Usnic acid is a novel Pim-1 inhibitor with the abilities of inhibiting growth and inducing apoptosis in human myeloid leukemia cells†
Yin-bo Fana,
Min Huanga,
Yu Caoa,
Ping Gongb,
Wen-bing Liua,
Shu-yu Jina,
Jia-chen Wena,
Yong-kui Jingbc,
Dan Liu*a and
Lin-xiang Zhao*a
aKey Laboratory of Structure-Based Drugs Design & Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang 110016, PR China. E-mail: linxiang.zhao@vip.sina.com; sammyld@163.com
bDepartment of Pharmacology, Shenyang Pharmaceutical University, Shenyang 110016, PR China
cDepartment of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA
First published on 22nd February 2016
Abstract
Usnic acid (UA) is a secondary metabolite of lichens with a unique dibenzofuran scaffold. The growth inhibitory and apoptotic effects of UA as well as the potential mechanisms of action were determined in human acute myeloid leukemia HL-60 cells and chronic myeloid leukemia K562 cells. UA inhibits growth and induces apoptosis in both cell lines with HL-60 cells more responsive. The apoptotic effects are associated with a decrease in the levels of the anti-apoptotic protein Mcl-1. UA inhibits mitogen-activated protein kinase-interacting kinase 1/eukaryotic translation-initiation factor 4E (Mnk1/eIF4E) and the proviral integration site of moloney murine leukemia virus-1/eIF4E-binding protein 1 (Pim-1/4E-BP1) signallings in both cell lines. A kinase inhibition assay reveals that UA is a potent Pim-1 inhibitor with limited activity to inhibit Mnk1. Considering the important role of Pim-1 in myeloid leukemia, UA represents a lead compound for developing effective therapeutics for myeloid leukemia treatment.
1. Introduction
Usnic acid (Fig. 1), a secondary metabolite isolated from the globally distributed lichens, is especially abundant in Alectoria, Cladonia, Usnea, Lecanora, Ramalina and Evernia.1 UA exhibits numerous pharmacological activities including antibiotic, antiviral, anti-inflammatory and antitumor activities.2,3 UA contains a unique dibenzofuran scaffold similar to cercosporamide (Fig. 1). Both compounds displayed similar antineoplastic activities in a wide variety of human cancer cell lines.4,5 Recently, cercosporamide was reported as a novel selective Mnk1/2 kinase inhibitor with antileukemia effects.6–8 In addition, cercosporamide also has been reported to inhibit several other enzymes including Pim-1.6,9 Based on the structural similarity of UA with cercosporamide, we propose that UA would also have similar biological activities to inhibit cell growth and to induce apoptosis in myeloid leukemia cells which were tested in two human myeloid leukemia cell lines HL-60 and K562. The potential mechanisms of action such as inhibition of signalling pathways of Mnk1 and Pim-1 were examined in cells treated with UA and using pure enzymes.2. Results
2.1 UA inhibits growth and induces apoptosis in HL-60 and K562 cells
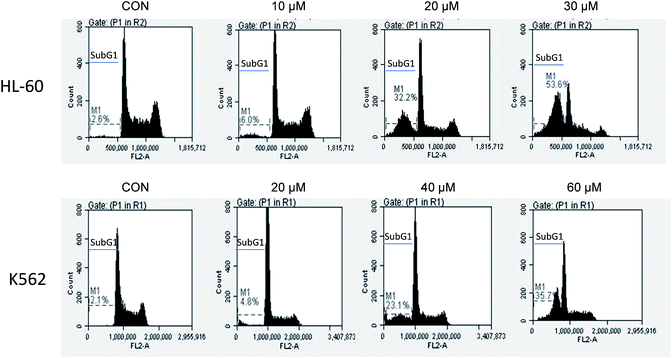
The growth inhibitory effects of UA towards myeloid leukemia HL-60 and K562 cells were determined by measuring cell numbers after treatment with UA at different concentrations for three days (ESI Fig. 1†). The IC50 values (the concentration of inhibiting 50% of cell growth) against HL-60 and K562 cells are 10.00 ± 1.03 μM and 10.39 ± 0.60 μM, respectively.The apoptosis induction abilities of UA in both cell lines were first measured by AO–EB staining to choose the appropriate dose and time for apoptosis occurrence (ESI Fig. 2†), and then by measuring fragmented DNA with PI staining using fluorescence-activated cell sorting (FACS). HL-60 cells are more sensitive than K562 cells to UA-induced apoptosis, but higher than 20 μM is required for apoptosis induction in both cell lines (Fig. 2). About 50% of apoptotic cells were detected in HL-60 cells after treatment with 30 μM of UA for 18 h. K562 cells were less sensitive to UA-induced apoptosis. UA at 60 μM induced 35.7% of cells undergoing apoptosis after 48 h treatment.
To elucidate the mechanism of UA-induced apoptosis, apoptosis-related proteins and activated caspases were measured with western blot analyses. HL-60 cells were treated with UA at concentrations of 10, 20 and 30 μM for 18 h and K562 cells were treated with UA at concentrations of 20, 40 and 60 μM for 48 h. Cleaved poly ADP ribose polymerase (PARP) was detected in HL-60 cells treated with 20 and 30 μM of UA and in K562 cells treated with 60 μM of UA. Correlated with PARP cleavage, cleaved pro-caspase-3, -8 and -9 were detected (Fig. 3), suggesting that a caspase activation-mediated apoptotic pathway leads to apoptosis induction. HL-60 cells express B-cell lymphoma 2 (Bcl-2) and Mcl-1 while K562 cells express B-cell lymphoma-extra-large (Bcl-xL) and Mcl-1.10 Consistent with the previous report, HL-60 cells do not have detectable Bcl-xL and K562 cells do not have detectable Bcl-2 (Fig. 3). The levels of Bcl-2 in HL-60 cells and the levels of Bcl-xL in K562 cells were not changed by UA treatment. However, the levels of Mcl-1 were decreased in both HL-60 and K562 cells, suggesting that down-regulation of Mcl-1 might play an important role in UA-induced apoptosis in both HL-60 and K562 cells.
2.2 UA inhibits Mnk1/eIF4E and Pim-1 signalling pathways
Extracellular signal-regulated kinase (ERK) and p38 MAPK have been found to activate Mnk1/2 which induce phosphorylation of eIF4E and increase the translation of Mcl-1.11–13 We tested the levels of ERK, p38, Mnk1, eIF4E and their phosphorylated forms in both cell lines treated with UA. UA did not have effects on those proteins in unphosphorylated forms (Fig. 4A). UA did not influence the forms of phosphorylated ERK and p38 in both cell lines (Fig. 4A). K562 cells, but not HL-60 cells, expressed phosphorylated Mnk1, which was inhibited by UA treatment. However, the levels of phosphorylated eIF4E were decreased in both HL-60 and K562 cells (Fig. 4A). Since eIF4E is phosphorylated by Mnk1/2, these data suggest that UA might work like cercosporamide as a Mnk1/2 inhibitor.The ability of eIF4E to regulate translation of protein is also controlled by binding with 4E-BP1 besides Mnk1 activation.14,15 4E-BP1 phosphorylation dissociates eIF4E and increases its ability of initiating translation. As we expand the range of proteins regulated by UA, it was found that the levels of phosphorylated 4E-BP1 were decreased by UA treatment (Fig. 4B). Many upstream factors affect the phosphorylation of 4E-BP1, with mammalian target of rapamycin (mTOR) as a main kinase to phosphorylate 4E-BP1.16 Although the levels of mTOR and p-mTOR are downregulated in HL-60 cells, they are not regulated by UA in K562 cells (Fig. 4B). It has been reported that the inhibition of p-4E-BP1 and suppression of protein translation process is the most representative effect of Pim kinase inhibition in leukemia cells.17 Pim kinases (Pim-1, 2, 3), a family of constitutively expressed and active serine/threonine kinases, are overexpressed in a wide range of hematopoietic malignancies and solid cancers.18 Pim-1 is the most highly expressed isoform in acute myeloid leukemia and chronic myeloid leukemia cells.19 Pim-1 exerts its oncogenic activities through regulation of myc-driven transcription, 4E-BP1-involved cap-dependent translation, and survival signalling by phosphoryting Bcl-2-associated agonist of cell death (BAD). We tested Pim-1 and its downstream targets including c-Myc and phosphorylated BAD with Western blotting (Fig. 4C). The levels of c-Myc and p-BAD were significantly decreased in K562 cells treated with UA. In addition, we found that the level of Pim-1 protein was also inhibited by UA treatment. These results indicate that UA is a potential Pim-1 inhibitor.
2.3 UA is a potent Pim-1 inhibitor with minimal inhibitory effects on Mnk1/2
To further test the abilities of UA to inhibit the activities of Mnk1/2 and Pim-1, the inhibitory effects of UA on Mnk1/2 and Pim-1 kinase activity were measured using pure enzymes. UA showed minimal inhibitory activity towards Mnk1/2 kinase in this biochemical inhibitory assay (IC50 > 100 μM). UA exhibited significant inhibitory effects on Pim-1 enzyme activity with an IC50 value of 202 ± 2.08 nM (Fig. 5A). The potential binding mode of UA with Pim-1 kinase was analyzed using the docking method. As shown in Fig. 5B, UA enters and occupies the ATP binding pocket of Pim-1, forming four hydrogen bonds with Glu121 and Asp186. These results reveal that UA is a unique and novel Pim-1 inhibitor. | ||
Fig. 5 UA inhibits Pim-1 activity. (A) Dose-response curves of Pim-1 activity inhibition by UA. Data shown are means ± SD of three independent experiments at ten concentrations ranging from 0.1–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nM. (B) Proposed binding mode of UA with Pim-1 kinase (PDB code: 4ALW) using docking analysis. 000 nM. (B) Proposed binding mode of UA with Pim-1 kinase (PDB code: 4ALW) using docking analysis. | ||
3. Discussion
Although a few reports show that UA inhibits tumour and leukemia cell growth, its mechanism of action is unknown.20,21 it selectively inhibits cancer cells growth, indicating great promise in cancer therapy.20 UA shares a similar structure with cercosporamide with abilities of inhibiting Mnk1 and Pim-1 (Fig. 1). Mnk1 is a kinase which phosphorylates eIF4E and then increases Mcl-1 translation. Mcl-1 is an important anti-apoptotic protein to block apoptosis in myeloid leukemia cells.22 Both HL-60 and K562 cells express high levels of Mnk1 and p-eIF4E (Fig. 4A). K562 cells have high levels of phosphorylated Mnk1 and are less sensitive to UA (Fig. 4A). UA decreased the levels of phosphorylated Mnk1 in K562 cells and the levels of phosphorylated eIF4E in both HL-60 and K562 cells, which is associated with the repression of Mcl-1 protein. Although these data suggest that UA might act as cercosporamide to inhibit Mnk1, UA did not inhibit Mnk1 activity at a concentration higher than 100 μM using pure Mnk1 enzyme assay. As reported recently, the decreased levels of Mnk1 may account for the decrease of phosphorylated eIF4E in both cell lines,23,24 we found that the levels of Mnk1 protein is decreased in both cell lines treated with UA (Fig. 4A). Since decreased levels of Mcl-1 are observed in HL-60 and K562 cells without repression of phosphorylated eIF4E (20 μM in HL-60 and 30 μM in K562 cells), UA could inhibit Mcl-1 translation through other mechanisms.mTOR has also been found to increase protein translation by phosphorylating p70S6K and 4E-BP1.16 UA treatment decreased the levels of p-4E-BP1, but not p-mTOR and pS70S6K. p-4E-BP1 is not only phosphorylated by mTOR, but also phosphorylated by Pim-1. We found that UA not only decreased the levels of p-4E-BP1, but also c-Myc, cyclin D1 and p-BAD as well as with increased levels of p27, the known events regulated by Pim-1 inhibitors.18,25 Using pure Pim-1 enzyme, we found that UA is a potent Pim-1 kinase inhibitor (Fig. 5A). Therefore, UA acts differently to cercosporamide with minimal inhibitory effects on Mnk1. The structure differences between the two compounds occur at three positions: an extra methyl group at C-8 of UA, the acetyl group at C-6 in UA versus a carbamoyl at that of cercosporamide, and the opposite configuration of methyl at C(9b). The weak inhibitory effect of UA on Mnk1 seems due to the absence of carbamoyl at C-6 position, as the carbamoyl group was reported to function as a necessary hinge binder in Mnk1/2 kinase catalysis pocket in the docking experiments.26 Since UA has similar anti-leukemia activities to reported results of cercosporamide,4,5 our data indicate that inhibition of Pim-1 would play a similar role with the inhibition of Mnk1 for myeloid leukemia therapy. Pim-1 is regulated by oncogenes such as FLT3-ITD in AML and BCR-ABL in CML.19,27 Pim-1 inhibitors are being developed with promising anti-leukemia effects.28,29 We found that UA not only inhibited the activity of Pim-1, but also decreased the levels of Pim-1 protein in K562 cells (Fig. 4C), suggesting that UA may also target BCR-ABL oncogenic signalling.
It's worth noting that some adverse health effects of UA as dietary supplements have been reported,30 different dose and the specialty of cancer treatment might make way for the development of UA in cancer therapy. Despite of that, chemical modifications are needed to improve its potency and property. In summary, UA represents a novel lead compound to develop Pim-1 inhibitors for myeloid leukemia therapy.
4. Materials and methods
4.1 Regents
Usnic acid (UA) was purchased from Aladdin Reagent Inc. (Shanghai, China) with a HPLC purity of 99%. 20 mM concentration stock solution for biological assays was prepared by dissolving in dimethylsulfoxide (Sigma Chemical Co.). RNase was purchased from Sigma Chemical Co. Propidium Iodide (PI) from BD technology, fetal bovine serum (FBS) from Tianjin TBD Co. HEPES and Typan blue from Amresco. Inc. (Solon, OH). Antibody to poly (ADP-ribose) polymerase (PARP), pro-caspase-3, 9 and cleaved-caspase-3, 9, caspase-8, Mcl-1, Bcl-2, Bcl-xL, p27 and β-actin were obtained from Santa Cruz Biotechnology Inc. Extracellular signal-regulated kinase (ERK)1/2, phospho-ERK(Thr202/Tyr204), eIF4E, phospho-eIF4E(Ser209), p38 MAPK, phospho-p38 MAPK(Thr180/Tyr182), phospho-Akt(Ser473), Akt, Mnk1 and phosphor-Mnk1(Thr197/202), 4E-BP1, phosphor-4E-BP1(Ser43/67), c-Myc and p-BAD (Ser112), BAD, phospho-mTOR(Ser2448), mTOR, phospho-p70S6K(Thr389), p70S6K, Pim-1, cyclin D1 from Cell Signaling Technology. Inc. Mouse and rabbit horseradish peroxidase (HRP)-conjugated secondary antibodies from BD Biosciences (San Diego, CA). ECL kit was purchased from Amersham Biosciences (England, UK). Full-length human Pim-1, Mnk1/2 kinases were bought from Carna Biosciences (Kobe, JP).4.2 Cell lines and cell culture treatment
Human acute myeloid leukemia HL-60 cells and human chronic myelogenous leukemia K562 cells were obtained from American Type Culture Collection (ATCC) and grown in Roswell Park Memorial Institute-1640 media supplemented with 100 μg mL−1 streptomycin, 100 U mL−1 penicillin, 1 mmol L−1 L-glutamine, 5.958 mg mL−1 HEPES free acid and 10% (v/v) heat-inactivated FBS.4.3 Cell growth inhibition assays
Cells were seeded at 5 × 104 cells per mL and incubated with the indicated concentrations of UA in 24-wells plate for three days. After treatment, cells were suspended and 50 μL suspension mixed with equal volume of 0.4% solution of trypan blue gently. The total number of cells and dead ones were determined with the aid of a hemocytometer. Live cells were round and bright, while dead cells appeared blue. Growth-inhibitory ability of the compounds was calculated and express as the ratio of the cell number of treated cells to that of untreated sells. The experiment was repeated at least three times.4.4 Quantitation of apoptotic cells
Apoptotic cells were determined by morphologic observation and fluorescence-activated cell sorting (FACS) analysis after staining with PI. For morphologic apoptosis quantification, cells were stained with AO and EB as described previously,31 and the percentage of apoptotic cells was calculated from 300 cells. For FACS analysis with PI staining, cells treated with different concentrations of UA were fixed with ice-cold 70% ethanol at a density of 1 × 105 cells per mL and treated with 1 mg mL−1 RNase for 30 min at 37 °C. PI was then added to a final concentration of 50 μg mL−1 and the DNA content was quantitated by flow cytometry with an excitation wavelength of 488 nm and an emission wavelength of 625 nm. Data were analyzed using CELLQuest software.4.5 Kinase assay
Ser/Thr KinEase assay kit (CisBio) was used to determine the kinase inhibition of the designed compounds using staurosporine and as positive control. 0.666 ng of Pim-1 or Mnk1/2 was incubated with different concentrations of test compounds in an 8 μL reaction mixture (1 μM substrate S3, 36.7 μM ATP, 5 mM MgCl2, 1 mM dithiothreitol and 1 × KinEASE enzymatic buffer) for 50 min at room temperature. Reactions were terminated through adding 10 μL EDTA-containing detection reagents following the kit protocol. The ratio between the HTRF signals of 615 and 665 nm recorded with an Infinite® F500 microplate reader (Tecan, Switzerland) and IC50 values were calculated from the inhibition curves.324.6 Western blot analysis
HL-60 and K562 cells were treated with UA for 18 h and 48 h, respectively. Protein extracts (30 μg) prepared with RIPA lysis buffer [50 mM Tris–HCl, 150 mM NaCl, 0.1% SDS, 1% NP-40, 0.5% sodium deoxycholate, 1 mM phenylmethylsulfonyl fluoride (PMSF), 100 μM leupeptin and 2 μg mL−1 aprotinin (pH 8.0)] were estimated by BCA kit and denatured on metal bath at 98 °C for 5 min. Samples were loaded onto 10% SDS PAGE and proteins resolved at constant voltage, then transferred to nitrocellulose membranes, which were stained with 0.2% Ponceau S red to assure equal loading and transfer. After blocking by 5% non-fat milk for 1 h, specific antibody to responding proteins and β-actin were incubated overnight at 4 °C. Then treated with appropriate HRP-linked secondary antibody and processed for further improved ECL detection.4.7 Molecular modeling
Sybyl software was used to perform in silico docking. The X-ray crystal structure of Pim-1 kinase in complex with a benzofuro[3,2-d]pyrimidin-4-one derivative was retrieved from Protein Data Bank (PDB code 4ALW), considering the similarity between the ligand and UA. All calculations and manipulations were performed with the Surflex-Dock modules in the Sybyl X 2.0 software package. All water molecules were removed and hydrogen added. Applying the default parameters, the best docking result was selected to analysis based on the favourable binding affinity rank in kcal mol−1 (docking score).Conflicts of interest
The authors declare no conflict of interest.Notes and references
- K. Ingólfsdóttir, Phytochemistry, 2002, 61, 729–736 CrossRef.
- M. Cocchietto, N. Skert, P. Nimis and G. Sava, Naturwissenschaften, 2002, 89, 137–146 CrossRef CAS PubMed.
- A. A. Araujo, M. G. de Melo, T. K. Rabelo, P. S. Nunes, S. L. Santos, M. R. Serafini, M. R. Santos, L. J. Quintans-Junior and D. P. Gelain, Nat. Prod. Res., 2015, 29, 2167–2180 CrossRef CAS PubMed.
- M. Bačkorová, M. Bačkor, J. Mikeš, R. Jendželovský and P. Fedoročko, Toxicol. in Vitro, 2011, 25, 37–44 CrossRef PubMed.
- L. W. Wang, B. G. Xu, J. Y. Wang, Z. Z. Su, F. C. Lin, C. L. Zhang and C. P. Kubicek, Appl. Microbiol. Biotechnol., 2011, 93, 1231–1239 CrossRef PubMed.
- B. W. Konicek, J. R. Stephens, A. M. McNulty, N. Robichaud, R. B. Peery, C. A. Dumstorf, M. S. Dowless, P. W. Iversen, S. Parsons, K. E. Ellis, D. J. McCann, J. Pelletier, L. Furic, J. M. Yingling, L. F. Stancato, N. Sonenberg and J. R. Graff, Cancer Res., 2011, 71, 1849–1857 CrossRef CAS PubMed.
- J. Altman, A. Szilard, B. Konicek, P. Iversen, B. Kroczynska, H. Glaser, A. Sassano, E. Vakana, J. Graff and L. Platanias, Blood, 2013, 121, 3675–3681 CrossRef CAS PubMed.
- S. Lim, T. Y. Saw, M. Zhang, M. R. Janes, K. Nacro, J. Hill, A. Q. Lim, C. T. Chang, D. A. Fruman, D. A. Rizzieri, S. Y. Tan, H. Fan, C. T. C. Chuah and S. T. Ong, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 2298–2307 CrossRef PubMed.
- J. E. Beggs, S. Tian, G. G. Jones, J. Xie, V. Iadevaia, V. Jenei, G. J. Thomas and C. G. Proud, Biochem. J., 2015, 467, 63–76 CrossRef CAS PubMed.
- S. Yin, R. Wang, F. Zhou, H. Zhang and Y. Jing, Mol. Pharmacol., 2011, 79, 1072–1083 CrossRef CAS PubMed.
- A. Waskiewicz, A. Flynn, C. Proud and J. Cooper, EMBO J., 1997, 16, 1909–1920 CrossRef CAS PubMed.
- R. Fukunaga and T. Hunter, EMBO J., 1997, 16, 1921–1933 CrossRef CAS PubMed.
- J. R. Graff, B. W. Konicek, J. H. Carter and E. G. Marcusson, Cancer Res., 2008, 68, 631–634 CrossRef CAS PubMed.
- J. D. Richter and N. Sonenberg, Nature, 2005, 433, 477–480 CrossRef CAS PubMed.
- A. Modrak-Wojcik, M. Gorka, K. Niedzwiecka, K. Zdanowski, J. Zuberek, A. Niedzwiecka and R. Stolarski, FEBS Lett., 2013, 587, 3928–3934 CrossRef CAS PubMed.
- H. Pópulo, J. M. Lopes and P. Soares, Int. J. Mol. Sci., 2012, 13, 1886–1918 CrossRef PubMed.
- C. Blanco-Aparicio, A. M. Collazo, J. Oyarzabal, J. F. Leal, M. I. Albaran, F. R. Lima, B. Pequeno, N. Ajenjo, M. Becerra, P. Alfonso, M. I. Reymundo, I. Palacios, G. Mateos, H. Quinones, A. Corrionero, A. Carnero, P. Pevarello, A. R. Lopez, J. Fominaya, J. Pastor and J. R. Bischoff, Cancer Lett., 2011, 300, 145–153 CrossRef CAS PubMed.
- M. C. Nawijn, A. Alendar and A. Berns, Nat. Rev. Cancer, 2011, 11, 23–34 CrossRef CAS PubMed.
- E. K. Keeton, K. McEachern, K. S. Dillman, S. Palakurthi, Y. Cao, M. R. Grondine, S. Kaur, S. Wang, Y. Chen, A. Wu, M. Shen, F. D. Gibbons, M. L. Lamb, X. Zheng, R. M. Stone, D. J. Deangelo, L. C. Platanias, L. A. Dakin, H. Chen, P. D. Lyne and D. Huszar, Blood, 2014, 123, 905–913 CrossRef CAS PubMed.
- T. T. Nguyen, S. Yoon, Y. Yang, H. B. Lee, S. Oh, M. H. Jeong, J. J. Kim, S. T. Yee, F. Crisan, C. Moon, K. Y. Lee, K. K. Kim, J. S. Hur and H. Kim, PLoS One, 2014, 9, e111575 Search PubMed.
- S. T. Zuo, L. P. Wang, Y. Zhang, D. N. Zhao, Q. S. Li, D. Shao and X. D. Fang, RSC Adv., 2014, 5, 153–162 RSC.
- S. P. Glaser, E. F. Lee, E. Trounson, P. Bouillet, A. Wei, W. D. Fairlie, D. J. Izon, J. Zuber, A. R. Rappaport, M. J. Herold, W. S. Alexander, S. W. Lowe, L. Robb and A. Strasser, Genes Dev., 2012, 26, 120–125 CrossRef CAS PubMed.
- S. Ramalingam, L. Gediya, A. K. Kwegyir-Afful, V. P. Ramamurthy, P. Purushottamachar, H. Mbatia and V. C. Njar, Oncotarget, 2014, 5, 530–543 CrossRef PubMed.
- H. W. Mbatia, S. Ramalingam, V. P. Ramamurthy, M. S. Martin, A. K. Kwegyir-Afful and V. C. Njar, J. Med. Chem., 2015, 58, 1900–1914 CrossRef CAS PubMed.
- S. S. T. Hnit, C. Xie, M. Yao, J. Holst, A. Bensoussan, P. De Souza, Z. Li and Q. Dong, Int. J. Biochem. Cell Biol., 2015, 68, 9–14 CrossRef CAS PubMed.
- J. Hou, F. Lam, C. Proud and S. Wang, Oncotarget, 2012, 3, 118–131 CrossRef PubMed.
- M. Zhang, W. Fu, S. Prabhu, J. C. Moore, J. Ko, J. W. Kim, B. J. Druker, V. Trapp, J. Fruehauf, H. Gram, H. Y. Fan and S. T. Ong, Mol. Cell. Biol., 2008, 28, 6496–6509 CrossRef CAS PubMed.
- N. A. Keane, M. Reidy, A. Natoni, M. S. Raab and M. O'Dwyer, Blood Cancer Journal, 2015, 5, e325 CrossRef CAS PubMed.
- P. Mondello, S. Cuzzocrea and M. Mian, J. Hematol. Oncol., 2014, 7, 95 CrossRef PubMed.
- W. Sanchez, J. T. Maple, L. J. Burqart and P. S. Kamath, Mayo Clin. Proc., 2006, 81, 541–544 CrossRef PubMed.
- X. Zhao, H. Zhong, R. Wang, D. Liu, S. Waxman, L. Zhao and Y. Jing, Oncotarget, 2015, 6, 5582–5596 CrossRef PubMed.
- Y. B. Fan, K. Li, M. Huang, Y. Cao, Y. Li, S. Y. Jin, W. B. Liu, J. C. Wen, D. Liu and L. X. Zhao, Bioorg. Med. Chem. Lett., 2016, 26, 1224–1228 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra01159d |
| This journal is © The Royal Society of Chemistry 2016 |