Lim, K.-M. Min, J.-C. Kim, H.-W. Shim, K.-S. Park and D.-W. Kim, RSC Adv., 2012, 2, 3315–3320 RSC.
- H. Zhang, H. Song, X. Chen and J. Zhou, J. Phys. Chem. C, 2012, 116, 22774–22779 CAS.
- Y. Xu, J. Guo and C. Wang, J. Mater. Chem., 2012, 22, 9562 RSC.
- X. Li, A. Dhanabalan, L. Gu and C. Wang, Adv. Energy Mater., 2012, 2, 238–244 CrossRef CAS.
- K.-C. Hsu, C.-E. Liu, P.-C. Chen, C.-Y. Lee and H.-T. Chiu, J. Mater. Chem., 2012, 22, 21533 RSC.
- S. Chen, Y. Wang, H. Ahn and G. Wang, J. Power Sources, 2012, 216, 22–27 CrossRef CAS.
- B. Luo, B. Wang, M. Liang, J. Ning, X. Li and L. Zhi, Adv. Mater., 2012, 24, 1405–1409 CrossRef CAS PubMed.
- J. Qin, C. He, N. Zhao, Z. Wang, C. Shi, E.-Z. Liu and J. Li, ACS Nano, 2014, 8, 1728–1738 CrossRef CAS PubMed.
- Y. Zou and Y. Wang, ACS Nano, 2011, 5, 8108–8114 CrossRef CAS PubMed.
- R. Kim, D. Nam and H. Kwon, J. Power Sources, 2010, 195, 5067–5070 CrossRef CAS.
- Y.-S. Lin, J.-G. Duh and H.-S. Sheu, J. Alloys Compd., 2011, 509, 123–127 CrossRef CAS.
- J. Park, J. Eom and H. Kwon, Electrochim. Acta, 2010, 55, 1825–1828 CrossRef CAS.
- L. Bazin, S. Mitra, P. L. Taberna, P. Poizot, M. Gressier, M. J. Menu, A. Barnabé, P. Simon and J. M. Tarascon, J. Power Sources, 2009, 188, 578–582 CrossRef CAS.
- Q. Li, S. Hu, H. Wang, F. Wang, X. Zhong and X. Wang, Electrochim. Acta, 2009, 54, 5884–5888 CrossRef CAS.
- L. Xue, Z. Fu, Y. Yao, T. Huang and A. Yu, Electrochim. Acta, 2010, 55, 7310–7314 CrossRef CAS.
- M. Yao, K. Okuno, T. Iwaki, T. Awazu and T. Sakai, J. Power Sources, 2010, 195, 2077–2081 CrossRef CAS.
- R. Lin, S. Zhang, Z. Du, H. Fang, Y. Ren and X. Wu, RSC Adv., 2015, 5, 87090–87097 RSC.
- N. Tamura, R. Ohshita, M. Fujimoto, S. Fujitani, M. Kamino and I. Yonezu, J. Power Sources, 2002, 107, 48–55 CrossRef CAS.
- Y. Qiu, K. Yan and S. Yang, Chem. Commun., 2010, 46, 8359–8361 RSC.
- Y. Xu, Q. Liu, Y. Zhu, Y. Liu, A. Langrock, M. R. Zachariah and C. Wang, Nano Lett., 2013, 13, 470–474 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |