Facile synthesis of NiCoMnO4 nanoparticles as novel electrode materials for high-performance asymmetric energy storage devices†
Afshin Pendashteh*a,
Jesus Palmaa,
Marc Andersonab and
Rebeca Marcillaa
aElectrochemical Processes Unit, IMDEA Energy Institute, Parque Tecnológico de Móstoles, Avda. Ramón de la Sagra, 3, 28935 Móstoles, Madrid, Spain. E-mail: afshin.pendashteh@imdea.org
bDepartment of Civil and Environmental Engineering, University of Wisconsin, Madison, USA
First published on 14th March 2016
Abstract
In attempt to reduce the amount of toxic Co in cobalt oxides, NiCoMnO4 nanoparticles were synthesized via a facile hydrothermal route and their electrochemical properties have been investigated as a novel electrode material for supercapacitors. Samples have been characterized using X-ray diffraction (XRD), energy dispersive X-ray analysis (EDAX), and X-ray photoelectron spectroscopy (XPS). N2 adsorption/desorption measurements demonstrated a high specific surface area of 175 m2 g−1. The morphology of the samples has been probed with scanning (SEM) and transmission (TEM) electron microscopy. The electrochemical properties of the NiCoMnO4 nanoparticles have been evaluated as electrode materials for energy storage application by means of various electrochemical techniques including cyclic voltammetry, galvanostatic charge–discharge measurements, and electrochemical impedance spectroscopy. According to the obtained results, the NiCoMnO4 electrodes showed significant improved capacitive performance in comparison with pure oxides (e.g. NiO, Co3O4, and Mn3O4). The NiCoMnO4 electrodes with a high mass loading of ∼10 mg cm−2 exhibited a high specific capacitance of 510 F g−1 at 1 A g−1, and a desirable rate capability (retaining 285 F g−1 at 10 A g−1). Finally, asymmetric devices based on NiCoMnO4 nanoparticles as positive electrodes and reduced graphene oxide nanosheets as negative electrodes were assembled, showing a remarkable performance including high energy (20 W h kg−1), and stunning power density (37.5 kW kg−1).
1. Introduction
Rapidly growing energy demands in our modern life have inspired a tremendous amount of scientific and industrial efforts to introduce and develop new electrode materials and technologies capable of fulfilling both high energy and high power requirements. Standing at the opposite ends of the electrochemical energy storage spectrum, batteries store charge based on faradaic redox reactions providing high energies while supercapacitors work by charge accumulation in the electrochemical double layer (EDLC) which yields inherently high power.1,2 However, differences between batteries and supercapacitors are becoming blurred by the appearance of hybrid/asymmetric devices, merging both energy storage mechanisms.3 As opposed to simple transition metal oxides (TMOs), mixed transition metal oxides (MTMOs) are placed at the summit of high-energy materials due to improved electrical and electrochemical behavior.4–6 Different varieties of MTMOs have been investigated and have shown impressive capacitances.7,8 Among the many transition metal oxides, cobalt oxides have attracted great attention, suggesting this family as very promising electrode materials in Li-ion batteries9 and excellent active materials for supercapacitors.10,11 Hence, considerable efforts have been made to partially substitute Co with less expensive and more benign elements to form the spinel structures with improved or comparable performance.12,13 For instance, NiCo2O4 and MnCo2O4 have been extensively investigated, demonstrating extremely high capacitances due to synergistic effect of different cations present in lattice structure.4,7,14–18 Very recently, we showed that substituting Co with common, abundant cations like Fe or Cu improves the electrochemical performance in comparison with pure Co3O4 synthesized in similar conditions.19,20 This is while that substituting Co with different cations not only reduces Co content of the final spinel, but also may enhance electrical and/or electrochemical properties. However, although cobaltites with more than one transition metal cation have been synthesised and applied in other fields, those which were tried so far in supercapacitor applications were only substituted with one transition metal cation. As an interesting example, spinel NiCoMnO4 includes three cations where all three are considered high capacitance materials in their simple oxide analogues.21,22 This particular spinel is known as a precursor for the synthesis of LiNi1/3Co1/3Mn1/3O2 as a cathode in Li-ion batteries.23 Furthermore, it has been already shown to be an efficient bifunctional catalyst for ORR/OER reactions and for CO oxidation as well.24 However, to the best of our knowledge, the electrochemical properties of this material have never been examined in alkaline asymmetric energy storage devices. Moreover, most of the previously studied systems were usually employing very small mass loadings (<1 mg cm−2), resulting in huge gravimetrical capacitances. This is while the performance of the electrode material is highly dependent on the mass loading and significantly decreases in highly loaded electrodes.25–27 Therefore, it is greatly important to introduce and develop high performance materials which can retain their properties under reasonable mass loading conditions (e.g. commercial scale of 8–10 mg cm−2).1,25–30Hydrothermal method, as a high-scale and very facile synthesis, has been extensively utilized to synthesize simple and binary metal oxides.31–33 Herein, this facile method was employed for the first time to synthesize the ternary transition metal oxide, NiCoMnO4 nanoparticles (NCMO NPs), without using any surfactants or templates. Electrochemical properties of NCMO NPs were studied as positive electrode materials in 3 M KOH solution using different electrochemical techniques. In order to assess the viability of NCMO NPs, electrodes with high mass-loading of NCMO NPs were prepared and used in asymmetric devices by their integration with highly conducting reduced graphene oxide (RGO) negative electrodes. Electrochemical performance of the asymmetric devices was evaluated and was compared to symmetric RGO-based supercapacitors, revealing excellent competence of the hybrid devices.
2. Experimental
2.1. Synthesis of NiCoMnO4 nanoparticles
NiCoMnO4 nanoparticles were synthesized via a facile hydrothermal route followed by thermal treatment (Scheme 1). Typically, equimolar amounts of NiCl2·6H2O, Mn(NO3)2·4H2O, and Co(NO3)2·6H2O were dissolved in a mixture of 20 ml of Milli-Q water and 20 ml of dimethylformamide (DMF) and stirred for 60 min to obtain a transparent solution. Afterwards, a 5% ammonium solution (NH4OH) was added to the mixture up to pH ≈ 9. The resultant dark green solution was kept stirring for a further 30 min. Next, the solution was transferred into a 200 ml Teflon-lined stainless steel autoclave reactor where the hydrothermal reaction took place at 120 °C for 2 h. When the reactor cooled to room temperature (RT), the resulting precipitate was vacuum filtered, rinsed thoroughly by using a water/ethanol mixture, and dried at 60 °C. Finally, the precipitate was calcined in an electrical furnace at 300 °C (rate of 2° min−1) for 2 h. For comparison, pure oxides (e.g. NiO, Co3O4, and Mn3O4) were also synthesized via the same route while only one of the metal salts was used as the precursor.2.2. Synthesis of reduced graphene oxide nanosheets (RGO NSs)
Graphene oxide (GO) was synthesized via modified Hummers method.34 In a typical synthesis, 8 g K2S2O8 and 8 g P2O5 were added to 24 ml of concentrated H2SO4 solution. Using a silicon oil bath, the solution was heated to 80 °C and 4 g graphite powder was gradually added followed by stirring for 6 h. After cooling to RT, the mixture was diluted by 300 ml Milli-Q water and then filtered and dried overnight at 60 °C. A 2 g quantity of the as-prepared powder (preoxidized graphite) was added to 92 ml concentrated H2SO4 solution, cooled in an ice bath. 12 g KMnO4 was gradually added under vigorous stirring. After 15 min, 2 g NaNO3 was added and the mixture was stirred at 35 °C for 2 h. Then 560 ml Milli-Q water and 10 ml H2O2 30% solution were introduced turning the solution to a bright yellow. The sample was filtered, rinsed several times, and dried at 100 °C. Next, it was dispersed again in Milli-Q water and the brown dispersion was dialyzed for at least 1 day to remove residual metal ions and acids. After vacuum filtration, the GO gel was dried at 60 °C.In order to reduce the GO sample, 100 mg of as-prepared sample was re-dispersed in 150 ml Milli-Q water and 70 μl of a hydrazine hydrate solution (50–60%) was added. The solution was then transferred to an oil bath where it was stirred for 6 h at 80 °C. The final sample was rinsed thoroughly with water and dried overnight at 60 °C.
2.3. Microstructural analysis
The prepared samples were structurally characterized using X-ray diffraction (XRD, PANalytical Empyream diffractometer utilizing Cu Kα radiation) at a generator voltage of 45 kV and an emission current of 40 mA. The crystalline size of the particles was calculated by Debye–Scherer equation:
d = Kλ/(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) θ)
| (1) |
Furthermore, energy dispersive spectroscopy (EDAX) was used to confirm the elemental composition of the sample. Nova Nano SEM230 FEG scanning electron microscopes (SEM) and FEI Tecnai 20 transmission electron microscope (TEM) with a BaF6 filament at an accelerating voltage of 200 kV were used to investigate morphological properties of the samples. X-ray photoelectron spectroscopy (XPS) was conducted to analyze the surface of the NiCoMnO4 samples by means of a monochromatic Al Kα radiation with a voltage of 12 kV, a current of 6 mA, and energy resolution of 0.1 eV for high resolution spectra. N2 adsorption–desorption measurements were conducted using a Quadrasorb instrument preceded by a thermal pretreatment of samples at 250 °C for 10 h.
2.4. Electrochemical characterizations
The electrochemical properties of the samples were investigated in a three-electrode configuration employing a 3 M KOH solution as the electrolyte. Working electrodes were fabricated by applying a paste containing the active material, carbon black, and PTFE with a weight ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, on cleaned pieces of nickel foam as the current collector. The mass loading of the samples ranged from 4.75 to 10.6 mg cm−2. A platinum mesh and a Hg/HgO (1 M NaOH, ALS Co., Ltd Japan) electrode were used as the counter and reference electrodes, respectively. The electrochemical behavior of the samples was evaluated on a Bio-Logic Science Instrument (VMP3) through cyclic voltammetry (CV), galvanostatic charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS) measurements. EIS measurements were conducted by applying an AC signal with amplitude of 10 mV in a frequency range from 200 kHz to 0.1 Hz.
1, on cleaned pieces of nickel foam as the current collector. The mass loading of the samples ranged from 4.75 to 10.6 mg cm−2. A platinum mesh and a Hg/HgO (1 M NaOH, ALS Co., Ltd Japan) electrode were used as the counter and reference electrodes, respectively. The electrochemical behavior of the samples was evaluated on a Bio-Logic Science Instrument (VMP3) through cyclic voltammetry (CV), galvanostatic charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS) measurements. EIS measurements were conducted by applying an AC signal with amplitude of 10 mV in a frequency range from 200 kHz to 0.1 Hz.
Specific capacitance of the samples was estimated form CV curves based on the charge stored during anodic and cathodic scans using the following equation:
 | (2) |
 | (3) |
2.5. Assembly of hybrid energy storage devices
Asymmetric hybrid devices were assembled by integrating a battery-like NiCoMnO4 electrode and a supercapacitor-like RGO electrode as the positive and negative electrodes respectively in 2-electrode Swagelok cells. A cellulosic filter paper was used as separator between two electrodes and was thoroughly soaked in 3 M KOH solution before measurements. Based on charge balance theory (Q+ = Q−), the mass ratio between positive and negative electrodes can be obtained as following:35
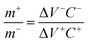 | (4) |
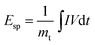 | (5) |
 | (6) |
3. Results and discussion
3.1. Microstructural and compositional analysis
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m and lattice parameters of a = b = c = 0.8145 nm (JCPDS 98-009-8472), in agreement with previous reports.23,37 Moreover, broad diffracted peaks in this sample suggest nanoscale crystalline domains according to Scherrer theory (c.a. 14 nm obtained for particle sizes). EDX results (Fig. S1†) further demonstrate the purity of the mixed metal oxide. N2 adsorption–desorption experiments were conducted for exploring textural properties of the NCMO NPs. As shown in Fig. 1b, the adsorption branch ascends continuously without leveling off, revealing interparticle adsorption. A small hysteresis (H3 type) appeared at high P/P0, typically seen in type-IV isotherms.38 The presence of such hysteresis suggests capillary condensation in mesopores (pore diameter of 2–50 nm).39 Accordingly, a high specific surface area of SBJH = 175 m2 g−1 and a total pore volume (Vtot) of 0.69 cm3 g−1 was achieved. Moreover, pore size distribution was estimated by BJH analysis (Fig. S2†), confirming the presence of mesopores mostly centred at ∼15 nm.
m and lattice parameters of a = b = c = 0.8145 nm (JCPDS 98-009-8472), in agreement with previous reports.23,37 Moreover, broad diffracted peaks in this sample suggest nanoscale crystalline domains according to Scherrer theory (c.a. 14 nm obtained for particle sizes). EDX results (Fig. S1†) further demonstrate the purity of the mixed metal oxide. N2 adsorption–desorption experiments were conducted for exploring textural properties of the NCMO NPs. As shown in Fig. 1b, the adsorption branch ascends continuously without leveling off, revealing interparticle adsorption. A small hysteresis (H3 type) appeared at high P/P0, typically seen in type-IV isotherms.38 The presence of such hysteresis suggests capillary condensation in mesopores (pore diameter of 2–50 nm).39 Accordingly, a high specific surface area of SBJH = 175 m2 g−1 and a total pore volume (Vtot) of 0.69 cm3 g−1 was achieved. Moreover, pore size distribution was estimated by BJH analysis (Fig. S2†), confirming the presence of mesopores mostly centred at ∼15 nm.
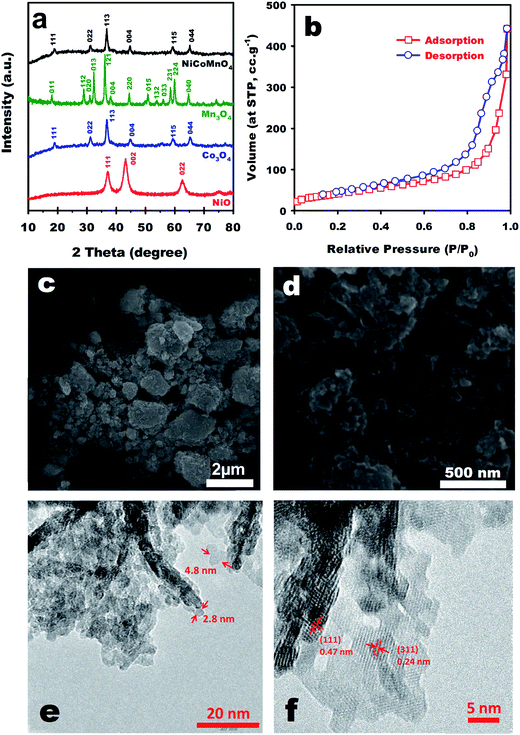 | ||
| Fig. 1 Microstructural analysis of the samples: (a) XRD patterns of NiO, Co3O4, Mn3O4, and NiCoMnO4; (b) N2 ad-/de-sorption isotherms; (c, d) SEM, and (e, f) TEM images of NCMO NPs. | ||
Morphology of the NCMO sample was explored by Scanning Electron Microscopy (SEM) and results were shown in Fig. 1c and d. As can be seen, the sample is almost uniform, comprising of particles with irregular shape (Fig. 1c). Amplified to higher magnification, in Fig. 1d, one can see that the large particles are themselves comprised of smaller nanoparticles agglomerated forming larger particles having different sizes in a cauliflower-like shape. Transmission Electron Microscopy (TEM) was employed to shed more light on morphological properties of the samples. Transmitting electron beam through NCMO specimen (Fig. 1e) shows very fine NPs in the range of 2–5 nm (shown by red arrows). These particles are connected to each other, forming a mesoporous structure in agreement with already observed textural properties. A high resolution TEM image of the NCMO NPs (Fig. 1f) reveals that the nanoparticles are composed of nanocrystalline domains of the spinel cobaltite. As it is seen, lattice fringes with d-spacing of 0.47 and 0.24 nm could be observed, corresponding to the (111) and (311) crystallographic planes, respectively.
The surface elemental composition and chemical state of atoms in the mixed transition metal oxide were probed by X-ray Photoelectron Spectroscopy (XPS). High-resolution Ni 2p spectrum (Fig. 2a) includes two main peaks centred at binding energies (B.E.) of 855.98 and 873.58 eV, assigned to Ni 2p3/2 and Ni 2p1/2, respectively. Two satellites (S1 and S2) also appear at 861.48 and 880.49 eV, indicating the presence of Ni2+.40 By scrutinizing the core region for Co 2p, one might note the presence of Co2+ and Co3+ species based on appearance of Co 2p3/2 at 780.18 eV and Co 2p1/2 at 795.58 eV (Fig. 2b).19 Fig. 2c shows the high-resolution spectra for Mn 2p. Mn 2p3/2 and Mn 2p1/2 emerged in 642.58 and 653.48 eV, unrevealing the existence of Mn3+ and Mn4+ in the spinel structure.37 O 1s spectrum (Fig. 2d) represents two peaks centred at 530.16 eV corresponding to M–O (M = Ni, Co, and Mn) and at 531.8 eV for –OH groups.37 Hence, these XPS results disclose the mixed-valence nature of the NCMO sample which could be beneficial in energy storage applications.
TEM analysis has been also used to explore the morphological properties of graphene layers. As can be seen, TEM image of RGO NSs (Fig. S3c†) depicts expected planar flaky forms of thin graphene layers wrinkled in some parts.
3.2. Electrochemical evaluation of the samples
The electrochemical behavior of samples was first evaluated in a three-electrode configuration using 3 M KOH as the electrolyte. The working electrodes were fabricated by active materials applied on Ni foam with high mass loadings of 4.75 to 10.6 mg cm−2. Cyclic voltammograms of NiO, Co3O4, Mn3O4, and NiCoMnO4 samples at a scan rate of 5 mV s−1 are shown in Fig. 3. In extending the potential range to a positive 600 mV, it can be clearly seen that all samples display a battery-like signature with strong redox peaks arising from faradaic reactions.43–45 Specific capacitances (Csp) of electrode materials were calculated based on the charge stored during forward and backward scans. Accordingly, by integrating the area under the curves, one obtains a high Csp of 425 F g−1 at 5 mV s−1 for NCMO NPs. This is significantly higher than that of obtained for pure oxides (e.g. 214, 173, and 105 F g−1 for NiO, Co3O4, and Mn3O4 samples, respectively). This improvement clearly shows the importance of the ternary mixed oxides in comparison with those of their single oxide analogues, possibly due to the synergistic effect of the combined structure of multi-cations in the lattice. | ||
| Fig. 3 Electrochemical properties of the samples: cyclic voltammograms of different samples at a scan rate of 5 mV s−1 in 3 M KOH solution. | ||
The shape of the CVs for pure oxides are in agreement with previously reported studies, suggesting that Co2+/Co3+ and Co3+/Co4+ species are involved in charge storage in Co3O4, while Ni2+/Ni3+ and Mn3+/Mn4+ redox couples are possibly transformed to each other during charge and discharge in NiO and Mn3O4 samples, respectively.21,46,47 Clarifying the exact redox mechanism in MTMOs is difficult due to complex structure and different possible phases in alkaline solutions. However, based on the obtained results from XPS analysis and previous reports on similar binary MOs,43,45 following mechanism can be proposed for faradaic reactions, responsible for charge storage in NCMO NPs:
| Ni2+(Co2+,Co3+)(Mn3+,Mn4+)O4 + H2O + OH− ↔ NiOOH + CoOOH + MnOOH + e− |
| MnOOH + OH− ↔ MnO2 + H2O + e− |
| CoOOH + OH− ↔ CoO2 + H2O + e− |
Cyclic voltammograms of NCMO NPs and pure oxides under various scan rates are shown in Fig. 4a and Fig. S4a–c,† respectively. Accordingly, specific capacitance values of the samples were plotted against scan rates, shown in Fig. S4d.† As seen, capacitance values decrease by increasing the scan rate in all samples. Capacitance fading can be attributed to kinetic polarization at higher scan rates, commonly seen in materials having a faradaic charge storage mechanism.48 Accordingly, Csp of NCMO NPs drops from 425 to 175 F g−1 (41% retention) as the scan rate undergoes a 20-fold increase. It is clear that the capacitance of NCMO NPs is much higher than those of single oxides at all measured scan rates.
Unlike the oxides, CVs of RGO NSs (Fig. 4b) are quite close to the rectangular shape which is characteristic of ideal electric double layer capacitors (EDLCs). More importantly, this semi-rectangular shape of the CV is well preserved by increasing the potential scan rate, demonstrating high electrical conductivity of the sample.49 This is in agreement with already obtained results in XRD and Raman which have suggested the significant removal of oxygen-containing functional groups.
According to calculations based on CV, it is been found that RGO NSs can deliver a capacitance of 145 F g−1 at 5 mV s−1 in negative potentials, which is a high capacitance for carbonaceous materials. Fig. 4c displays specific capacitance values of RGO NSs in comparison with NCMO NPs at various scan rates. The evolution of Csp corresponding to different scan rates in RGO sample reveals excellent capacitance retention even after increasing the scan rate by a factor of 20 (70% retention). This can be understood from EDLC-based charge storage in RGO NSs and their high electric conductivity as well. Galvanostatic charge–discharge (GCD) measurements were performed at various specific currents (Fig. 4d and e) to monitor the energy storage behavior of NCMO NPs as positive and RGO NSs as negative electrodes. As seen in Fig. 4d, NCMO NPs have a non-triangular profile, indicating the faradaic nature of reaction as already understood from cyclic voltammetry experiments. Moreover, the voltage plateaus are exactly in accordance with the redox peaks in CV curves (Fig. 4a). High columbic efficiency of the sample can be also realized by almost symmetrical counterparts in charge–discharge profiles. Using discharge profiles, NCMO NPs showed a high specific capacitance of 510 F g−1 at a current density of 1 A g−1. Rate capability of the samples is also shown in Fig. 4f, revealing around 56% capacitance retention at 10 A g−1 for NCMO NPs. In contrary, the nearly linear GCD profile of RGO NSs (Fig. 4e) portrays an explicit difference in charge storing mechanism, typical for EDLC-based electrodes. A specific capacitance value of 145 F g−1 at 1 A g−1 was obtained. This decreases to 80 F g−1 at 10 A g−1 (Fig. 4f).
To further illuminate the reasons of excellent electrochemical behavior of the NCMO NPs, Electrochemical Impedance Spectroscopy (EIS) measurements were conducted. Nyquist plot of the sample is depicted in Fig. S5.† In the high frequencies, the intercept of the curve with the real axis is considered as equivalent series resistance (ESR), a combination of the electrolyte resistance, intrinsic resistance of the active material, and the interfacial contact resistances. Accordingly, ESR value for NCMO electrode is as small as 0.944 Ω, revealing excellent electrical conductivity of the sample. The semicircle at high/medium frequency ranges can be attributed to charge transfer resistance (Rct) of the faradaic reactions and its small diameter demonstrates rapid nature of the reaction kinetics in NCMO NPs. Moreover, in low frequency region, linear part corresponds to diffusion-controlled movement of ions which is close to ideal vertical line and reveals excellent electrochemical properties of NCMO NPs for energy storage applications.20 All these indicate the viability of NCMO NPs as a promising electrode material with small impedance.
We also compared the obtained results for NCMO NPs with some corresponding previous reports, listed in Table 1, demonstrating the promising properties of the NCMO NPs as electrode materials for energy storage. As can be seen from the table and to our best knowledge, the energy storage performance of the NiCoMnO4 as a ternary metal oxide has been investigated in alkaline solutions for the first time in the present work. Moreover, NCMO NPs showed superior or comparable specific capacitance value in comparison with previously reported simple or mixed binary cobalt oxides. All of these results suggest that the synthesized NCMO NPs can promisingly serve as positive electrode materials while RGO NSs can be employed as an ideal negative electrode in hybrid energy storage applications.
| Sample | Synthesis route | Electrolyte | Csp (F g−1) | Potential window | Discharge regime | Ref. |
|---|---|---|---|---|---|---|
| MnO2@CCNs | Pyrolysis and hydrothermal | 1 M Na2SO4 | 262 | 0.9 | 0.2 A g−1 | 51 |
| Co3O4/RGO | Hydrothermal | 1 M KOH | 445 | 0.55 | 0.5 A g−1 | 52 |
| NiCo2O4 NS | Solvothermal | 2 M KOH | 578 | 0.5 | 1 A g−1 | 53 |
| MnCo2O4 NS | Electrodeposition | 1 M NaOH | 400 | 1.0 | 1 A g−1 | 12 |
| MnCo2O4 NS | Hydrothermal | 6 M KOH | 410 | 0.5 | 1 A g−1 | 54 |
| MnCo2O4 nanostructures | Sol–gel | 2 M KOH | 405 | 0.4 | 0.62 A g−1 | 55 |
| Ordered porous CuCo2O4 | SBA-15 template | 6 M KOH | 1210 | 0.5 | 2 A g−1 | 6 |
| Ultrathin mesoporous NiCo2O4 | Electrodeposition | 3 M KOH | 2010 | 0.4 | 2 A g−1 | 4 |
| Co0.72Ni0.28LDH | Electrodeposition | 1 M KOH | 2104 | 0.6 | 1 A g−1 | 26 |
| NiCo-LDH@CNT/NF | CVD and electrodeposition | 1 M KOH | 2046 | 0.5 | 1 A g−1 | 28 |
| MnO2/CFP | Hydrothermal | 1 M Na2SO4 | 251 | 1.0 | 1 A g−1 | 56 |
| Co3O4 nanoplate arrays | Electrodeposition | 1 M KOH | 146 | 0.5 | 1 A g−1 | 57 |
| NiCoMnO4 NPs | Hydrothermal | 3 M KOH | 510 | 0.55 | 1 A g−1 | This study |
| 425 | 5 mV s−1 |
3.3. Performance of NCMO NPs//RGO NSs asymmetric devices
To take advantage of both high-energy faradaic reaction and high-power EDLC phenomenon, two different charge storage fashions were integrated in hybrid NCMO NPs//RGO NSs devices. These positive and negative electrodes were gravimetrically balanced (m+/m− = 0.56) according to charge balance theory to reassure that we have achieved the maximum potential window. According to the CV signature of these electrodes in our three-electrode configuration (Fig. S6†), by integrating the two electrodes with different working potential windows, one can expand the operating voltage window to 1.5 V (above the thermodynamic decomposition voltage of water, i.e. >1.2 V).50 This is much higher than the available operating voltage in a symmetric RGO NSs//RGO NSs (e.g. 1 V, Fig. S7†). Fig. 5a shows CV curves of hybrid NCMO NPs//RGO NSs device at different voltage windows at 50 mV s−1 in 3 M KOH solution. As can be seen, EDLC plays a dominant role in storing the electric charge up to 800 mV. Beyond this, faradaic reactions take charge of the process and appearance of redox peaks lead to a significant deviation from the ideal rectangular shape typically seen in EDLC symmetric devices (e.g. RGO//RGO in S7a). Conducting CV measurements at various scan rates on the hybrid cell (Fig. 5b) reveals insignificant change in shape of the curve by increasing the sweep rate, demonstrating excellent rate capability and reversibility of this energy storage device.Rate capability of the asymmetric NCMO NPs//RGO NSs and symmetric RGO NSs//RGO NSs is shown in Fig. 5c. A high specific capacitance value (based on the mass of both electrodes, c.a. 14.9 mg cm−2) of 45 F g−1 was achieved at a scan rate of 5 mV s−1. This value is 1.7 times greater than the value obtained for a symmetric RGO NSs//RGO NSs cell (26 F g−1). This is due to embedding NCMO NPs as the positive electrode in the cell which provides high-capacitance as well as fast faradaic reactions. As can be seen, the asymmetric cell could retain 75% of its initial capacitance at a high scan rate of 100 mV s−1, and the capacitance was still much higher than the symmetric cell (34 F g−1 against 17 F g−1). Fig. 5d displays GCD profile of the asymmetric NCMO NPs//RGO NSs cell at a current density of 0.5 A g−1. Nearly identical wings in charge–discharge counterparts demonstrate the excellent reversibility and columbic efficiency of the cell. The charge–discharge wings showed a slightly deviated linear shape where the slope-changing points are in accordance with the observed peaks in CV curves (Fig. 5a). Furthermore, the behavior of individual electrodes in the full cell was monitored via a Hg/HgO reference electrode placed in a T-shape Swagelock® cell to insure that the charge of electrodes are well balanced by adjusting the mass of electrodes; the signals for positive and negative electrodes are shown in Fig. 5d. As can be seen, each electrode behaved in almost the same way as it performed in the three-electrode system. This confirms that mass of electrodes are well balanced with each electrode performing in its corresponding potential region. Clearly, the overall profile is a combination of the two charge storage mechanisms.
A Ragone plot is considered as a common tool for comparing the performance of energy storage devices. The corresponding Ragone chart of our hybrid NCMO NPs//RGO NSs device is displayed in Fig. 6a. (according to GCD profiles at Fig. S8†). As can be seen, the hybrid cell could attain a high specific energy of c.a. 20 W h kg−1 at a specific power of 0.377 kW kg−1 at a current density of 0.5 A g−1. This is more than 6 times greater than the maximum energy of the RGO//RGO cell, originating from significantly increased capacitance and operating voltage by embedding the novel NCMO electrodes in the positive terminal. By increasing the current load to 50 A g−1 (∼385 mA cm−2), an impressively high specific power of 37.5 kW kg−1 was achieved at 3.68 W h kg−1; this is much higher (15 times greater) than the RGO//RGO cell for the same energy density. These results demonstrate that by enclosing the NCMO electrode in the cell one not only increases available energy but also enhances significantly the attainable power.
The capability of providing a prolonged charge is an important factor in energy storage devices. On that account, we inspected capacitance retention of our devices for 2000 cycles at a current density of 2 A g−1 and the outcome is shown in Fig. 6b. Accordingly, a capacitance retention of around 87% was obtained after 2000 cycles, showing the competence of the hybrid device. Moreover, no significant change was realized in the shape of profiles as shown in first three and last cycles (inset of Fig. 6b), revealing good reversibility of the system during consecutive long cycles. Table 2 summarizes a comparison of the performance of NCMO NPs as an electrode material in an asymmetric NCMO NPs//RGO NSs device with some recent corresponding reports for similar systems, showing superior or comparable characteristics of the assembled devices. Moreover, Fig. 6c provides an indication regarding the viability of NCMO NPs//RGO NSs for practical applications. Here, blue and red LEDs (20 mA) were powered by connecting 3 cells in series for around 10 min (see video file in ESI†).
| Sample | Electrolyte | Csp (F g−1) | Potential window (V) | Max. specific energy (W h kg−1) | Max. specific power (kW kg−1) | Ref. |
|---|---|---|---|---|---|---|
| MnO2//graphene | 1 M Na2SO4 | 37 (5 mA cm−2) | 2 | 25.2 | 2.5 | 58 |
| Ordered porous CuO//AC | 6 M KOH | 72.4 (1 A g−1) | 1.4 | 19.7 | 7 | 50 |
| MnO2//3D graphene | 0.5 M Na2SO4 | 30 (1.5 mA cm−2) | 1 | 6.8 | 2.5 | 59 |
| NiCo2S4//AC | 2 M KOH | ∼80 (2 A g−1) | 1.5 | 24.7 | 17.2 | 60 |
| CoO/Co3O4//AC | 3 M KOH | 38.6 (0.2 A g−1) | 1.4 | 10.5 | 4.1 | 61 |
| NiCoDH//AC | 2 M KOH | N/A | 1.5 | 17.5 | ∼10 | 62 |
| NiCoMnO4 NPs//RGO NSs | 3 M KOH | 66 (0.5 A g−1) | 1.5 | 20 | 37.5 | This study |
| 45 (5 mV s−1) |
All of these results suggest NiCoMnO4 NPs as an impressive positive electrode material for aqueous asymmetric devices. Accordingly, combination of NCMO NPs as a novel high capacitance electrode material with a highly conductive RGO NSs which assure high-power, leads to a high-performance promising hybrid device. More importantly, facile synthesis routes and high mass loadings employed during this study ensure suitable applicability of the electrode materials in commercial devices. Despite the competence of NCMO NPs as a novel promising positive electrode material, it should be mentioned that there is obviously room to improve its performance; e.g. by utilization of ordered porous structures to ensure utilization of full capacitance of material or by taking advantage of synergistic effects in nanocomposite materials. Attempts for improving the merits of this material are currently being pursued.
4. Conclusion
In summary, NiCoMnO4 nanoparticles were synthesized via facile hydrothermal route and their electrochemical properties as electrode materials for energy storage application have been investigated in alkaline medium. The NiCoMnO4 nanoparticles showed excellent electrochemical properties with a high capacitance of 510 F g−1 at 1 A g−1, much higher than the pure oxide analogues (e.g. NiO, Co3O4, and Mn3O4) synthesized via the same route. NiCoMnO4 electrodes were integrated with RGO nanosheets in asymmetric devices, resulting a high energy density of 20 W h kg−1 and a maximum power density of 37.5 kW kg−1. Moreover, NCMO NPs//RGO NSs hybrid devices showed excellent rate capability at various current loads and cycling stability over 2000 cycles.Acknowledgements
Authors gratefully acknowledge financial support from the European Commission through the Marie-Curie AMAROUT II Fellowship program (A. P.), MINECO (former MICINN) through the Ramon y Cajal Program (RYC-2011-08093), ENE2012-31516, European Union structural funds and the Comunidad de Madrid MAD2D-CM Program (S2013/MIT-3007).References
- P. Simon, Y. Gogotsi and B. Dunn, Science, 2014, 343, 1210 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845 CrossRef CAS PubMed.
- V. Augustyn, P. Simon and B. Dunn, Energy Environ. Sci., 2014, 7, 1597 CAS.
- C. Yuan, J. Li, L. Hou, X. Zhang, L. Shen and X. W. Lou, Adv. Funct. Mater., 2012, 22, 4592 CrossRef CAS.
- G. Zhang and X. W. Lou, Adv. Mater., 2013, 25, 976 CrossRef CAS PubMed.
- A. Pendashteh, S. E. Moosavifard, M. S. Rahmanifar, Y. Wang, M. F. El-Kady, R. B. Kaner and M. F. Mousavi, Chem. Mater., 2015, 27, 3919 CrossRef CAS.
- Y. Zhu, Z. Wu, M. Jing, H. Hou, Y. Yang, Y. Zhang, X. Yang, W. Song, X. Jia and X. Ji, J. Mater. Chem. A, 2015, 3, 866 CAS.
- W. Kang, Y. Tang, W. Li, Z. Li, X. Yang, J. Xu and C.-S. Lee, Nanoscale, 2014, 6, 6551 RSC.
- N. Yan, L. Hu, Y. Li, Y. Wang, H. Zhong, X. Hu, X. Kong and Q. Chen, J. Phys. Chem. C, 2012, 116, 7227 CAS.
- Y. Wang, T. Zhou, K. Jiang, P. Da, Z. Peng, J. Tang, B. Kong, W.-B. Cai, Z. Yang and G. Zheng, Adv. Energy Mater., 2014, 4, 1400696 Search PubMed.
- C. Yuan, H. B. Wu, Y. Xie and X. W. Lou, Angew. Chem., Int. Ed., 2014, 53, 1488 CrossRef CAS PubMed.
- T. Nguyen, M. Boudard, L. Rapenne, O. Chaix-Pluchery, M. J. Carmezim and M. F. Montemor, RSC Adv., 2015, 5, 27844 RSC.
- B. Liu, B. Liu, Q. Wang, X. Wang, Q. Xiang, D. Chen and G. Shen, ACS Appl. Mater. Interfaces, 2013, 5, 10011 CAS.
- X. Liu, S. Shi, Q. Xiong, L. Li, Y. Zhang, H. Tang, C. Gu, X. Wang and J. Tu, ACS Appl. Mater. Interfaces, 2013, 5, 8790 CAS.
- K. Zhang, W. Zeng, G. Zhang, S. Hou, F. Wang, T. Wang and H. Duan, RSC Adv., 2015, 5, 69636 RSC.
- S. Khalid, C. Cao, A. Ahmad, L. Wang, M. Tanveer, I. Aslam, M. Tahir, F. Idrees and Y. Zhu, RSC Adv., 2015, 5, 33146 RSC.
- F. Li, G. Li, H. Chen, J. Q. Jia, F. Dong, Y. B. Hu, Z. G. Shang and Y. X. Zhang, J. Power Sources, 2015, 296, 86 CrossRef CAS.
- N. Padmanathan and S. Selladurai, Ionics, 2014, 20, 479 CrossRef CAS.
- A. Pendashteh, J. Palma, M. Anderson and R. Marcilla, J. Mater. Chem. A, 2015, 3, 16849 CAS.
- A. Pendashteh, M. S. Rahmanifar, R. B. Kaner and M. F. Mousavi, Chem. Commun., 2014, 50, 1972 RSC.
- S. K. Meher and G. R. Rao, J. Phys. Chem. C, 2011, 115, 15646 CAS.
- A. Paravannoor, S. Naira, P. Pattathil, M. Manca and A. Balakrishnan, Chem. Commun., 2015, 51, 6092 RSC.
- Y. Ren, Z. Ma and P. G. Bruce, J. Mater. Chem., 2012, 22, 15121 RSC.
- X. Yu and A. Manthiram, Catal. Sci. Technol., 2015, 5, 2072 CAS.
- Z. Tang, C.-h. Tang and H. Gong, Adv. Funct. Mater., 2012, 22, 1272 CrossRef CAS.
- V. Gupta, S. Gupta and N. Miura, J. Power Sources, 2008, 175, 680 CrossRef CAS.
- Z. Lu, Q. Yang, W. Zhu, Z. Chang, J. Liu, X. Sun, D. G. Evans and X. Duan, Nano Res., 2012, 5, 369 CrossRef CAS.
- X. Li, J. Shen, W. Sun, X. Hong, R. Wang, X. Zhao and X. Yan, J. Mater. Chem. A, 2015, 3, 13244 CAS.
- H. Chen, J. Jiang, L. Zhang, D. Xia, Y. Zhao, D. Guo, T. Qi and H. Wan, J. Power Sources, 2014, 254, 249 CrossRef CAS.
- C.-h. Tang, X. Yin and H. Gong, ACS Appl. Mater. Interfaces, 2013, 5, 10574 CAS.
- J. Wang, X. Zhang, Q. Wei, H. Lv, Y. Tian, Z. Tong, X. Liu, J. Hao, H. Qu, J. Zhao, Y. Li and L. Mai, Nano Energy, 2016, 19, 222 CrossRef CAS.
- S. Vijayakumar, S.-H. Lee and K.-S. Ryu, RSC Adv., 2015, 5, 91822 RSC.
- X. Ren, C. Guo, L. Xu, T. Li, L. Hou and Y. Wei, ACS Appl. Mater. Interfaces, 2015, 7, 19930 CAS.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- J. Zhang and X. S. Zhao, ChemSusChem, 2012, 5, 818 CrossRef CAS PubMed.
- P. Navalpotro, J. Palma, M. Anderson and R. Marcilla, J. Power Sources, 2016, 306, 711 CrossRef CAS.
- K. Li, X. Luo, X. Lin, F. Qi and P. Wu, J. Mol. Catal. A: Chem., 2014, 383–384, 1 CAS.
- A. S. Poyraz, W. A. Hines, C.-H. Kuo, N. Li, D. M. Perry and S. L. Suib, J. Appl. Phys., 2014, 115, 114309 CrossRef.
- G. Wang, H. Liu, J. Horvat, B. Wang, S. Qiao, J. Park and H. Ahn, Chem.–Eur. J., 2010, 16, 11020 CrossRef CAS PubMed.
- J. Ji, L. L. Zhang, H. Ji, Y. Li, X. Zhao, X. Bai, X. Fan, F. Zhang and R. S. Ruoff, ACS Nano, 2013, 7, 6237 CrossRef CAS PubMed.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806 CrossRef CAS PubMed.
- Z. Bo, X. Shuai, S. Mao, H. Yang, J. Qian, J. Chen, J. Yan and K. Cen, Sci. Rep., 2014, 4, 4684 Search PubMed.
- M. Yu, J. Chen, J. Liu, S. Li, Y. Ma, J. Zhang and J. An, Electrochim. Acta, 2015, 151, 99 CrossRef CAS.
- T. Brousse, D. Bélanger and J. W. Long, J. Electrochem. Soc., 2015, 162, A5185 CrossRef CAS.
- A. N. Naveen and S. Selladurai, RSC Adv., 2015, 5, 65139 RSC.
- D. Li, F. Meng, X. Yan, L. Yang, H. Heng and Y. Zhu, Nanoscale Res. Lett., 2013, 8, 1 CrossRef PubMed.
- K. Liang, X. Tang and W. Hu, J. Mater. Chem., 2012, 22, 11062 RSC.
- M. F. El-Kady and R. B. Kaner, Nat. Commun., 2013, 4, 1475 CrossRef PubMed.
- A. Pendashteh, M. F. Mousavi and M. S. Rahmanifar, Electrochim. Acta, 2013, 88, 347 CrossRef CAS.
- S. E. Moosavifard, M. F. El-Kady, M. S. Rahmanifar, R. B. Kaner and M. F. Mousavi, ACS Appl. Mater. Interfaces, 2015, 7, 4851 CAS.
- Y. Li, N. Yu, P. Yan, Y. Li, X. Zhou, S. Chen, G. Wang, T. Wei and Z. Fan, J. Power Sources, 2015, 300, 309 CrossRef CAS.
- Z. Song, Y. Zhang, W. Liu, S. Zhang, G. Liu, H. Chen and J. Qiu, Electrochim. Acta, 2013, 112, 120 CrossRef CAS.
- M. Kuang, Y. X. Zhang, T. T. Li, K. F. Li, S. M. Zhang, G. Li and W. Zhang, J. Power Sources, 2015, 283, 270 CrossRef CAS.
- L. Li, F. He, S. Gai, S. Zhang, P. Gao, M. Zhang, Y. Chen and P. Yang, CrystEngComm, 2014, 16, 9873 RSC.
- L.-B. Kong, C. Lu, M.-C. Liu, Y.-C. Luo, L. Kang, X. Li and F. C. Walsh, Electrochim. Acta, 2014, 115, 22 CrossRef CAS.
- X. Su, L. Yu, G. Cheng, H. Zhang, M. Sun and X. Zhang, Appl. Energy, 2015, 153, 94 CrossRef CAS.
- G. Nagaraju, Y. H. Ko and J. S. Yu, J. Power Sources, 2015, 283, 251 CrossRef CAS.
- J. Cao, Y. Wang, Y. Zhou, J.-H. Ouyang, D. Jia and L. Guo, J. Electroanal. Chem., 2013, 689, 201 CrossRef CAS.
- Y. He, W. Chen, X. Li, Z. Zhang, J. Fu, C. Zhao and E. Xie, ACS Nano, 2012, 7, 174 CrossRef PubMed.
- Y. Zhu, X. Ji, Z. Wu and Y. Liu, Electrochim. Acta, 2015, 186, 562 CrossRef CAS.
- M. Pang, G. Long, S. Jiang, Y. Ji, W. Han, B. Wang, X. Liu, Y. Xi, D. Wang and F. Xu, Chem. Eng. J., 2015, 280, 377 CrossRef CAS.
- M. Jing, H. Hou, C. E. Banks, Y. Yang, Y. Zhang and X. Ji, ACS Appl. Mater. Interfaces, 2015, 7, 22741 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra00960c |
| This journal is © The Royal Society of Chemistry 2016 |





