Improved cycling stability of lithium–sulphur batteries by enhancing the retention of active material with a sandwiched hydrothermally treated graphite film
Yuede Pan,
Shu-Lei Chou*,
Hua-Kun Liu and
Shi-Xue Dou
Institute for Superconducting and Electronic Materials, University of Wollongong, New South Wales 2522, Australia. E-mail: shulei@uow.edu.au; Tel: +61 4298 1405
First published on 21st March 2016
Abstract
A new lithium–sulphur battery with a hydrothermally treated graphite film sandwiched between the separator and the sulphur cathode shows increased capacity, enhanced cycling stability and improved coulombic efficiency. After 50 cycles, a high capacity of 631 mA h g−1 is maintained, compared to 203 mA h g−1 for the Li–S battery with conventional configuration. Moreover, the coulombic efficiency is increased to near 100% from around 94%. This improved electrochemical performance could be attributed to the new cell configuration, because the graphite film greatly retains the active material by alleviating the polysulphide shuttling problem and providing extra reaction sites for sulphur species.
Introduction
Li–S batteries have been considered as promising next-generation batteries owing to their high theoretical specific capacity of 1675 mA h g−1, energy density of 2500 W h kg−1, in addition to the abundance and low price of sulphur.1 The theoretical energy density of Li–S batteries is 3–5 times higher than that of conventional lithium ion batteries.2 These attractive advantages make Li–S batteries important candidates for energy storage systems intended for wide applications in electric vehicles and back-up energy storage in renewable and intermittent energy sources such as solar and wind. However, compared to conventional electrode materials (LiCoO2, LiFePO4), there is a unique problem of low utilization of the active material sulphur as the cathode material.3 First, the intrinsic high electrical resistivity of sulphur makes the active material utilization very low, particularly at high rates.4 Because of the poor transport of both lithium ions and electrons, a certain percentage of the sulphur in the cathode is not able to participate in the electrochemical reactions. This is one main reason that for a long time, the reported capacity of Li–S batteries was low until Nazar et al. reported a high-capacity Li–S cathode of S/CMK-3 composite, prepared by a melt-diffusion method.5 Second, apart from the low utilization of sulphur in the cathode, sulphur is lost in the form of polysulphides, which are the soluble intermediate products in the transformation between the two end products of sulphur and Li2S. The intermediate polysulphides are dissolved in the ether-based liquid electrolyte and shuttle between the sulphur cathode and the lithium anode, causing quick decay of the capacity.6 To increase the active material utilization, varied approaches have been applied. For example, sulphur has been incorporated with different carbon materials,7 conductive polymers8 and metal oxides.9 Moreover, the interlayer strategy proves effective in decreasing the polysulphide shuttling problem.10Herein, we report a new configuration of Li–S batteries using a graphite film sandwiched between the sulphur cathode and the separator (Scheme 1). The cathode material is sulphur/Super P composite (S/SP). The graphite film is hydrothermally treated to anchor functional carbonyl and carboxyl groups, which have strong affinity for the polysulphides. The graphite film suppresses the polysulphide shuttling problem and provides extra reaction sites for the sulphur species, thereby improving the active material utilization, the capacity and the cycling stability of the Li–S battery.
Experimental
Hydrothermal treatment of the graphite film
Graphite films were ultrasonically treated in acetone and then put into a 100 mL Teflon-lined stainless-steel autoclave. 50 mL of deionized water were added and heated at 120 °C for 10 h. After the hydrothermal treatment, the films were ultrasonicated in acetone and dried at 100 °C for 4 h and then put into a glove box filled with argon. Before the cells were assembled, the graphite films were soaked in the electrolyte for 24 h.Material characterizations
The S/SP composite was prepared through the reaction between sodium thiosulphate and oxalic acid. Raman scattering experiments were performed on a JOBIN YVON HR800 Confocal Raman system with a 632.8 nm He–Ne laser. The morphology and structure of the samples were investigated by X-ray diffraction (XRD) (GBC MMA), field emission scanning electron microscopy (FE-SEM; JEOL JSM-7500FA) with an energy dispersive X-ray spectroscopy (EDS) detector and transmission electron microscopy (TEM, JEM 1200EX). X-ray photoelectron spectroscopy (XPS) was conducted with a SPECS PHOIBOS 100 Analyser installed in a high-vacuum chamber with the base pressure below 10−8 mbar, X-ray excitation was provided by Al Kα radiation with photon energy hν = 1486.6 eV at the voltage of 12 kV and power of 120 W. XPS spectra were obtained with a 20 eV constant pass energy. The binding energy scale was calibrated using the C 1s peak at 284.6 eV.Electrochemical measurements
The S/SP composite was mixed with Super P and poly(vinylidene fluoride) (PVDF) with a weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 in N-methyl-2-pyrrolidone (NMP) to form a slurry, which was pasted on aluminium foil and then dried in a vacuum oven at 50 °C for 24 h. The sulphur content in a typical electrode is around 0.8 mg cm−1. CR 2032 coin-type cells were assembled in an Ar-filled glove box (Mbraun, Unilab, Germany) using lithium metal foil as the counter electrode. For the new configuration, a piece of graphite film was placed between the sulphur cathode and the separator. The electrolyte was 1 M bis(trifluoromethane)sulfonimide lithium salt (LiTFSI) in a 1
10 in N-methyl-2-pyrrolidone (NMP) to form a slurry, which was pasted on aluminium foil and then dried in a vacuum oven at 50 °C for 24 h. The sulphur content in a typical electrode is around 0.8 mg cm−1. CR 2032 coin-type cells were assembled in an Ar-filled glove box (Mbraun, Unilab, Germany) using lithium metal foil as the counter electrode. For the new configuration, a piece of graphite film was placed between the sulphur cathode and the separator. The electrolyte was 1 M bis(trifluoromethane)sulfonimide lithium salt (LiTFSI) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vol/vol mixture of 1,3-dioxolane (DOL)/dimethoxyethane (DME). Cell disassembly was carried out in the glove box; the sandwiched graphite film and the cathode were washed with DOL and dried at room temperature. The cells were charged/discharged at 168 mA g−1 using a charger system manufactured by Land Battery Testers. Cyclic voltammetry (CV) was conducted on a Biologic VMP3 electrochemistry workstation at a scanning rate of 0.1 mV s−1.
1 vol/vol mixture of 1,3-dioxolane (DOL)/dimethoxyethane (DME). Cell disassembly was carried out in the glove box; the sandwiched graphite film and the cathode were washed with DOL and dried at room temperature. The cells were charged/discharged at 168 mA g−1 using a charger system manufactured by Land Battery Testers. Cyclic voltammetry (CV) was conducted on a Biologic VMP3 electrochemistry workstation at a scanning rate of 0.1 mV s−1.
Results and discussion
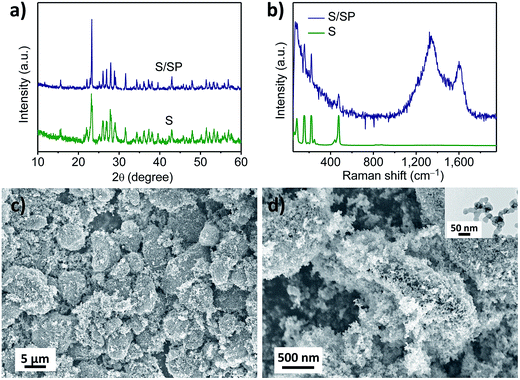
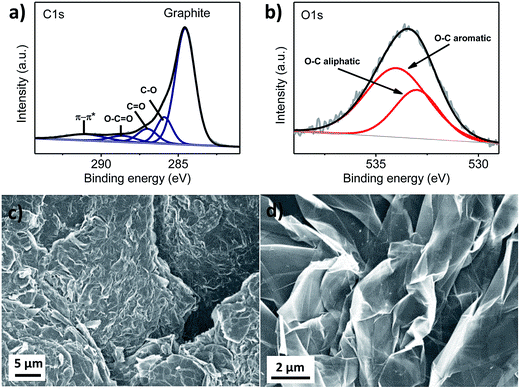
For the cathode material, a new structure of S/SP composite was prepared. The sulphur in the S/SP composite was confirmed by X-ray diffraction (Fig. 1a) and Raman spectra (Fig. 1b). The two peaks at 1345 and 1604 nm−1 are assigned to Super P. As determined by thermogravimetric analysis, the content of sulphur in the composite is 75 wt%. The core–shell morphology of the composite can be observed from the scanning electron microscopy (SEM) images. The size of the composite is around 10 μm (Fig. 1c), at the surface of which are linked nanoparticles of Super P (Fig. 1d). The linked nanoparticles have a diameter of around 40 nm (inset). The S/SP composite is composed of the micrometer-sized sulphur particle as the core and the linked nanoparticle (∼50 nm) of Super P as the wrapping layer. We assume that the contact coating of Super P improves the conductivity of the micrometer-sized sulphur particles.The graphite film, after hydrothermal treatment, was analysed by XPS and SEM. The XPS C 1s spectrum of graphite is presented in Fig. 2a. The main peak at 284.6 eV can be assigned to aromatic C–C bonds and the broad low-intensity peak centred at 291.0 eV corresponds to the π–π* shake up satellite.11 Between them are the other three peaks, which are at 285.9, 287.0 and 288.6 eV. It was reported12 that compared to the graphite C 1s peak, single-bonded C–O, double-bonded C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and carboxylic O–C
O and carboxylic O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O are shifted by ∼1.5 eV, 3.0 eV and 4.5 eV, respectively. It can be observed that the shifts for the three components match very well with these values. These organic functional groups are also reflected by the XPS O 1s spectrum in Fig. 2c. The peaks at 533 and 534 eV are assigned to O–C sp3 and O–C sp2 species, respectively.13 These oxygen species might have been introduced into the graphite under the hydrothermal treatment. It is noteworthy that during the charging and discharging of the modified cell, the organic functional groups on the graphite film might play a glue-like role in retaining sulphur and polysulphides.14 Moreover, in the graphite film, there are evident cracks (Fig. 2c), which might have been introduced by the hydrothermal treatment. In the closed environment of the autoclave at 120 °C, a temperature above the boiling point of water, the water vapour might strongly interact with the graphite film and produce some channels in it. In another study,15 a certain ratio of carbon black was mixed with reduced graphene oxide to form a free-standing paper for a new Li–S battery configuration, and the function of the carbon black was to produce channels, which enabled the transportation of electrolyte and lithium ions. As can be observed from Fig. 2d, the graphite film is composed of carbon nanosheets stacking with each other. The nanosheets behave as the reaction sites for the active sulphur species.
O are shifted by ∼1.5 eV, 3.0 eV and 4.5 eV, respectively. It can be observed that the shifts for the three components match very well with these values. These organic functional groups are also reflected by the XPS O 1s spectrum in Fig. 2c. The peaks at 533 and 534 eV are assigned to O–C sp3 and O–C sp2 species, respectively.13 These oxygen species might have been introduced into the graphite under the hydrothermal treatment. It is noteworthy that during the charging and discharging of the modified cell, the organic functional groups on the graphite film might play a glue-like role in retaining sulphur and polysulphides.14 Moreover, in the graphite film, there are evident cracks (Fig. 2c), which might have been introduced by the hydrothermal treatment. In the closed environment of the autoclave at 120 °C, a temperature above the boiling point of water, the water vapour might strongly interact with the graphite film and produce some channels in it. In another study,15 a certain ratio of carbon black was mixed with reduced graphene oxide to form a free-standing paper for a new Li–S battery configuration, and the function of the carbon black was to produce channels, which enabled the transportation of electrolyte and lithium ions. As can be observed from Fig. 2d, the graphite film is composed of carbon nanosheets stacking with each other. The nanosheets behave as the reaction sites for the active sulphur species.
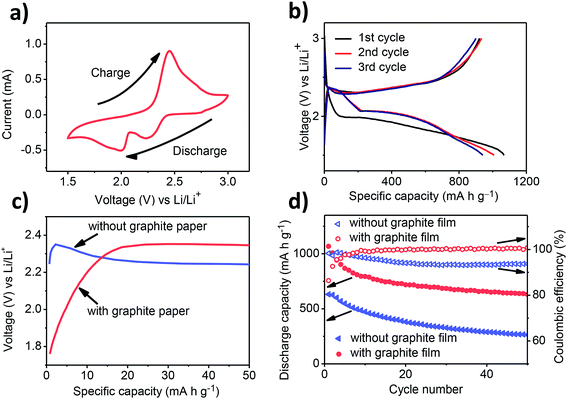
The electrochemical performances of cells with and without a graphite film are presented in Fig. 3. Typical electrochemical reactions are revealed by the CV results (Fig. 3a). The cathodic peaks at 2.00 V and 2.23 V correspond to the two-step reaction from elemental sulphur to insoluble Li2S2 or Li2S through intermediate soluble polysulfides (e.g., Li2S8, Li2S6 and Li2S4). The overlapping anodic peaks at 2.45 V relate to the inverse reactions, i.e., lithium sulfides are oxidized to lithium polysulphides and elemental sulphur. Initial discharge/charge voltage profiles for the first cycles present one discharge plateau at ∼2.0 V, followed by a long slope (Fig. 3b), whereas for the following two cycles, there are two discharge plateaus: the first at ∼2.3 V and the second at 2.1 V. The two cycles show high discharge capacities of 1008 and 942 mA h g−1, respectively. It is noted that for the first cycle, the discharge voltage plateau is more negative than the following cycles. This phenomenon can be attributed to the affected electrochemical reactions between lithium ions and sulphur, owing to the addition of the graphite film.16 Further investigation is needed to unveil the exact mechanism. Moreover, compared to the cell without the graphite film, the beginning stage of the charge process exhibits a much lower polarization, demonstrating that the graphite film functions as a pseudo-substrate for the sulphur cathode (Fig. 3c). The graphite film enables much higher capacity, markedly decreased capacity decay and higher electrochemical stability (Fig. 3d). The cell with the graphite film exhibits a high initial capacity of 1068 mA h g−1. After 50 cycles, the retained capacity is 631 mA h g−1, triple that of the 203 mA h g−1 for the cell without the graphite film. To exclude the capacity contributed by the graphite film, a Li/graphite film battery was assembled. The contribution from the graphite/Li+ intercalation process was 36 mA h per gram of sulphur, which occurs only ten percent of the whole improvement of capacity. It is revealed that the graphite film only functions as a polysulfide blocking layer instead of an electrode. It is noted that the graphite/Li+ intercalation happens at a voltage below 0.2 V, whereas the voltage window applied herein is from 1.7 V to 2.6 V. Moreover, the coulombic efficiency of the Li–S battery with the new configuration stabilizes at nearly 100%, compared to the 94% for the conventional cell configuration. The improved coulombic efficiency indicates that the sandwiched graphite film plays an important role in suppressing the polysulphide shuttling.
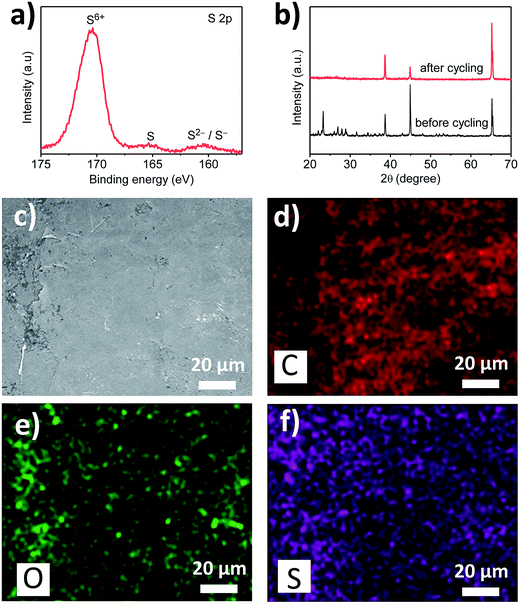
The function of the graphite film was further studied by disassembling the cell and examining the cathode and the graphite film using XRD, XPS, SEM and EDS (Fig. 4). After 10 cycles, the XRD peaks for sulphur disappear, with only peaks for the aluminium substrate remained, showing that the sulphur in the cathode has turned amorphous (Fig. 4b). Moreover, the XPS S 2p spectrum shows three peaks centred at around 170, 165 and 160 eV (Fig. 4a) and these values of three peaks match very well with the literature,17 from which they can be assigned to S6+, elemental S, and S2−/S−, respectively. The S6+ species could be assigned to residual lithium salt from the electrolyte, whereas S, S− and S2− are ascribed to the products of the electrochemical reactions that occur on the graphite film. This observation proves that the graphite film located between the separator and the cathode provides an extra place for the electrochemical reactions in the Li–S battery. The sulphur species on the graphite film originate from the soluble polysulphides, i.e., the intermediate products in the transformation between the end products of sulphur and Li2S (Fig. 4c–f). The polysulphides are retained by the functional groups in the graphite film. Therefore, they can stay on the graphite film and be oxidized or reduced in the charge/discharge process. In this way, the graphite film functions as a pseudo-substrate, greatly enhancing the retention of active material, improving the capacity, electrochemical stability and cycling capability.
Conclusions
A hydrothermally treated graphite film was sandwiched between the sulphur cathode and the separator. The functional groups on the graphite film play an important role in retaining the soluble sulphur species. Owing to the improved utilization of the active material, the specific capacity was significantly improved for the new configuration. The graphite film in the Li–S cell acts as a blocking layer against soluble polysulphides and provides additional reaction sites for the sulphur species. After 50 cycles, the cell with the graphite film retained a capacity almost triple that of the cell without the graphite film. Moreover, the coulombic efficiency was increased from around 94% to near 100%, indicating a much more stable Li–S battery.Acknowledgements
The study is supported through Auto CRC 2020, project 1-111. We would like to acknowledge the utilization of facilities and assistance of staff at the University of Wollongong Electron Microscopy Centre. We also thank Dr Tania Silver for critical reading of the manuscript.References
- J. B. Goodenough and Y. Kim, Chem. Mater., 2010, 22, 587–603 CrossRef CAS.
- (a) D. Aurbach, E. Pollak, R. Elazari, G. Salitra, C. S. Kelley and J. Affinito, J. Electrochem. Soc., 2009, 156, A694–A702 CrossRef CAS; (b) A. Manthiram, Y. Fu, S.-H. Chung, C. Zu and Y.-S. Su, Chem. Rev., 2014, 114, 11751–11787 CrossRef CAS PubMed.
- (a) Y. X. Yin, S. Xin, Y. G. Guo and L. J. Wan, Angew. Chem., Int. Ed., 2013, 52, 13186–13200 CrossRef CAS PubMed; (b) G. Zhou, D.-W. Wang, F. Li, P.-X. Hou, L. Yin, C. Liu, G. Q. Lu, I. R. Gentle and H.-M. Cheng, Energy Environ. Sci., 2012, 5, 8901–8906 RSC.
- S. E. Cheon, K. S. Ko, J. H. Cho, S. W. Kim, E. Y. Chin and H. T. Kim, J. Electrochem. Soc., 2003, 150, A800–A805 CrossRef CAS.
- X. L. Ji, K. T. Lee and L. F. Nazar, Nat. Mater., 2009, 8, 500–506 CrossRef CAS PubMed.
- (a) M. Vijayakumar, N. Govind, E. Walter, S. D. Burton, A. Shukla, A. Devaraj, J. Xiao, J. Liu, C. Wang, A. Karim and S. Thevuthasan, Phys. Chem. Chem. Phys., 2014, 16, 10923–10932 RSC; (b) G.-C. Li, G.-R. Li, S.-H. Ye and X.-P. Gao, Adv. Energy Mater., 2012, 2, 1238–1245 CrossRef CAS.
- (a) C. F. Zhang, H. B. Wu, C. Z. Yuan, Z. P. Guo and X. W. Lou, Angew. Chem., Int. Ed., 2012, 51, 9592–9595 CrossRef CAS PubMed; (b) K. Zhang, Q. Zhao, Z. L. Tao and J. Chen, Nano Res., 2013, 6, 38–46 CrossRef CAS; (c) J. Xu, J. Shui, J. Wang, M. Wang, H.-K. Liu, S. X. Dou, I.-Y. Jeon, J.-M. Seo, J.-B. Baek and L. Dai, ACS Nano, 2014, 8, 10920–10930 CrossRef CAS PubMed.
- (a) X. M. He, W. H. Pu, J. G. Ren, L. Wang, J. L. Wang, C. Y. Jiang and C. R. Wan, Electrochim. Acta, 2007, 52, 7372–7376 CrossRef CAS; (b) W. Li, Q. Zhang, G. Zheng, Z. W. Seh, H. Yao and Y. Cui, Nano Lett., 2013, 13, 5534–5540 CrossRef CAS PubMed; (c) L. Xiao, Y. Cao, J. Xiao, B. Schwenzer, M. H. Engelhard, L. V. Saraf, Z. Nie, G. J. Exarhos and J. Liu, Adv. Mater., 2012, 24, 1176–1181 CrossRef CAS PubMed.
- (a) Z. W. Seh, W. Y. Li, J. J. Cha, G. Y. Zheng, Y. Yang, M. T. McDowell, P. C. Hsu and Y. Cui, Nat. Commun., 2013, 4, 1331 CrossRef PubMed; (b) Q. Pang, D. Kundu, M. Cuisinier and L. F. Nazar, Nat. Commun., 2014, 5, 4759 CrossRef CAS PubMed.
- (a) Y. S. Su and A. Manthiram, Nat. Commun., 2012, 3, 1166 CrossRef PubMed; (b) M. R. Kaiser, J. Wang, X. Liang, H.-K. Liu and S.-X. Dou, J. Power Sources, 2015, 279, 231–237 CrossRef CAS.
- M. R. Cuervo, E. Asedegbega-Nieto, E. Diaz, S. Ordonez, A. Vega, A. B. Dongil and I. Rodriguez-Ramos, Carbon, 2008, 46, 2096–2106 CrossRef CAS.
- M. Phaner-Goutorbe, A. Sartre and L. Porte, Microsc., Microanal., Microstruct., 1994, 5, 283–290 CrossRef CAS.
- J. L. Hueso, J. P. Espinos, A. Caballero, J. Cotrino and A. R. Gonzalez-Elipe, Carbon, 2007, 45, 89–96 CrossRef CAS.
- L. Wang, Z. H. Dong, D. Wang, F. X. Zhang and J. Jin, Nano Lett., 2013, 13, 6244–6250 CrossRef CAS PubMed.
- X. F. Wang, Z. X. Wang and L. Q. Chen, J. Power Sources, 2013, 242, 65–69 CrossRef CAS.
- J. Wang, J. Yang, C. Wan, K. Du, J. Xie and N. Xu, Adv. Funct. Mater., 2003, 13, 487–492 CrossRef CAS.
- E. Sosa, R. Cabrera-Sierra, M. T. Oropeza, F. Hernandez, N. Casillas, R. Tremont, C. Cabrera and I. Gonzalez, J. Electrochem. Soc., 2003, 150, B530–B535 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |