Microwave-assisted one-step synthesis and characterization of a slow release nitrogen fertilizer with inorganic and organic composites
Peng Wena,
Zhansheng Wu*a,
Yanhui Hea,
Bang-Ce Yea,
Yajie Han*a,
Xinyuan Guanb and
Jun Wangb
aSchool of Chemistry and Chemical Engineering, Shihezi University, Shihezi 832003, PR China. E-mail: wuzhans@126.com; yaji1978@126.com; Fax: +86-993-2057270; Tel: +86-993-2055015
bAgricultural Techniques Extension Center, Xinjiang Agricultural Reclamation Academy of Sciences, Shihezi, 832000, PR China
First published on 30th March 2016
Abstract
A slow-release fertilizer (SRF) was synthesized in one step based on urea incorporated in a polymer matrix composed of sodium alginate (NaAlg), acrylic acid (AA), acrylamide (AM) and bentonite (Bent) via microwave irradiation. The proposed microwave-assisted method yielded high reaction rates with less reaction time of 5 minutes at 300 W. The raw materials and final products were characterized in terms of the structure and properties through Fourier transform infrared spectroscopy, X-ray diffraction, thermogravimetric analysis, scanning electron microscopy, and uniaxial compression measurements. The application potential was verified on the basis of swelling in different environments, largest water-holding ratio and water-retention capacity of soil, release study in soil, and the effect of the proposed SRF product on the germination rate of cotton seed. Results indicated that the addition of Bent not only contributed to the increase in water absorbency, largest water-holding ratio and water-retention capacity of soil, but also caused the system to liberate the nutrient in a more prolonged manner based on a Case II release mechanism with skeleton erosion. Thus, microwave irradiation would be a possible method to produce SRFs for potential agricultural and horticultural applications.
1. Introduction
Fertilizers, as one of the most important products of the agrochemical industry, are one of the most vital input materials for crop production both in quality and quantity.1 Nitrogen, the essential nutrients for good soil fertility and plant growth, is a key aspect in the productivity of agriculture, although the utilization efficiency is only about 30–50%.2,3 Urea is the most economically viable fertilizer in global agriculture due to its high nitrogen content and comparatively low production costs.2 However, ammonia volatilization and nitrate leaching obtains a high rate of loss and low efficiency for urea, consequently, resulting in large economic losses and severe environmental hazards.4 To improve the utilization efficiency of fertilizers while reducing environmental problems, slow-release fertilizers (SRFs) have emerged as a good alternative. SRFs are designed to release nutrients gradually to match the nutrient requirements of plants and thus it can make the nutrient available in the field for a longer period of time than conventional fertilizers can.1 The use of SRFs has been proved to be an effective method to reduce the economic losses and environmental pollution.5The growth and quality of plants not only rely on fertilizers but also on water. Many farmlands, especially in arid and semiarid regions, suffer from water shortage. Superabsorbent polymers (SAP) have been extensively investigated as a water-managing material. SAP is a three-dimensionally cross-linked hydrophilic functional material that can absorb aqueous fluids up to thousands of times their original weight without dissolving and can hold liquid even under pressure.6 The utilization of SAP improves the water-holding capacity and fertility of soil.1 However, synthetic polymers derived from petroleum-based polyacrylate or polyacrylamide types with poor degradability dominate the market and cause environmental problems.6 With increasing environmental awareness and diminishing petroleum-based resources, studies have focused on the incorporation of biodegradable, eco-friendly polysaccharides into SAP via green processing.7
Sodium alginate (NaAlg), composed of poly-β-1,4-D-mannuronic acid and α-1,4-L-glucuronic acid, in varying proportions by 1–4 linkages, provides a wide range of commercial applications because this substance can undergo gelatinization and exhibits advantageous properties such as relatively inert behavior in aqueous environments within a matrix and high gel porosity.8–10 In addition, NaAlg has always been used as a matrix to entrap and deliver proteins, drugs, and cells because this substance is soluble in water, renewable, nontoxic, biodegradable, and biocompatible.9 By virtue of these advantages, NaAlg has been extensively investigated in industrial and medical fields. However, NaAlg applications remain unfeasible because of its high price, gas permeability, low modulus, and thermal stability. To solve these problems, researchers synthesized clay/polymer nanocomposites with clays added in high proportions.1 The incorporation of clays not only decreases production costs, but also improves the water-retention ability, swelling ability, gel strength, mechanical and thermal stabilities.1 Thus, demands for economically and environmentally sustainable NaAlg-based clay/polymer materials have increased. For instance, bentonite (Bent), which is mainly composed of montmorillonite (MMT), whose structural unit consists of two tetrahedral sheets (Si–O) separated by an octahedral sheet (Al–O–OH), is a good substrate for SAP with reactive –OH groups on the surface, high specific surface area, high swelling capacity, and a valuable cation exchange capacity.11–14 Furthermore, Bent is environmentally friendly and readily available in large quantities at a low cost.
Microwave irradiation (MW) has been considered as a potential alternative to preparing SAPs. Compared with conventional, time-consuming, thermally induced polymerization by conductive heating with an external heat source, microwave irradiation via a commercial household microwave oven can provide various advantages such as simple experimental apparatus, uniform heating, reaction rate increases, time and energy savings, and convenient process control.7 However, studies are yet to develop on the combination of SAP and SRFs in single formulation on the basis of NaAlg, acrylic acid (AA), acrylamide (AM), Bent, and urea as raw materials via microwave irradiation. Thus, SRF synthesis by a microwave technique should be developed and their properties should be investigated to improve the utilization efficiency of water resources and fertilizer nutrients and compared with conventional fertilizers.
This study mainly aimed to synthesize a Bent-based SRF by using AA, AM, NaAlg and urea via microwave irradiation. The physical and chemical properties of the SRF were characterized using Fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD), thermogravimetric analysis (TGA), scanning electron microscopy (SEM) and uniaxial compression measurements. The water absorbency of the samples in different environments, largest water-holding ratio, water-retention capacity of soil, slow-release behavior of the SRF in soil, and the effect of the proposed SRFs product on the germination rate of cotton seed were also systematically investigated. The release kinetics was also obtained. Results revealed that microwave irradiation may be a significant strategy to produce SRFs that could be widely applicable in modern agriculture and horticulture.
2. Experimental
2.1 Materials
AA was provided by Tianjin Fuyu Fine Chemical Co., Ltd. AA was distilled under reduced pressure before use to remove the polymerization inhibitor and stored in a brown reagent bottle. NaAlg, AM, urea, potassium persulfate (KPS) and N,N′-methylenebis acrylamide (MBA) were purchased from Tianjin Fuchen Chemical Reagent Co., Ltd. All reagents were of analytical grade and all solutions were prepared with distilled water. The raw Bent samples were collected from XiaZiJie deposits in the Xinjiang Uyghur Autonomous Region of China. The Bent used was purified using the method of Sun et al.12 The resultant Bent has a composition (%, by mass) of A12O3 13.06, SiO2 64.62, Na2O 2.66, K2O 2.43, CaO 1.92, MgO 2.38, Fe2O3 4.93, TiO2 0.59, MnO 0.26, and P2O5 0.18, and an ignition loss of 6.20. The MMT content was 93.0 g based on 100 g Bent, the cation exchange capacity was 98.4 mmol based on 100 g Bent and the swelling index was 89.5 mL g−1.2.2 Preparation of SRFs
In this procedure, 3.0 g of NaAlg was mixed with 100 mL of distilled water in a three-necked flask equipped with an electric blender, a reflux condenser, and a nitrogen line. A NaAlg solution as obtained by stirring at 60 °C in a water bath for 1 h until the NaAlg dissolved completely. Subsequently, 1.5 g of Bent was added to the NaAlg solution while constantly stirring to disperse Bent uniformly. The mixture was then cooled to room temperature. A solution containing 5.0 g of AA, 5.0 g of AM, 3.6 g of urea, 0.1 g of MBA, and 3.3 mL of 0.04 mol L−1 KPS was subsequently introduced to the cooled NaAlg/Bent mixture while agitating for 30 min. All these procedures were performed under a nitrogen atmosphere. Furthermore, the mixture was transferred into a 250 mL round bottom flask and then was placed inside a MW oven (MM823LA6-NS, Midea, China) with the following appropriate modifications. One hole was drilled into the oven to provide a straight tube with a reflux condenser and the hole was jacketed with copper tubing to avoid microwave leakage. The MW oven possessed a 2450 MHz frequency, a maximum 900 W output power, and the oven was equipped with six adjustable power levels and a time controller with a maximum time range of 30 min. The solution was subjected to irradiation at 300 W for 5 min to complete polymerization under a nitrogen atmosphere. The resulting NaAlg-g-p(AA-co-AM)/Bent/urea samples were washed with 70% ethanol and dried to a constant weight in a vacuum oven at 70 °C and were then grounded and passed through 40–80 mesh sieves.2.3 Characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) and then compressed to form KBr tablets for spectroscopic characterization using a Fourier transform infrared spectrometer (Nicolet Avatar 360, USA) in the wavenumber range of 4000–400 cm−1 with a resolution of 4 cm−1 and number of scans 50.
200) and then compressed to form KBr tablets for spectroscopic characterization using a Fourier transform infrared spectrometer (Nicolet Avatar 360, USA) in the wavenumber range of 4000–400 cm−1 with a resolution of 4 cm−1 and number of scans 50.2.4 Swelling measurement
About 0.1 g of the pre-dried samples were immersed entirely in 250 mL of distilled water, various saline solutions (NaCl, KCl, NH4Cl, MgCl2, FeCl3 and Na2SO4, and Na3PO4) with different concentrations ranging from 0.05 to 0.25 mol L−1 and various pH value solutions ranging from 2.0 to 12.0 at room temperature until the swelling equilibrium was reached. The swollen samples were filtered through a 100-mesh stainless steel screen and weighed. The equilibrium water absorbency Qeq (g g−1) was calculated by eqn (1).
 | (1) |
2.5 Slow release behavior in soil
1 g of SRF was embedded into a nylon bag and then buried 5 cm beneath the surface of the soil in a plastic cup containing 150 g of dry soil and incubated for different periods at room temperature. Throughout the experiment, the moisture content of the soil was maintained at 30% by weighing and adding distilled water if necessary, periodically. After 1, 3, 5, 10, 15, 20, 25, and 30 days of incubation, the bag with remaining SRF were picked out and then estimated for the contents of nitrogen after being dried at room temperature to a constant weight. The remaining amount of nitrogen was determined by an elemental analysis instrument.With the aim of gaining insights into the slow release kinetics and mechanism in soil, the first order release kinetics,15 Higuchi release kinetics,16 and Ritger–Peppas release kinetics models were employed.17
First order release kinetics model:
 | (2) |
Higuchi release kinetics model:
 | (3) |
Ritger–Peppas release kinetics model:
 | (4) |
2.6 Largest water-holding ratio of soil with SRFs
Different amounts of SRFs with application ratios of 0.1% and 0.2% were mixed well with 200 g of dry soil and placed in a PVC tube with a diameter of 4.5 cm. The bottom of the tube was sealed with 200-mesh nylon fabric and weighed (marked W0). Subsequently, an appropriate amount of distilled water was slowly added from the top of the tube until the soil samples reached saturation. The tube was weighed a second time (marked W1). A control experiment in soil without SRFs was also conducted. The largest water-holding ratio (WH%) of the soil was calculated using eqn (5).
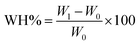 | (5) |
2.7 Measurement of the water-retention of soil with SRFs
The water retention of the soil with SRFs was measured at different treatments: control, 200 g of dry soil only; 200 g of dry soil mixed well with 2.0 g of sample; and 200 g of dry soil mixed well with 4.0 g of sample. Each sample was placed in a glass beaker and weighed (marked W0). The soil samples were slowly drenched with distilled water to saturate the soil with the largest WH% determined in a previous step, and then the beaker was weighed again (marked W1). The beakers were kept under identical conditions at room temperature and weighed every three days (Wi) for 30 days. The water-retention ratio (WR%) of the soil with SRFs was calculated by eqn (6).
 | (6) |
2.8 Pot experiments
Pot culture experiments on the cotton plant were carried out with treatments of SRFs and pure urea. Different amounts of SRFs and pure urea with the same nitrogen content were mixed well with 200 g of vermiculite (60% humidity) per pot and then placed into separate germination boxes. Ten cotton seeds were sown in each pot and eight replicates were treated for each treatment. The pots were placed in an artificial climatic box with day/night temperature of 30 °C and 25 °C, respectively, and 400 μmol (m2 s)−1 photons of light was supplied for 14 h during the day time. The germination rate of cotton seeds was calculated after 7 days. The results were the averages of eight measurements.3. Results and discussion
3.1 Characterization
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of acrylamide unit. Furthermore, the bands observed at approximately 1718 cm−1 and 1452 cm−1 of the samples in Fig. 1d–f corresponded to the C
O of acrylamide unit. Furthermore, the bands observed at approximately 1718 cm−1 and 1452 cm−1 of the samples in Fig. 1d–f corresponded to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O symmetric and asymmetric stretching vibrations of the acrylate unit, respectively. The abovementioned information demonstrated that the grafting reaction of AA and AM with NaAlg occurred.19 In Fig. 1d–f, the bands between 3300 cm−1 and 3500 cm−1 were assigned to the overlapped stretching vibrations of O–H and N–H; the bands at 2941, 2950, and 2946 cm−1 were associated with the combined stretching of CH2 in AA and AM in the SAP structure.1 The following characteristic bands of urea were observed in the NaAlg-g-p(AA-co-AM)/Bent/urea: 3442 cm−1 and 3346 cm−1 for the asymmetric and symmetric stretching vibration of NH2, respectively, 1681 cm−1 for the C
O symmetric and asymmetric stretching vibrations of the acrylate unit, respectively. The abovementioned information demonstrated that the grafting reaction of AA and AM with NaAlg occurred.19 In Fig. 1d–f, the bands between 3300 cm−1 and 3500 cm−1 were assigned to the overlapped stretching vibrations of O–H and N–H; the bands at 2941, 2950, and 2946 cm−1 were associated with the combined stretching of CH2 in AA and AM in the SAP structure.1 The following characteristic bands of urea were observed in the NaAlg-g-p(AA-co-AM)/Bent/urea: 3442 cm−1 and 3346 cm−1 for the asymmetric and symmetric stretching vibration of NH2, respectively, 1681 cm−1 for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, and 557 cm−1 for the N–CO–N bending vibration, implying the involvement of urea in NaAlg-g-p(AA-co-AM)/Bent/urea.2
O stretching, and 557 cm−1 for the N–CO–N bending vibration, implying the involvement of urea in NaAlg-g-p(AA-co-AM)/Bent/urea.2
3.2 Water absorbency studies
The pH levels, ion types and concentrations could have a profound effect on the swelling behavior. Therefore, an examination of water absorbency in different environments was of vital importance in view of their agricultural and horticultural applications. | ||
| Fig. 6 Effect of various ions and concentrations on the water absorbency of NaAlg-g-p(AA-co-AM)/urea (a) and NaAlg-g-p(AA-co-AM)/Bent/urea (b). | ||
Moreover, divalent and trivalent anion salt solutions greatly affected the water absorbency, and the effect of the three anions on the swelling followed the tendency: Cl− < SO42− < PO43−. This is due to the negative charge of the added anions strongly repelling the negative charge of the anions in the polymeric chains. As a result, the added anions barely diffuse into the polymer network. Therefore, the effect law was mainly manipulated by the ionic strength of the salt solutions.24
3.3 Slow release behavior of SRF in soil
It was noted that the slow-release tendency for both samples in soil were similar (Fig. 8). The fertilizer in NaAlg-g-p(AA-co-AM)/urea and NaAlg-g-p(AA-co-AM)/Bent/urea released 13.4%, 25.8%, 60.3% and 2.34%, 8.27%, 56.1% on the 1st, 3rd, and 30th day, respectively, which indicated NaAlg-g-p(AA-co-AM)/Bent/urea exhibited a more efficient slow release property than NaAlg-g-p(AA-co-AM)/urea. This was ascribed to the addition of Bent creating a highly porous structure and a tortuous path that retarded the diffusion of fertilizers through the network to the medium.1 Therefore, the incorporation of Bent not only produced a porous structure in the sample, but also controlled the release of fertilizer from the sample. Interestingly, many cracks were observed on the surface of the SRF and the SRF became fragile with an increase in burial time, which was mainly due to the disruption of covalently linked (1–4) glycoside bonds of NaAlg and the degradation/decomposition of the polymer skeleton by microorganisms and enzymes in soil.353.4 Water-holding ratio of soil with SRFs
The largest water-holding (WH) ratios of soil with different application ratios of fertilizers are shown in Fig. 9. The largest WH ratio of soil without samples was 28.5%, whereas the largest WH ratio reached 42.3% and 50.7% of the soil with different dosages of NaAlg-g-p(AA-co-AM)/urea and 58.4% and 75.4% for the soil with different dosages of NaAlg-g-p(AA-co-AM)/Bent/urea. It was concluded that the largest WH ratio of the soil was remarkably improved by the addition of the samples to the soil and the water content increased as the dosage of the samples in the soil increased. This fact was due to the excellent water absorbance capacities of the polymer matrices. In addition, the experimental data also demonstrated that the introduction of Bent to the fertilizer could prominently enhance the largest WH ratio of the soil with NaAlg-g-p(AA-co-AM)/Bent/urea. The reason may be that the exfoliation and dispersion of Bent, as confirmed in our XRD and SEM analyses, in the polymeric network resulted in the water holding capacity of the sample to increased.22 Consequently, once the samples were applied to the soil, the soil could store and manage more rainwater or irrigation water. As a result, water loss could be efficiently reduced and water utilization efficiency could be improved.3.5 Water-retention capacity of soil with SRFs
Fig. 10 presents the water-retention capacity of the soil with and without SRFs. The soil without the samples lost all the absorbed water after 12 days, while the soil with different dosages of NaAlg-g-p(AA-co-AM)/urea lost all the absorbed water after 18 and 24 days. In addition, the water retention rate of the soil dosed with different amounts of NaAlg-g-p(AA-co-AM)/Bent/urea were 5.6% and 19.3% on the 30th day. These results indicated that the addition of samples to the soil could increase the water-retention capacity of the soil and decrease water evaporation. The water-retention capacity of the soil also increased as the dosage of the samples in the soil increased. The reason was that the polymer matrix endows the soil with excellent water absorbency and water-retention capacity and could absorb and store a large amount of water for prolonged used by plants.2 Simultaneously, fertilizer nutrients could also be released slowly with water.2 Therefore, the swollen SRF resembled a micro-reservoir to retain and supply moisture and nutrition to plants, and thus, the utilization efficiencies of water and fertilizer were greatly improved.2,36 Consequently, application of the samples could prolong irrigation cycles, reduce irrigation frequencies and provide protection from drought. Experimentation also indicated that the addition of Bent could significantly enhance the water-retention ability due to the exfoliation and dispersion of Bent into the polymer matrix, as verified in our previous XRD and SEM analyses. Moreover, the soil without the samples became hardened and cracked, while the soil with the samples maintained a continuous configuration to enhance soil aeration and permeability to prevent soil erosion. As a result, a favorable environment was established for crop growth.3.6 Slow release kinetics
The release fractions of the two fertilizers for different models were plotted and the respective kinetic parameters of various models were summarized in Table 1. The goodness of linear fit with the Ritger–Peppas release model was found by the correlation coefficients (R2) for both of two SRFs released into soil. For spherical matrices, according to the classification of the diffusion mechanism when n ≤ 0.43, the urea release mechanism approaches Fickian diffusion, whereas when n ≥ 0.85, Case II transport occurs, leading to a zero order (swelling or erosion controlled) release mechanism. When 0.43 ≤ n < 0.85, anomalous transport was observed involving both Fickian diffusion and polymer chain relaxation.37 As evident from Table 1, all diffusion exponents (n) were greater than 0.85 for both two fertilizers in soil, which was an indication of the Case II release mechanism with skeleton erosion.| Samples | First order | Higuchi | Ritger–Peppas | ||||
|---|---|---|---|---|---|---|---|
| R2 | K1 | R2 | KH × 10−2 | R2 | K × 10−2 | n | |
| NaAlg-g-p(AA-co-AM)/urea | 0.865 | 35.22 | 0.916 | 9.15 | 0.945 | 0.266 | 2.195 |
| NaAlg-g-p(AA-co-AM)/Bent/urea | 0.865 | 35.22 | 0.972 | 7.52 | 0.973 | 36.4 | 1.026 |
3.7 Effect of SRFs on the germination rate of cotton seeds
The germination rate of the SRF treatments increased by 18.32% in comparison to that of the pure urea treatments (Table 2). This phenomenon was assigned to the fact that the SRF product not only possessed good slow-release properties, but also excellent water absorbency and water retention capacities, which could supply the cotton seeds with essential nutritional elements and plenty of water during the growth period. Consequently, this decreases the nitrate leaching and improves the availabilities of water and fertilizer and eventually improves the germination rate.38 The proposed SRF product was beneficial for seed germination and could be widely applicable in modern agriculture and horticulture, especially in arid and desert regions.| Fertilizers | Germination rate (%) |
|---|---|
| Urea | 58.32 ± 1.34 |
| NaAlg-g-p(AA-co-AM)/Bent/urea | 76.64 ± 2.24 |
4. Conclusions
A novel NaAlg-g-p(AA-co-AM)/Bent/urea slow-release fertilizer was successfully prepared via microwave irradiation. With the microwave technique, the reaction rates were enhanced for a reduced reaction time of only 5 minutes at 300 W. FTIR, XRD, TGA, and SEM characterization results, and uniaxial compression measurement results revealed that a grafting reaction occurred as along with incorporation of Bent and urea into the polymer matrix of NaAlg-g-p(AA-co-AM)/Bent/urea with good nanodispersion, exfoliation of Bent into the polymer matrix, excellent thermal stability, a highly porous structure, and good mechanical properties. The swelling behavior was strongly dependent on the type and concentration of salt added to the swelling medium and the solution pH. The incorporation of Bent not only provided an improvement in the swelling capacity, largest water-holding ratio and water-retention capacity of soil, but also remarkably endowed the product to liberate nutrients in a more prolonged manner than without Bent. In addition, the release mechanism was based on a Case II release mechanism with skeleton erosion. The research on germination rate of cotton seeds indicated that the SRF has great feasibility in agriculture. Thus, the sample not only exhibited a good slow-release property, but also an excellent water absorbency and water retention capacity. The proposed products with these properties could effectively improve the utilization of nutrients from fertilizer and water by plants and contribute to sustainable development in agriculture.Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (21466034), Science and Technology Fund Projects of Shihezi University (2013ZRKXJQ01), and Science and Technology Innovation Team Project of Eighth Division in Xinjiang Group (2015TD03).References
- A. Rashidzadeh and A. Olad, Carbohydr. Polym., 2014, 114, 269–278 CrossRef CAS PubMed.
- B. L. Ni, M. Z. Liu and S. Y. Lü, Chem. Eng. J., 2009, 155, 892–898 CrossRef CAS.
- A. Bortolin, F. A. Aouada, L. H. Mattoso and C. Ribeiro, J. Agric. Food Chem., 2013, 61, 7431–7439 CrossRef CAS PubMed.
- M. M. Costa, E. C. Cabral-Albuquerque, T. L. Alves, J. C. Pinto and R. L. Fialho, J. Agric. Food Chem., 2013, 61, 9984–9991 CrossRef CAS PubMed.
- M. Y. Guo, M. Z. Liu, Z. Hu, F. L. Zhan and L. Wu, J. Appl. Polym. Sci., 2005, 96, 2132–2138 CrossRef CAS.
- Z. X. Liu, Y. G. Miao, Z. Y. Wang and G. H. Yin, Carbohydr. Polym., 2009, 77, 131–135 CrossRef CAS.
- C. X. Lin, H. Y. Zhan, M. H. Liu, S. Y. Fu and L. H. Huang, J. Appl. Polym. Sci., 2010, 118, 399–404 CrossRef CAS.
- S. B. Hua and A. Q. Wang, Carbohydr. Polym., 2009, 75, 79–84 CrossRef CAS.
- Z. Q. Li, J. F. Shen, H. W. Ma, X. Lu, M. Shi, N. Li and M. X. Ye, Polym. Bull., 2012, 68, 1153–1169 CrossRef CAS.
- W. B. Wang and A. Q. Wang, Carbohydr. Polym., 2010, 80, 1028–1036 CrossRef CAS.
- Z. S. Wu, C. Li, X. F. Sun, X. L. Xu, B. Dai, J. E. Li and H. S. Zhao, Chin. J. Chem. Eng., 2006, 14, 253–258 CrossRef CAS.
- X. F. Sun, C. Li, Z. S. Wu, X. L. Xu, L. Ren and H. S. Zhao, Chin. J. Chem. Eng., 2007, 15, 632–638 CrossRef CAS.
- Q. Y. Chen, F. Tian, J. Feng and Z. S. Wu, J. Shihezi Univ., Nat. Sci., 2015, 33, 346–350 CAS.
- S. H. Qin, Z. S. Wu, A. Rasool and C. Li, J. Appl. Polym. Sci., 2012, 126, 1687–1697 CrossRef CAS.
- S. Benita, Kinetic model identification of drug release from microcapsules using the nonlinear regression search procedure, in Microencapsulation and Artificial Cells, Springer, 1984, pp. 255–258 Search PubMed.
- P. R. Ravi, U. K. Kotreka and R. N. Saha, AAPS PharmSciTech, 2008, 9, 302–313 CrossRef CAS PubMed.
- P. L. Ritger and N. A. Peppas, J. Controlled Release, 1987, 5, 37–42 CrossRef CAS.
- S. Salem, A. Salem and A. A. Babaei, Chem. Eng. J., 2015, 260, 368–376 CrossRef CAS.
- Y. M. Zhou, S. Y. Fu, L. L. Zhang and H. Y. Zhan, Carbohydr. Polym., 2013, 97, 429–435 CrossRef CAS PubMed.
- L. H. Xie, M. Z. Liu, B. L. Ni and Y. F. Wang, J. Agric. Food Chem., 2012, 60, 6921–6928 CrossRef CAS PubMed.
- T. Wan, R. Q. Huang, Q. H. Zhao, L. Xiong, L. L. Qin, X. M. Tan and G. J. Cai, J. Appl. Polym. Sci., 2013, 130, 3404–3410 CrossRef CAS.
- M. Likhitha, R. R. N. Sailaja, V. S. Priyambika and M. V. Ravibabu, Int. J. Biol. Macromol., 2014, 65, 500–508 CrossRef CAS PubMed.
- A. E. Mucientes, F. Santiago, A. M. Carrero and B. Talavera, J. Polym. Eng., 2013, 33, 61–69 CAS.
- M. Y. Zhang, Z. Q. Cheng, T. Q. Zhao, M. Z. Liu, M. J. Hu and J. F. Li, J. Agric. Food Chem., 2014, 62, 8867–8874 CrossRef CAS PubMed.
- A. Sawut, M. Yimit, W. Sun and I. Nurulla, Carbohydr. Polym., 2014, 101, 231–239 CrossRef CAS PubMed.
- T. P. Gao, W. B. Wang and A. Q. Wang, Macromol. Res., 2011, 19, 739–748 CrossRef CAS.
- H. X. Yang, W. B. Wang and A. Q. Wang, J. Dispersion Sci. Technol., 2012, 33, 1154–1162 CrossRef CAS.
- M. Y. Zhang, Z. Q. Cheng, M. Z. Liu, Y. Q. Zhang, M. J. Hu and J. F. Li, J. Appl. Polym. Sci., 2014, 131 DOI:10.1002/app.40471.
- Y. Bao, J. Z. Ma and N. Li, Carbohydr. Polym., 2011, 84, 76–82 CrossRef CAS.
- A. Bortolin, F. A. Aouada, L. H. C. Mattoso and C. Ribeiro, J. Agric. Food Chem., 2013, 61, 7431–7439 CrossRef CAS PubMed.
- T. L. Zhang, F. J. Zhang, S. S. Dai, Z. F. Li, B. G. Wang, H. P. Quan and Z. Y. Huang, J. Appl. Polym. Sci., 2016, 133 DOI:10.1002/app.43219.
- J. L. Wang, W. B. Wang, Y. A. Zheng and A. Q. Wang, J. Polym. Res., 2011, 18, 401–408 CrossRef CAS.
- M. Sadeghi and H. Hosseinzadeh, Turk. J. Chem., 2008, 32, 375–388 CAS.
- W. B. Wang, J. Wang, Y. R. Kang and A. Q. Wang, Composites, Part B, 2011, 42, 809–818 CrossRef.
- Y. H. He, Z. S. Wu, L. Tu, Y. J. Han, G. L. Zhang and C. Li, Appl. Clay Sci., 2015, 109–110, 68–75 CrossRef CAS.
- R. Liang, M. Z. Liu and L. Wu, React. Funct. Polym., 2007, 67, 769–779 CrossRef CAS.
- H. Kaygusuz and F. B. Erim, React. Funct. Polym., 2013, 73, 1420–1425 CrossRef CAS.
- W. Luo, W. A. Zhang, P. Chen and Y. E. Fang, J. Appl. Polym. Sci., 2005, 96, 1341–1346 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |









