Preparation of glycerol monostearate from glycerol carbonate and stearic acid
Abstract
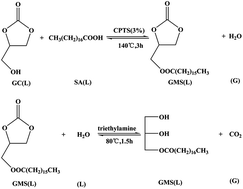
The chemical equilibrium for the preparation of glycerol monostearate (GMS) from glycerol carbonate (GC) and stearic acid (SA) was investigated. The chemical equilibrium constant K of the base-catalyzed synthesis of GMS from GC and SA was much smaller than that of the acid-catalyzed synthesis of (2-oxo-1,3-dioxolan-4-yl) methyl stearate (ODOMS) from GC and SA. In other words, it was thermodynamically difficult to obtain GMS with a high yield from GC and SA catalysed by basic catalysts. To prove this argument, we used magnesium oxide (MgO) as a catalyst to synthesize GMS from GC and SA. As expected, the yield of GMS was quite low. To increase the yield of GMS, a two-step procedure was proposed. First, pure ODOMS was synthesized by the esterification of GC with SA using copper p-toluenesulfonate (CPTS) as the catalyst. The conversion of SA reached 96.14% under the following conditions: reaction temperature, 140 °C; catalyst amount, 3% CPTS (based on the SA weight); reaction time, 3 h; GC-to-SA molar ratio, 1.5 : 1. Second, GMS was produced at a yield of 64.4% by the hydrolysis of ODOMS in the presence of triethylamine. The syntheses of ODOMS and GMS were confirmed by 1H- and 13C-NMR, FTIR and LC-MS analysis.

 Please wait while we load your content...
Please wait while we load your content...