Analysis of the water extract of waste papaya bark ash and its implications as an in situ base in the ligand-free recyclable Suzuki–Miyaura coupling reaction†
Manashi Sarmaha,
Anindita Dewana,
Manoj Mondalb,
Ashim J. Thakura and
Utpal Bora*a
aDepartment of Chemical Sciences, Tezpur University, Napaam, Tezpur, Assam 784028, India. E-mail: ubora@tezu.ernet.in; utbora@yahoo.co.in
bDepartment of Chemistry, Dibrugarh University, Dibrugarh-786004, Assam, India
First published on 10th March 2016
Abstract
The conversion of waste papaya-bark to ash–water extract via low-temperature combustion, and its utilisation as an efficient and environmentally friendly in situ basic medium for the Suzuki–Miyaura cross-coupling reaction at room temperature are reported. The papaya-bark ash was characterized by EDX, ion-exchange chromatography and flame photometry to reveal a broad range of active metal oxides. The chemical analysis reports of ash showed the presence of oxides of K, Ca, Na, Li and Mg, which possibly in the presence of water produce the corresponding hydroxides in situ, responsible for the basicity. Application of the ash–water extract as a base was highly effective for ligand-free Pd(OAc)2 catalyzed Suzuki–Miyaura cross-coupling reaction. The reaction proceeds smoothly without any promoter/ligand to give excellent yields. Moreover, after completion of the reaction, the catalytic system could be easily recovered by simple fractional separation, and recyclable at least five times, with the loss of some catalytic activity from the 3rd cycle onward.
The palladium catalyzed Suzuki–Miyaura cross-coupling reaction is one of the most useful and explored methodologies for the synthesis of diversified biaryl motifs.1 Typically, this reaction involves selective cross-coupling between aryl halides and arylboronic acids to give symmetrical and unsymmetrical biaryls in high yields. In recent years, the importance of biaryl derivatives as structural components of numerous natural products, drugs, dyes, optical devices, agrochemicals, as well as engineering materials, such as liquid crystal, conducting polymers, molecular wire and advanced functional materials, has attracted huge attention from the scientific community.1
The general mechanism of this reaction involves distinct three-stages; oxidative addition, transmetalation and reductive elimination. Since, the oxidative addition is the rate-determining step, attention particularly being focused on to increase the electron density of palladium, which thereby accelerates the rate of oxidative addition. This can be done by using sterically crowded and electron rich ligands. Literature reveals that the careful selection of ligand can promote both first and last steps of the catalytic cycle.2 Numerous electron-rich phosphine-based ligands,3 sterically crowded NHC,4 oximes,5 imines,6 and palladacycles7 were developed and employed as catalyst precursors under both conventional organic or biphasic media. Although, these ligand assisting system bear excellent activity, the principle drawbacks associated with them are availability, stability, air/moisture-sensitivity and tedious preparation steps.
In the most crucial stage of the mechanism, the transmetalation stage, the transfer of the organoboron species to the palladium in the presence of external base occurs. However, added base often competes with many functional groups, and also causes protodeboronation with electron-deficient arylboronic acids.8
Thus, with simple and economical methodologies, the exercise of “ligand-free” catalytic system, moderate basicity and wide compatibility has become the need of current manufacturing process to establish greener and sustainable strategy.9 The use of a natural base is elegant for this reaction media.
In pursuance to the principle of green chemistry, and with the aim to minimize the chemical waste, we decided to study in detail the use of an agro-waste stuff that significantly fulfills the desired expectation of safe reaction strategy. Herewith, we opt for dead papaya tree/bark that is thrown to rot/deteriorate/decay. Traditionally, its ash is used as detergents, so considering these view, it can be an appropriate alternative for moderate base. To the best of our knowledge, there are only two methods which significantly deals with the use of water extract of burned-ash of banana peels and rice straw as solvent system.10 However, as most of the aryl halides are hydrophobic in nature, the use of pure water medium often fails to furnish utmost efficiency.11 Indeed, several reports mentioned the use of organic–water system, which aids in the complete dissolution of the organic substrates, and also in the stabilization of the metal.
Herein, we report our preliminary research on the combustion of milled agro-waste papaya bark (Carica papaya) to ash, which can be used as water-extract to produce in situ base system. The milled agro-waste papaya bark-derived ash and its corresponding water extract were analyzed by energy-dispersive X-ray spectroscopy (EDX), Ion-Exchange Chromatography (IEC) and flame photometry. Moreover, an attempt was also made to investigate the basic property of the ash–water extract employing Suzuki–Miyaura cross-coupling reaction. The reaction mixtures were subjected to gas chromatography-mass spectroscopy (GC-MS) to investigate the trace of possible side-products due to protodeboronation. The coupling products were isolated and characterized using Fourier transform-infrared spectroscopy (FT-IR), GC-MS, 1H and 13C nuclear magnetic spectroscopy. Moreover, the catalytic system, after separation of the products, could be recycled and reused for many consecutive cycles with some loss in its catalytic activity.
Results and discussion
The water-extract was prepared by burning oven dried papaya bark to ashes. Thereafter, 10 g of the ash was suspended in 100 mL distilled water and stirred for half hour at room temperature. The suspension was then filtered and a light yellow colored extract was isolated (Fig. 1).The distribution of elements as based on the EDX analysis of the papaya bark ash is shown in Fig. 2. The report reveals a very high distribution of the oxides of Na, Mg, K and Ca. This distribution is comparably higher than that of the waste biomass such as banana peels,12 rice,13 and wheat straw.14
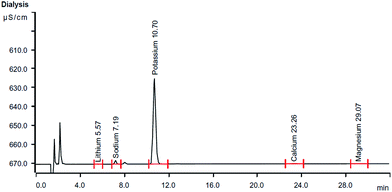
The results of metal concentration and metal contents analysis of the water-extract determined by IEC and flame photometry are summarized in Fig. 3 and Table 1.
| Entry | Metal | Metal concentration (FP) | Metal concentration (IC) |
|---|---|---|---|
| 1 | K | 1876.24 | 2042.95 |
| 2 | Ca | 6.812 | 6.76 |
| 3 | Na | 21.98 | 27.24 |
| 4 | Li | Trace | Trace |
| 5 | Mg | Trace | Trace |
Since, metal oxides can react with water to produce metal hydroxides, the above result highlights the basicity of the water-extract. To confirm this, we investigated the Suzuki–Miyaura cross-coupling reaction, which is highly depended on the presence of external base. To explore the effectiveness of water-extract in Suzuki–Miyaura cross-coupling, the reaction of 4-bromonitrobenzene (0.5 mmol), phenylboronic acid (0.6 mmol), and Pd(OAc)2 (1 mol%) was chosen as the prototype and performed under water-extract at room temperature. The results are summarized in Table 2.
| Entry | Pd(OAc)2 (mol%) | Water-extract (mL) | EtOH (mL) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 4-bromonitrobenzene (0.5 mmol), phenylboronic acid (0.6 mmol), ca. 28 °C in air unless otherwise noted.b Isolated yields.c Without extract.d Water extracted was diluted with water (2 mL). | |||||
| 1 | 1 | 3 | — | 60 | 80 |
| 2 | 1 | 5 | — | 60 | 80 |
| 3 | 1 | 2 | 2 | 5 | 98 |
| 4c | 1 | 3 | 360 | — | |
| 5 | 1 | 3 | 1 | 30 | 85 |
| 6 | 1 | 3 | 2 | 7 | 94 |
| 7 | 0.5 | 2 | 2 | 7 | 97 |
| 8 | 0.25 | 2 | 2 | 30 | 30 |
| 9d | 0.5 | 2 | 2 | 360 | 30 |
On performing the reaction using 1 mol% Pd(OAc)2 and in water-extract (3 mL) at room temperature, we were able to isolate 80% of cross-coupling product (Table 2, entry 1). On increasing the amount of the extract (to 5 mL) no further increase in yield was noticed (Table 2, entry 2). Based on some recent reports on Suzuki–Miyaura coupling, that explains the effect of different co-solvents,15 we next tried the reaction using ethanol and water-extract in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. To our delight, the reaction proceeds significantly within 5 minutes, and we were able to isolate 98% coupling product (Table 2, entry 3). The improved result may be due to the enhanced solubility of the halide substrate in biphasic medium. However the reaction did not proceed at all in absence of water extract (Table 2, entry 4). Upon revising the ratio of ethanol and water-extract, no appreciable improvement in the yield and reaction time was noticed (Table 2, entries 5 & 6). Moreover, on optimizing the reaction conditions using different palladium loading, we noted that the cross-coupling proceeds efficiently with 0.5 mol% Pd(OAc)2 (Table 2, entries 7 vs. 8). To reveal the effectiveness of water extract, we performed the reaction by diluting the water-extract with water (2 mL); however the yield of the cross coupling product was drastically reduced under this reaction conditions. This may be due the decrease in basic strength of the water-extract on further dilution (Table 2, entry 9).
1 ratio. To our delight, the reaction proceeds significantly within 5 minutes, and we were able to isolate 98% coupling product (Table 2, entry 3). The improved result may be due to the enhanced solubility of the halide substrate in biphasic medium. However the reaction did not proceed at all in absence of water extract (Table 2, entry 4). Upon revising the ratio of ethanol and water-extract, no appreciable improvement in the yield and reaction time was noticed (Table 2, entries 5 & 6). Moreover, on optimizing the reaction conditions using different palladium loading, we noted that the cross-coupling proceeds efficiently with 0.5 mol% Pd(OAc)2 (Table 2, entries 7 vs. 8). To reveal the effectiveness of water extract, we performed the reaction by diluting the water-extract with water (2 mL); however the yield of the cross coupling product was drastically reduced under this reaction conditions. This may be due the decrease in basic strength of the water-extract on further dilution (Table 2, entry 9).
In our earlier report, we have reported that the addition of additives such as sodium sulfate, sodium chloride and sodium acetate to the Pd-catalyzed ligand free Suzuki–Miyaura reaction enhances the rate of cross-coupling reaction in water.16 Han and co-workers have also presented strong evidences17 for the existence of interactions between the salt particle with palladium and aryl halide. These interactions were found significant for the synergistic effect between the salts and the catalyst. It is believed that such interactions are very much possible under present reaction conditions.
With this optimized condition in hand, we further studied the generality of Suzuki–Miyaura coupling using diverse range of aryl bromides and arylboronic acids (Table 3). The catalytic system delivers excellent and rapid cross-coupling with both electron-rich and electron-deficient aryl bromides at room temperature (Table 3, entries 1–17). However the application of the catalytic system to 4-bromoaniline and 4-bromobenzoic acid gave moderate and low yields of cross coupling product respectively (Table 3, entry 18 & 19). This method is compatible with bromo substituted heteroaryl derivatives (Table 3, entry 20). However, the reaction with aryl chloride did not proceed under the current reaction conditions. The scope of the reaction was further evaluated by employing differently substituted arylboronic acids. Interestingly, both electron rich and electron-deficient organoboron derivatives furnished high yield of products (Table 3, entries 2–15). Moreover, on GC-MS analysis of the above-performed reactions, no protodeboronation product i.e. arenes were identified.
| Entry | R1 | R2 | Time (min) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: aryl bromide (0.5 mmol), arylboronic acid (0.6 mmol), Pd(OAc)2 (0.5 mol%), extract (2 mL), EtOH (2 mL), ca. 28 °C in air unless otherwise noted.b Isolated yields. | ||||
| 1 | 4-NO2 | H | 7 | 97 |
| 2 | 4-NO2 | 4-Cl | 20 | 95 |
| 3 | 4-NO2 | 4-OMe | 10 | 95 |
| 4 | 4-NO2 | 4-t-Bu | 10 | 98 |
| 5 | 4-OMe | H | 5 | 97 |
| 6 | 2-OMe | H | 30 | 96 |
| 7 | 4-OMe | 3-Me | 5 | 97 |
| 8 | 4-OMe | 4-Cl | 30 | 92 |
| 9 | 4-OCH3 | 4-t-Bu | 5 | 98 |
| 10 | 4-CHO | H | 7 | 96 |
| 11 | 4-CHO | Cl | 30 | 96 |
| 12 | 2-Me | H | 5 | 99 |
| 13 | 2-Me | 4-OMe | 5 | 96 |
| 14 | 4-COMe | H | 5 | 97 |
| 15 | 4-COMe | 4-Cl | 40 | 95 |
| 16 | 4-Me | H | 5 | 99 |
| 17 | 4-OH | H | 20 | 90 |
| 18 | 4-NH2 | H | 60 | 85 |
| 19 | 4-COOH | H | 24 h | 30 |
| 20 | 3-Bromopyridine | H | 2 h | 85 |
The major challenge of a metal-mediated catalytic system is its ability for recyclability, which receives widespread application and avoids metal contamination to the final products. The reusability and efficiency of water extract-Pd(OAc)2 catalytic systems were investigated on the model coupling reactions of 4-bromonitrobenzene with phenylboronic acid. Since, biaryl derivatives are insoluble in water, the product was easily isolated by extraction with ethyl acetate. The residue catalyst was recycled using fresh reactants, water extract and EtOH, and the efficiency of the recyclability was shown in Table 4. Reusability test for Suzuki–Miyaura coupling reactions was carried out for five consecutive cycles and some loss of its catalytic activity was observed from 3rd catalytic cycle onwards.
In conclusion, we have developed environmentally benign and natural-base system using papaya bark-ash for Suzuki–Miyaura coupling reaction, whose significant features are as follows: (i) use of expensive and environmentally unfavorable organic ligands is avoided, (ii) high efficiency under mild reaction conditions in aqueous media in open air, (iii) no side-products due to protodeboronation of arylboronic acids, and (iv) catalyst recycling. The central attraction of our reaction system is a highly abundant natural feedstock, which excellently fits the global urge for sustainable development. Further efforts to utilize these catalytic systems in other synthetic reactions are in progress in our laboratory.
Experimental
General experimental procedure for Suzuki reaction: a mixture of aryl bromide (0.5 mmol), arylboronic acid (0.6 mmol), Pd(OAc)2 (0.5 mol%), water-extract (2 mL) and EtOH (2 mL) was stirred at room temperature in a 50 mL round bottom flask. After completion (vide TLC); the reaction mixture was extracted with ethyl acetate (3 × 10 mL) and the combined organic layer was washed with brine (2 × 10 mL), dried over Na2SO4 and concentrated in vacuo. The residue were purified by column chromatography on silica gel (eluent: EtOAc–hexane) to give the corresponding biaryl compound. The desired products are characterized by comparing 1H, 13C NMR and mass spectral data with authentic samples.Procedure for recycling the catalytic system for Suzuki reaction: the activity of the catalytic system was investigated in 4-bromonitrobenzene (1 mmol) and phenylboronic acid (1.2 mmol) with 0.5 mol% Pd(OAc)2, 4 mL water-extract and 4 mL EtOH. After the first cycle, the reaction mixture was extracted with ethylacetate (3 × 15 mL) followed by centrifugation. The clearly separated organic fraction has been removed from the system and evaporated to get the crude product. The residue catalyst was directly used for the next reaction cycle followed by addition of fresh reactants, water-extract and solvent. After 5 consecutive run the catalytic activity significantly decreases with a lower yield of cross-coupled product.
Acknowledgements
M. S. thanks Tezpur University for providing institutional fellowship. A. D. acknowledges UGC-New Delhi for Dr D. S. Kothari Postdoctoral fellowship.References and notes
- (a) A. Suzuki, Angew. Chem., Int. Ed., 2011, 50, 6722 CrossRef CAS PubMed; (b) A. Suzuki and Y. Yamamoto, Chem. Lett., 2011, 40, 894 CrossRef CAS; (c) A. Balanta, C. Godard and C. Claver, Chem. Soc. Rev., 2011, 40, 4973 RSC; (d) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS; (e) A. F. Littke and G. C. Fu, Angew. Chem., Int. Ed., 2002, 41, 4176 CrossRef CAS; (f) J. Dupont, C. S. Consorti and Spencer, Chem. Rev., 2005, 105, 2527 CrossRef CAS PubMed; (g) A. V. Gaikwad, A. Holuigue, M. B. Thathagar, J. E. Elshof and G. Rothenberg, Chem.–Eur. J., 2007, 13, 6908 CrossRef CAS PubMed; (h) J. D. Sellars and P. G. Steel, Chem. Soc. Rev., 2011, 40, 5170 RSC; (i) A. Fihri, M. Bouhrara, B. Nekoueishahraki, J.-M. Basset and V. Polshettiwar, Chem. Soc. Rev., 2011, 40, 5181 RSC.
- R. Martin and S. L. Buchwald, Acc. Chem. Res., 2008, 41, 1461 CrossRef CAS PubMed.
- (a) T. Schareina and R. Kepme, Angew. Chem., Int. Ed., 2002, 41, 1521 CrossRef CAS; (b) S. Urgaonkar, M. Nagarajan and J. G. Verkade, Tetrahedron Lett., 2002, 43, 8921 CrossRef CAS; (c) N. Biricik, F. Durap, C. Kayan, B. Gumgum, N. Gurbuz, I. Ozdemir, W. H. Ang, Z. Fei and R. J. Scopelliti, Organomet. Chem., 2008, 693, 2693 CrossRef CAS; (d) G. A. Molander and B. Canturk, Angew. Chem., Int. Ed., 2009, 48, 9240 CrossRef CAS PubMed; (e) B. Milde, D. Schaarschmidt, T. Rüffer and H. Lang, Dalton Trans., 2012, 41, 5377 RSC; (f) P. Das, U. Bora, A. Tairai and C. Sharma, Tetrahedron Lett., 2010, 51, 1479 CrossRef CAS; (g) G. Borah, D. Boruah, G. Sarmah, S. Bharadwaj and U. Bora, Appl. Organomet. Chem., 2013, 27, 688 CrossRef CAS.
- C. W. K. Gstöttmayr, V. P. W. Böhm, E. Herdtweck, M. Grosche and W. A. Herrmann, Angew. Chem., Int. Ed., 2002, 41, 1363 CrossRef.
- (a) L. Botella and C. Nájera, Angew. Chem., Int. Ed., 2002, 41, 179 CrossRef CAS; (b) L. Botella and C. Nájera, J. Organomet. Chem., 2002, 663, 46 CrossRef CAS.
- B. Mu, T. Li, W. Xu, G. Zeng, P. Liu and Y. Wu, Tetrahedron, 2007, 63, 11475 CrossRef CAS.
- (a) R. B. Bedford and S. L. Welch, Chem. Commun., 2001, 129 RSC; (b) R. B. Bedford, Chem. Commun., 2003, 1787 RSC.
- (a) H. G. Kuvila and K. V. Nahabedian, J. Am. Chem. Soc., 1961, 83, 2159 CrossRef; (b) H. G. Kuivila, J. F. Reuwer and J. A. Mangravite, J. Am. Chem. Soc., 1964, 86, 2666 CrossRef CAS.
- (a) V. Polshettiwar, C. Len and A. Fihri, Coord. Chem. Rev., 2009, 253, 2599 CrossRef CAS; (b) V. Polshettiwar, A. Decottignies, C. Len and A. Fihri, ChemSusChem, 2010, 3, 502 CrossRef CAS PubMed; (c) G. Hervé and C. Len, RSC Adv., 2014, 4, 46926 RSC; (d) A. Fihri, D. Luart, C. Len, A. Solhy, C. Chevrin and V. Polshettiwar, Dalton Trans., 2011, 40, 3116 RSC; (e) G. Sartori, G. Enderlin, G. Hervé and C. Len, Synthesis, 2012, 44, 767 CrossRef CAS; (f) A. Hassine, S. Sebti, A. Solhy, M. Zahouily, C. Len, M. N. Hedhili and A. Fihri, Appl. Catal., A, 2013, 450, 13–18 CrossRef CAS; (g) G. Sartori, G. Hervé, G. Enderlin and C. Len, Synthesis, 2013, 330 CAS; (h) A. Decottignies, A. Fihri, G. Azemar, F. Djedaini-Pilard and C. Len, Catal. Commun., 2013, 32, 101 CrossRef CAS; (i) S. Gallagher-Duval, G. Hervé, G. Sartori, G. Enderlin and C. Len, New J. Chem., 2013, 37, 1989 RSC; (j) T. Lussier, G. Hervé, G. Enderlin and C. Len, RSC Adv., 2014, 4, 46218 RSC.
- (a) P. R. Boruah, A. A. Ali, B. Saikia and D. Sarma, Green Chem., 2015, 17, 260 RSC; (b) P. R. Boruah, A. A. Ali, M. Chetia, B. Saikia and D. Sarma, Chem. Commun., 2015, 51, 11489 RSC.
- C. Röhlich, A. S. Wirth and K. Köhler, Chem.–Eur. J., 2012, 18, 15485 CrossRef PubMed.
- D. C. Deka and N. N. Talukdar, Indian J. Tradit. Knowle., 2007, 6, 72 Search PubMed.
- B. M. Jenkins, R. R. Bakker and J. B. Wei, Biomass Bioenergy, 1996, 4, 177 CrossRef.
- V. L. Budarin, J. H. Clark, B. A. Lanigan, P. Shuttleworth, S. W. Breeden, A. J. Wilson, D. J. Macquarrie, K. Milkowski, J. Jones, T. Bridgeman and A. Ross, Bioresour. Technol., 2009, 100, 6064 CrossRef CAS PubMed.
- (a) G. Sarmah and U. Bora, Tetrahedron Lett., 2015, 56, 2906 CrossRef CAS; (b) G. Sarmah, M. Mondal and U. Bora, Appl. Organomet. Chem., 2015, 29, 495 CrossRef CAS.
- M. Mondal and U. Bora, Green Chem., 2012, 14, 1873 RSC.
- B. Zhang, J. Song, H. Liu, J. Shi, J. Ma, H. Fan, W. Wang, P. Zhang and B. Han, Green Chem., 2014, 16, 1198 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra for all the products. See DOI: 10.1039/c6ra00454g |
| This journal is © The Royal Society of Chemistry 2016 |