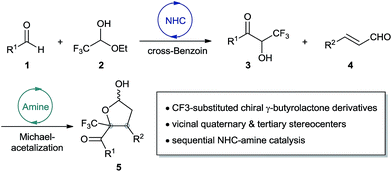
Catalytic cross-benzoin/Michael/acetalization cascade for asymmetric synthesis of trifluoromethylated γ-butyrolactones†
Xiang-Hong He‡
a,
Lei Yang‡b,
Wei Huanga,
Qian Zhaoa,
Xiao-Li Pana,
Dao-Feng Jiangb,
Ming-Cheng Yangb,
Cheng Peng*ab and
Bo Han*a
aState Key Laboratory Breeding Base of Systematic Research, Development and Utilization of Chinese Medicine Resources, Chengdu University of Traditional Chinese Medicine, Chengdu 611137, China. E-mail: hanbo@cdutcm.edu.cn
bMinistry of Education Key Laboratory of Standardization of Chinese Medicine, School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu 611137, China. E-mail: pengcheng@cdutcm.edu.cn
First published on 15th March 2016
Abstract
A sequential NHC-amine catalytic cascade reaction has been developed to assemble aromatic aldehydes, trifluoroacetaldehyde ethyl hemiacetal and enals asymmetrically into CF3-substituted chiral γ-butyrolactone derivatives featuring vicinal quaternary and tertiary stereocenters. This approach incorporates a highly chemoselective intermolecular cross-benzoin reaction and a highly regioselective Michael-acetalization cascade. Various multi-functionalized tetrahydrofuran scaffolds were readily prepared from hemiacetal intermediates through convenient organic transformations.
Introduction
One of the most fascinating areas of organofluorine chemistry is the asymmetric synthesis of trifluoro-methylated compounds.1 Stereoselective introduction of trifluoromethyl groups into organic molecules may significantly improve their usefulness as drugs, such as by improving the metabolic stability, binding affinity, membrane permeability and bioavailability.2 To this end, several methods have been developed to generate medicinally important chiral γ-butyrolactone derivatives3 with a trifluoromethyl-substituted quaternary stereocenter.4–6 In 2004, Glorius's group reported the synthesis of racemic trifluoro-methylated γ-butyrolactone via a process involving N-heterocyclic carbine (NHC)-catalyzed conjugate umpolung of α,β-unsaturated aldehydes, with trifluoro-acetophenone as the electrophilic reaction partner.4 Subsequently, the Glorius group, as well as the groups of Ishida, Saigo, Fiksdahl and others used chiral NHC catalysts to achieve the asymmetric version of this [3 + 2] annulation, albeit with unsatisfactory stereocontrol (Scheme 1, path a).5 Chi and co-workers have since achieved good enantioselectivity and moderate diastereoselectivity when synthesizing CF3-substituted γ-butyrolactones; their method involves NHC-catalyzed ester β-activation of a mixture of saturated ester and trifluoroketone (Scheme 1, path b).6 These advances, though important, are not likely to generate the full diversity of pharmacologically interesting, multi-functionalized CF3-substituted γ-butyrolactone derivatives,7 highlighting the need for more research into asymmetric catalytic syntheses that show good efficiency and high stereoselectivity.Several groups have exploited the inherent basicity and compatibility of chiral amines and NHCs to develop organocatalytic domino or cascade reactions based on sequential amine-NHC catalytic reactions in order to construct stereochemically complex molecules.8 However few studies have explored the potential of a reverse relay NHC-amine catalytic pathway to synthesize enantio-enriched architectures.9 In 2015, Anand's group described a highly chemoselective, NHC-catalyzed cross-benzoin reaction of aromatic aldehydes with trifluoroacetaldehyde ethyl hemiacetal to afford trifluoromethyl-containing acyloins.10 This achievement, coupled with our own recent success developing organocatalytic cascade reactions to assemble synthetically important cyclic molecules,11 led us to ask whether trifluoromethylated acyloins could serve as the basis for subsequent aminocatalytic cascade reactions (Scheme 1, path c). If successful, this approach might broaden the applications of sequential NHC-amine catalysis.
We envisioned a two-step organocatalytic cascade as a way to asymmetrically synthesize CF3-substituted γ-butyrolactone derivatives (Scheme 2). The protocol begins with NHC-catalyzed cross-benzoin condensation of aromatic aldehyde 1 and CF3CH(OH)OEt 2. The resulting trifluoromethylated α-hydroxyketone intermediate 3 participates directly in the second aminocatalytic cycle by serving as the 1,2-bisnucleophile. Subsequent Michael-acetalization cyclization of α-hydroxyketone with chiral secondary amine-mediated α,β-unsaturated iminium forms the desired chiral hemiacetal 5, which features vicinal quaternary and tertiary stereocenters.
Results and discussion
To probe the feasibility of the proposed cascade, we performed preliminary experiments using benzaldehyde 1a, CF3CH(OH)OEt 2a and cinnamaldehyde 4a as substrates. The first crossed acyloin condensation proceeded smoothly in the presence of 10 mol% of catalyst Ia in THF at room temperature for 4 h. Next we added benzoic acid to neutralize the reaction mixture and induce formation of the enol nucleophile. This acidic additive also serves as co-catalyst in the subsequent iminium catalytic cycle. Finally, we added the Jørgensen–Hayashi catalyst IIa and α,β-unsaturated aldehyde 4a, and the cascade process afforded the hemiacetal 5a, albeit in moderate yield. Direct oxidation of the hemiacetal with PCC gave the more stable corresponding γ-butyrolactone as a 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 mixture of diastereomers, with 90% ee for the major isomer 6a and 85% ee for the minor isomer 6a′ (Table 1, entry 1).
20 mixture of diastereomers, with 90% ee for the major isomer 6a and 85% ee for the minor isomer 6a′ (Table 1, entry 1).
| Entry | Cat. I | Cat. II | Solvent | Acid | Yieldb (%) | drc 6a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6a′ 6a′ |
eed (%) 6a/6a′ |
|---|---|---|---|---|---|---|---|
| a Unless indicated otherwise, the reaction was performed with precatalyst I (0.05 mmol), DBU (0.1 mmol), 1a (0.25 mmol) and 2a (0.5 mmol) in solvent (2 mL) at room temperature, after which acidic additive (0.2 mmol), catalyst II (0.1 mmol) and 4a (0.5 mmol) were added.b Yield of isolated 6a and 6a′.c The diastereomeric ratio (dr) was calculated by 1H NMR analysis of the crude reaction mixture.d Determined by HPLC using a chiral stationary phase.e Reaction performed at 70 °C.f 1 mL THF and 1 mL toluene were used.g 1 mL DMF and 1 mL toluene were used. | |||||||
| 1 | Ia | IIa | THF | BzOH | 40 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
90/85 |
| 2 | Ib | IIa | THF | BzOH | 26 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
88/85 |
| 3 | Ic | IIa | THF | BzOH | <10 | — | — |
| 4 | Ia | IIb | THF | BzOH | <10 | — | — |
| 5e | Ia | IIa | THF | BzOH | 52 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
90/84 |
| 6e | Ia | IIa | DMF | BzOH | <10 | — | — |
| 7e | Ia | IIa | MeCN | BzOH | 32 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
90/84 |
| 8e | Ia | IIa | Toluene | BzOH | <10 | — | — |
| 9e | Ia | IIa | Mix.f | BzOH | 35 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
85/80 |
| 10e | Ia | IIa | Mix.g | BzOH | 40 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
89/86 |
| 11e | Ia | IIa | THF | p-FBA | 38 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
88/84 |
| 12e | Ia | IIa | THF | AcOH | 61 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
93/85 |
Next we screened additional parameters in order to optimize the reaction (Table 1). This was challenging because the sequential NHC-amine catalytic systems in the cascade are mixed together in the same pot, meaning that reaction conditions must simultaneously maximize the catalytic efficiency of both systems. Screening a few catalyst combinations (entries 2–4) identified triazolium salt Ia and diphenyl prolinol TMS ether IIa as the most efficient pair for this tandem reaction. Increasing reaction temperature increased reaction yield without compromising dr or ee (entry 5). DMF, acetonitrile, toluene, and some mixed solvents were unsatisfactory because they led to lower yields of final product or to unacceptably slow reactions (entries 6–10). Screening acidic additives in the second catalytic cycle affected reaction efficiency (entries 11 and 12), with acetic acid giving the best results.
Once we had optimized reaction conditions (Table 1, entry 12), we examined substrate scope, beginning with a wide range of aromatic aldehydes 1 (Table 2). The reaction proceeded with good yield and high stereoselectivity with nearly any electron-withdrawing substitution at the ortho-, meta-, or para-position on the aromatic aldehydes (entries 2–7). Reaction was slightly slow when aromatic aldehyde was substituted at the ortho-position (entry 6), which may reflect steric hindrance. Good yield and high stereoselectivity were obtained in the case of aromatic aldehyde bearing an electron-donating group (entry 8). Heteroaryl aldehydes gave better yields of products 6i and 6j than aryl aldehydes did, and with greater diastereoselectivities (entries 9 and 10). Cinnamaldehyde reacted efficiently with CF3CH(OH)OEt, and the product 6k containing a cinnamoyl group was generated with good stereocontrol (entry 11). Unfortunately aliphatic aldehydes did not work in the first cross-benzoin step,12 suggesting that the overall cascade reaction is compatible only with aromatic aldehydes and aromatic enals.
| Entry | R1 | R2 | Product | Yieldb (%) | drc 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′ 6′ |
eed (%) 6/6′ | |
|---|---|---|---|---|---|---|---|
| a See entry 12 and footnote a in Table 1.b Yield of isolated 6 and 6′.c The diastereomeric ratio (dr) was calculated by 1H NMR analysis of the crude reaction mixture.d Determined by HPLC using a chiral stationary phase. | |||||||
| 1 | Ph | Ph | 6a/6a′ | 61 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :22 :22 |
93/85 | |
| 2 | 3-Cl–C6H4 | Ph | 6b/6b′ | 66 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
97/91 | |
| 3 | 4-Cl–C6H4 | Ph | 6c/6c′ | 69 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
92/85 | |
| 4 | 3-Br–C6H4 | Ph | 6d/6d′ | 65 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
89/88 | |
| 5 | 4-Br–C6H4 | Ph | 6e/6e′ | 63 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
90/88 | |
| 6 | 2-F–C6H4 | Ph | 6f/6f′ | 48 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
90/85 | |
| 7 | 4-F–C6H4 | Ph | 6g/6g′ | 56 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
85/88 | |
| 8 | 4-iPr–C6H4 | Ph | 6h/6h′ | 58 | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
85/83 | |
| 9 | 2-Furyl | Ph | 6i/6i′ | 75 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
88/88 | |
| 10 | 2-Thienyl | Ph | 6j/6j′ | 78 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
89/86 | |
| 11 | Cinnamoyl | Ph | 6k/6k′ | 59 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
89/87 | |
| 12 | Ph | 2-Cl–C6H4 | 6l/6l′ | 68 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
94/81 | |
| 13 | Ph | 4-Cl–C6H4 | 6m/6m′ | 71 | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23 |
86/95 | |
| 14 | Ph | 4-Br–C6H4 | 6n/6n′ | 67 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
86/86 | |
| 15 | Ph | 2-F–C6H4 | 6o/6o′ | 70 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
86/91 | |
| 16 | Ph | 3-F–C6H4 | 6p/6p′ | 69 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
85/88 | |
| 17 | Ph | 4-F–C6H4 | 6q/6q′ | 65 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
88/84 | |
| 18 | Ph | 4-NO2–C6H4 | 6r/6r′ | 62 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
90/86 | |
| 19 | Ph | 4-CH3–C6H4 | 6s/6s′ | 55 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27 |
83/81 | |
| 20 | Ph | 2-CH3O–C6H4 | 6t/6t′ | 54 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
85/85 | |
| 21 | Ph | 2-Furyl | 6u/6u′ | 75 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
60/61 | |
| 22 | Ph | CH3 | 6v/6v′ | 51 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
92/80 | |
After defining the substrate scope for component 1, we turned to the scope for the α,β-unsaturated aldehyde partner. A broad array of β-aryl-substituted enals 4 proved to be competent substrates (entries 12–21). Nevertheless, the electronic properties of the substituent on the aromatic ring of substrate 4 slightly affected the reaction. When the aromatic ring carried an electron-withdrawing substituent, the reactions proceeded smoothly to completion, affording products 6l–6r in good yield with high to excellent stereoselectivity (entries 12–18). In contrast, when the aromatic ring carried an electron-donating substituent, the reactions were relatively slow, and the products 6s and 6t were attained in moderate yields (entries 19 and 20). Heteroaryl enal also led to the target product 6u in high yield with good diastereoselectivity, but the ee value was relatively low (entry 21). Even the less reactive crotonaldehyde participated in the reaction, giving product 6v with high enantioselectivity, albeit in lower yield (entry 22). The absolute configuration of 6c was determined by X-ray crystallographic analysis,13 and the absolute configurations of other products were tentatively assigned by analogy.
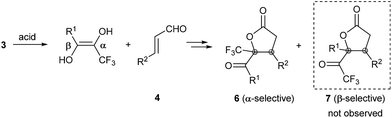
Remarkably, acid-promoted formation of the asymmetric enediol intermediate assured a steady supply of nucleophile for the second catalytic cycle. Although both the α- and β-position of the CF3 can, in principle, serve as the nucleophilic site (Scheme 3), giving rise to the respective cyclization products 6 and 7, we detected only α-selective cyclization products (two diastereoisomers) using 13C- and 19F-NMR. We never detected β-selective cyclization products under our reaction conditions.
We propose the transition state model in Scheme 4 to explain the high diastereo- and enantioselectivities observed in our cascade process with a variety of substrates. In this model, which is analogous to the one proposed by the Jørgensen group for amine-catalyzed epoxidation14 or Michael addition,15 the asymmetric enediol is able to approach the β-position of the chiral iminium intermediate close enough to allow carbon–carbon bond formation selectively between the Re face of the enediol and the Si face of the iminium. This approach minimizes steric repulsion among the enediol, bulky diphenylsiloxymethyl group on the pyrrolidine ring, and β-substituent on the enal. The resulting hemiacetal is generated in the S,S configuration with high stereoselectivity.
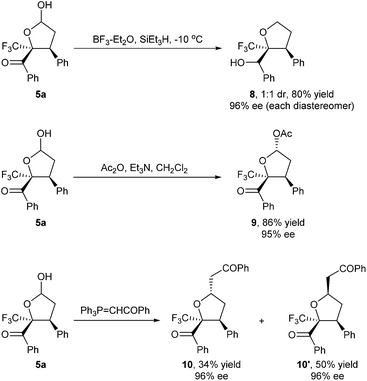
To demonstrate the synthetic utility of our asymmetric cascade process, we smoothly converted the chiral hemiacetal 5a into some versatile building blocks different from γ-butyrolactones (Scheme 5). Treating 5a with Et3SiH and BF3–Et2O in dichloromethane at −10 °C led to simultaneous removal of the hydroxyl group and reduction of the benzoyl carbonyl group, generating the chiral tetrahydrofuran derivative 8. We were also able to acetylate the hydroxyl group of hemiacetal 5a to give stable 9 in high yield. Treating 5a with 1-phenyl-2-(triphenylphosphoranylidene)-ethanone led to an apparent Wittig-oxa-Michael process, affording the multi-functionalized tetrahydrofuran 10 with three chiral centers.
Conclusions
In conclusion, we have developed an efficient and practical one-pot cascade process that delivers synthetically useful, multi-functionalized chiral hydrogenated γ-butyrolactone derivatives featuring a CF3-substituted quaternary stereocenter in good yield with high stereoselectivity. The process comprises a highly chemoselective NHC-catalyzed intermolecular cross-benzoin reaction of aromatic aldehydes with trifluoroacetaldehyde ethyl hemiacetal; followed by a highly regioselective amine-catalyzed Michael-acetalization cascade involving α,β-unsaturated aldehyde. This novel approach broadens the useful scope of sequential dual catalysis.16 Further study of asymmetric domino reactions involving NHC-amine catalysis are underway in our laboratory.Acknowledgements
We are grateful for financial support from NSFC (21302016, 81573588 and 81573589), the Science & Technology Department of Sichuan Province (2014JQ0020) and the Foundation for the Author of National Excellent Doctoral Dissertation of PR China (201487).Notes and references
- For recent reviews on the asymmetric synthesis of trifluoro-methylated compounds, see: (a) T. Furuya, A. S. Kamlet and T. Ritter, Nature, 2011, 473, 470–477 CrossRef CAS PubMed; (b) J. Nie, H.-C. Guo, D. Cahard and J.-A. Ma, Chem. Rev., 2011, 111, 455–529 CrossRef CAS PubMed; (c) O. A. Tomashenko and V. V. Grushin, Chem. Rev., 2011, 111, 4475–4521 CrossRef CAS PubMed; (d) A. Studer, Angew. Chem., Int. Ed., 2012, 51, 8950–8958 CrossRef CAS PubMed; (e) T. Liang, C. N. Neumann and T. Ritter, Angew. Chem., Int. Ed., 2013, 52, 8214–8264 CrossRef CAS PubMed; (f) E. Merino and C. Nevado, Chem. Soc. Rev., 2014, 43, 6598–6608 RSC.
- For recent reviews on the applications of fluorine in medicinal chemistry, see: (a) C. Isanbor and D. O'Hagan, J. Fluorine Chem., 2006, 127, 303–319 CrossRef CAS; (b) K. L. Kirk, J. Fluorine Chem., 2006, 127, 1013–1029 CrossRef CAS; (c) K. Müeller, C. Faeh and F. Diederich, Science, 2007, 317, 1881–1886 CrossRef PubMed; (d) W. K. Hagmann, J. Med. Chem., 2008, 51, 4359–4369 CrossRef CAS PubMed; (e) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320–330 RSC; (f) J. Wang, M. Sánchez-Roselló, J. Luis Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432–2506 CrossRef CAS PubMed; (g) E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly and N. A. Meanwell, J. Med. Chem., 2015, 58, 8315–8359 CrossRef CAS PubMed.
- For recent reviews on the synthesis of chiral γ-butyrolactone derivatives, see: (a) M. Seitz and O. Reiser, Curr. Opin. Chem. Biol., 2005, 9, 285–292 CrossRef CAS PubMed; (b) S. Gil, M. Parra, P. Rodriguez and J. Segura, Mini-Rev. Org. Chem., 2009, 6, 345–358 CrossRef CAS; (c) R. R. A. Kitson, A. Millemaggi and R. J. K. Taylor, Angew. Chem., Int. Ed., 2009, 48, 9426–9451 CrossRef CAS PubMed; (d) J. M. J. Nolsøe and T. V. Hansen, Eur. J. Org. Chem., 2014, 3051–3065 CrossRef.
- (a) C. Burstein and F. Glorius, Angew. Chem., Int. Ed., 2004, 43, 6205–6208 CrossRef CAS PubMed; (b) K. Hirano, I. Piel and F. Glorius, Adv. Synth. Catal., 2008, 350, 984–988 CrossRef CAS.
- (a) C. Burstein, S. Tschan, X. Xie and F. Glorius, Synthesis, 2006, 2418–2439 CAS; (b) Y. Matsuoka, Y. Ishida and K. Saigo, Tetrahedron Lett., 2008, 49, 2985–2989 CrossRef CAS; (c) Y. Matsuoka, Y. Ishida, D. Sasaki and K. Saigo, Chem.–Eur. J., 2008, 14, 9215–9222 CrossRef CAS PubMed; (d) R. B. Strand, T. Helgerud, T. Solvang, A. Dolva, C. A. Sperger and A. Fiksdahl, Tetrahedron: Asymmetry, 2012, 23, 1350–1359 CrossRef CAS.
- Z. Fu, J. Xu, T. Zhu, W. W. Y. Leong and Y. R. Chi, Nat. Chem., 2013, 5, 835–839 CrossRef CAS PubMed.
- For some other approaches to CF3-substituted chiral γ-butyrolactone derivatives, see: (a) P. V. Ramachandran, K. J. Padiya, V. Rauniyar, M. V. R. Reddy and H. C. Brown, Tetrahedron Lett., 2004, 45, 1015–1017 CrossRef CAS; (b) P. Bravo, M. Frigerio, A. Melloni, W. Panzeri, C. Pesenti, F. Viani and M. Zanda, Eur. J. Org. Chem., 2002, 1895–1902 CrossRef CAS; (c) Y. Komatsu, F. Sasaki, S. Takei and T. Kitazume, J. Org. Chem., 1998, 63, 8058–8061 CrossRef CAS.
- (a) S. P. Lathrop and T. Rovis, J. Am. Chem. Soc., 2009, 131, 13628–13630 CrossRef CAS PubMed; (b) L. Deiana, P. Dziedzic, G.-L. Zhao, J. Vesely, I. Ibrahem, R. Rios, J. Sun and A. Cordova, Chem.–Eur. J., 2011, 17, 7904–7917 CrossRef CAS PubMed; (c) D. Enders, A. Grossmann, H. Huang and G. Raabe, Eur. J. Org. Chem., 2011, 4298–4301 CrossRef CAS; (d) C. B. Jacobsen, K. L. Jensen, J. Udmark and K. A. Jørgensen, Org. Lett., 2011, 13, 4790–4793 CrossRef CAS PubMed; (e) Y.-Z. Liu, J. Zhang, P.-F. Xu and Y.-C. Luo, J. Org. Chem., 2011, 76, 7551–7555 CrossRef CAS PubMed; (f) K. E. Ozboya and T. Rovis, Chem. Sci., 2011, 2, 1835–1838 RSC; (g) C. B. Jacobsen, Ł. Albrecht, J. Udmark and K. A. Jørgensen, Org. Lett., 2012, 14, 5526–5529 CrossRef CAS PubMed; (h) Y. Liu, M. Nappi, E. C. Escudero-Adán and P. Melchiorre, Org. Lett., 2012, 14, 1310–1313 CrossRef CAS PubMed; (i) L. Dell'Amico, G. Rassu, V. Zambrano, A. Sartori, C. Curti, L. Battistini, G. Pelosi, G. Casiraghi and F. Zanardi, J. Am. Chem. Soc., 2014, 136, 11107–11114 CrossRef PubMed.
- (a) B.-C. Hong, N. S. Dange, C.-S. Hsu and J.-H. Liao, Org. Lett., 2010, 12, 4812–4815 CrossRef CAS PubMed; (b) B.-C. Hong, N. S. Dange, C.-S. Hsu, J.-H. Liao and G.-H. Lee, Org. Lett., 2011, 13, 1338–1341 CrossRef CAS PubMed; (c) G. He, F. Wu, W. Huang, R. Zhou, L. Ouyang and B. Han, Adv. Synth. Catal., 2014, 356, 2311–2319 CrossRef CAS.
- B. T. Ramanjaneyulu, S. Mahesh and R. V. Anand, Org. Lett., 2015, 17, 6–9 CrossRef CAS PubMed.
- (a) X. Xie, C. Peng, G. He, H.-J. Leng, B. Wang, W. Huang and B. Han, Chem. Commun., 2012, 48, 10487–10489 RSC; (b) X. Li, L. Yang, C. Peng, X. Xie, H.-J. Leng, B. Wang, Z.-W. Tang, G. He, L. Ouyang, W. Huang and B. Han, Chem. Commun., 2013, 49, 8692–8694 RSC; (c) B. Han, X. Xie, W. Huang, X. Li, L. Yang and C. Peng, Adv. Synth. Catal., 2014, 356, 3676–3682 CrossRef CAS; (d) B. Han, W. Huang, W. Ren, G. He, J.-h. Wang and C. Peng, Adv. Synth. Catal., 2015, 357, 561–568 CrossRef CAS; (e) R. Zhou, Q. Wu, M. Guo, W. Huang, X. He, L. Yang, F. Peng, G. He and B. Han, Chem. Commun., 2015, 51, 13113–13116 RSC.
- We screened 2-phenylacetaldehyde and 3-phenylpropanal.
- CCDC 1440711† (6c) contains the ESI crystallographic data for this article.
- (a) M. Marigo, J. Franzen, T. B. Poulsen, W. Zhuang and K. A. Jørgensen, J. Am. Chem. Soc., 2005, 127, 6964–6965 CrossRef CAS PubMed; (b) W. Zhuang, M. Marigo and K. A. Jørgensen, Org. Biomol. Chem., 2005, 3, 3883–3885 RSC.
- S. Brandau, A. Landa, J. Franzen, M. Marigo and K. A. Jørgensen, Angew. Chem., Int. Ed., 2006, 45, 4305–4309 CrossRef CAS PubMed.
- (a) C. Grondal, M. Jeanty and D. Enders, Nat. Chem., 2010, 2, 167–178 CrossRef CAS PubMed; (b) M. Rueping, R. M. Koenigs and I. Atodiresei, Chem.–Eur. J., 2010, 16, 9350–9365 CrossRef CAS PubMed; (c) Ł. Albrecht, H. Jiang and K. A. Jørgensen, Angew. Chem., Int. Ed., 2011, 50, 8492–8509 CrossRef PubMed; (d) C. C. J. Loh and D. Enders, Chem.–Eur. J., 2012, 18, 10212–10225 CrossRef CAS PubMed; (e) C. M. Marson, Chem. Soc. Rev., 2012, 41, 7712–7722 RSC; (f) H. Pellissier, Adv. Synth. Catal., 2012, 354, 237–294 CrossRef CAS; (g) Z. Du and Z. Shao, Chem. Soc. Rev., 2013, 42, 1337–1378 RSC; (h) H. Pellissier, Tetrahedron, 2013, 69, 7171–7210 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization data for new compounds. CCDC 1440711. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra03571j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |