High performance oxygen-bridged N-shaped semiconductors with a stabilized crystal phase and blue luminescence†
Abstract
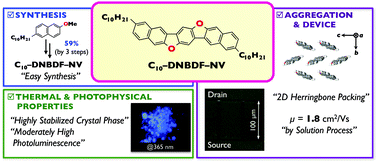
Here, we describe an oxygen-bridged N-shaped π-electron core, dinaphtho[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]difuran (DNBDF), as a new entity of organic semiconducting materials. Interestingly, by introduction of flexible alkyl chains at appropriate positions, DNBDF π-cores exhibit solution processability, a highly stabilized crystal phase, high mobility, and blue luminescence as a solid.

 Please wait while we load your content...
Please wait while we load your content...