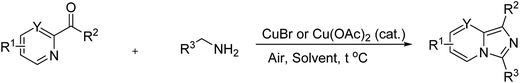
Synthesis of imidazo[1,5-a]pyridines via oxidative amination of the C(sp3)–H bond under air using metal–organic framework Cu-MOF-74 as an efficient heterogeneous catalyst†
Hanh T. H. Nguyen,
Oanh T. K. Nguyen,
Thanh Truong* and
Nam T. S. Phan*
Department of Chemical Engineering, HCMC University of Technology, VNU-HCM, 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Vietnam. E-mail: tvthanh@hcmut.edu.vn; ptsnam@hcmut.edu.vn; Fax: +84 8 38637504; Tel: +84 8 38647256 ext. 5681
First published on 6th April 2016
Abstract
A crystalline porous copper-based metal–organic framework Cu-MOF-74 was synthesized, and was characterized by X-ray powder diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), thermogravimetric analysis (TGA), Fourier transform infrared (FT-IR), atomic absorption spectrophotometry (AAS), and nitrogen physisorption measurements. The Cu-MOF-74 used was a recyclable catalyst for the condensation–cyclization reaction between 2-benzoyl pyridine and different benzylamines via oxidative amination of the C(sp3)–H bond to form 1,3-diarylated imidazo[1,5-a]pyridines using air as the oxidant. The Cu-MOF-74 exhibited better catalytic activity for the transformation than other MOFs including Cu(BDC), Cu2(BDC)2(DABCO), Cu3(BTC)2, Cu2(BPDC)2(BPY), Cu(OBA), Cu2(OBA)2(BPY), and Cu(INA)2. The solid catalyst could be recovered and reused several times without a significant degradation in catalytic activity. To the best of our knowledge, the synthesis of 1,3-diarylated imidazo[1,5-a]pyridines via oxidative amination of the C(sp3)–H bond was not previously performed under heterogeneous catalysis conditions.
1. Introduction
Imidazo[1,5-a]pyridines have emerged as valuable scaffolds in a variety of pharmaceutical candidates and agrochemicals, as well as in numerous functional materials.1–5 Traditionally, these fused heterocycles could be achieved by the Vilsmeier-type cyclizations of N-2-pyridylmethylamides or N-2-pyridylmethylthioamides with several reagents.6,7 Alternative synthetic approaches have been explored to produce imidazo[1,5-a]pyridines. Murai and co-workers reported the oxidative condensation–cyclization of aldehydes and aryl-2-pyridylmethylamines in the presence of a stoichiometric amount of elemental sulfur as an oxidant.8 Paoletti and Crawforth previously demonstrated a one-pot synthesis of imidazo[1,5-a]pyridines starting from a carboxylic acid and 2-methylaminopyridines.9 Shen and co-workers developed a base-mediated protocol to synthesize these heterocycles from the reaction of 1,1-dibromo-1-alkenes with 2-aminomethylpyridines.10 Manivannan and Fulwa pointed out that 3-substituted imidazo[1,5-a]pyridines could be synthesized from the three-component cyclization of aldehydes, 2-cyanopyridine, and ammonium acetate.11 As described in Scheme 1, Zeng and co-workers recently reported the first example of a CuBr-catalyzed direct transannulation of N-heteroaryl aldehydes or N-heteroaryl ketones with alkylamines via Csp3–H amination to produce imidazo[1,5-a]pyridines using molecular oxygen as a sole oxidant.12 Xu and Adimurthy also performed the similar transformation using Cu(OAc)2 and CuI as catalysts, respectively.13 To achieve more environmentally benign approaches to imidazo[1,5-a]pyridines in terms of the ease of handling, simple workup, recyclability and reusability, heterogeneous catalysts should be explored to replace conventional copper salts.Metal–organic frameworks (MOFs), also known as porous coordination polymers, are a new class of hybrid network supramolecular solid materials, consisting of organic linkers and metal-connecting points.14–19 Compared to conventional crystalline materials, MOFs would exhibit many advantages in terms of well-defined structures, high surface areas, high porosity, structural diversity, and the ability to tune the surface hydrophobicity/hydrophilicity.14–23 Although challenges in commercialization still remain to be solved, potential applications of MOFs in several fields have been extensively investigated.14,15,24–29 During the last few years, MOFs as heterogeneous catalysts have been one of the hot topics.30–36 The combination of organic linkers and metal cations in the framework could maximize the opportunity for high dispersion and high loading of catalytically active sites.31,37–43 Indeed, both carbon–carbon35,44–53 and carbon–heteroatom forming organic transformations54–62 using MOFs as heterogeneous catalysts or catalyst supports have been mentioned in the literature, and this topic would be extensively explored in the near future.30–32,63,64 Among several popular MOFs, copper-based frameworks have attracted significant attention as catalysts for numerous organic transformations due to their unsaturated open copper metal sites.53,57,65–71 In this work, we wish to report the synthesis of 1,3-diarylated imidazo[1,5-a]pyridines via oxidative amination of C(sp3)–H bond using a copper-based metal–organic framework Cu-MOF-74 as an efficient heterogeneous catalyst with molecular oxygen in air as the stoichiometric oxidant. To the best of our knowledge, this transformation was not previously carried out under heterogeneous catalysis conditions.
2. Experimental
2.1. Materials and instrumentation
All reagents and starting materials were obtained commercially from Sigma-Aldrich and Merck, and were used as received without any further purification unless otherwise noted. Nitrogen physisorption measurements were conducted using a Micromeritics 2020 volumetric adsorption analyzer system. Samples were pretreated by heating under vacuum at 150 °C for 3 h. A Netzsch Thermoanalyzer STA 409 was used for thermogravimetric analysis (TGA) with a heating rate of 10 °C min−1 under a nitrogen atmosphere. X-ray powder diffraction (XRD) patterns were recorded using a Cu Kα radiation source on a D8 Advance Bruker powder diffractometer. Scanning electron microscopy studies were conducted on a S4800 Scanning Electron Microscope (SEM). Transmission electron microscopy studies were performed using a JEOL JEM 1400 Transmission Electron Microscope (TEM) at 100 kV. The Cu-MOF-74 sample was dispersed on holey carbon grids for TEM observation. Elemental analysis with atomic absorption spectrophotometry (AAS) was performed on an AA-6800 Shimadzu. Fourier transform infrared (FT-IR) spectra were obtained on a Nicolet 6700 instrument, with samples being dispersed on potassium bromide pallets.Gas chromatographic (GC) analyses were performed using a Shimadzu GC 2010-Plus equipped with a flame ionization detector (FID) and an SPB-5 column (length = 30 m, inner diameter = 0.25 mm, and film thickness = 0.25 μm). The temperature program for GC analysis held samples at 100 °C for 1 min; heated them from 100 to 280 °C at 40 °C min−1; and held them at 280 °C for 8.5 min. Inlet and detector temperatures were set constant at 280 °C. Diphenyl ether was used as an internal standard to calculate GC yield. GC-MS analyses were performed using a Shimadzu GCMS-QP2010Ultra with a ZB-5MS column (length = 30 m, inner diameter = 0.25 mm, and film thickness = 0.25 μm). The temperature program for GC-MS analysis held samples at 50 °C for 2 min; heated samples from 50 to 280 °C at 10 °C min−1 and held them at 280 °C for 10 min. Inlet temperature was set constant at 280 °C. MS spectra were compared with the spectra gathered in the NIST library. The 1H NMR and 13C NMR were recorded on Bruker AV 500 spectrometers using residual solvent peak as a reference.
2.2. Synthesis of the metal–organic framework Cu-MOF-74
In a typical preparation, a solid mixture of H2dhtp (H2dhtp = 2,5-dihydroxyterephthalic acid; 0.186 g, 0.97 mmol), and Cu(NO3)2·3H2O (0.500 g, 2.07 mmol) was dissolved in a mixture of DMF (DMF = N,N′-dimethylformamide; 20 ml), and water (1 ml). The resulting solution was then distributed to three 10 ml vials. The vials were then heated at 85 °C in an isothermal oven for 18 h. After cooling the vials to room temperature, the solid product was removed by decanting with mother liquor and washed in DMF (3 × 20 ml) for 3 days. Solvent exchange was carried out with methanol (3 × 20 ml) at room temperature for 3 days. The material was then evacuated under vacuum at 150 °C for 5 h, yielding 0.260 g of Cu-MOF-74 in the form of reddish black crystals (60% based on H2dhtp).2.3. Catalytic studies
In a typical experiment, a mixture of 2-benzoyl pyridine (0.037 g, 0.2 mmol) and diphenyl ether (0.034 g, 0.2 mmol) as an internal standard in DMF (1 ml) was added into a 10 ml screw tube containing the pre-determining amount of Cu-MOF-74 catalyst and benzylamine (0.066 g, 0.6 mmol) at room temperature. The catalyst concentration was calculated with respect to the copper/2-benzoyl pyridine molar ratio. The reaction mixture was stirred at 120 °C for 8 h. Reaction yield was monitored by withdrawing aliquots from the reaction mixture, quenching with water (1 ml). The organic components were then extracted into ethyl acetate (2 ml), dried over anhydrous Na2SO4, and analyzed by GC with reference diphenyl ether. The combined organic layers were concentrated under reduced pressure. The resulting residue was purified by column chromatography (ethyl acetate/hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to afford 1,3-diphenylimidazo[1,5-a]pyridine. The product identity was further confirmed by GC-MS, 1H NMR and 13C NMR. To investigate the recyclability of Cu-MOF-74, the catalyst was separated from the reaction mixture by simple centrifugation, washed with copious amounts of DMF and methanol, dried 150 °C under vacuum in 6 h, and reused if necessary. For the leaching test, upon completion of the reaction, the Cu-MOF-74 catalyst was separated from the reaction mixture by simple centrifugation. The liquid phase was transferred to a new reactor vessel, and fresh reagents were added to the solution. Reaction progress, if any, was monitored by GC as previously described.
8) to afford 1,3-diphenylimidazo[1,5-a]pyridine. The product identity was further confirmed by GC-MS, 1H NMR and 13C NMR. To investigate the recyclability of Cu-MOF-74, the catalyst was separated from the reaction mixture by simple centrifugation, washed with copious amounts of DMF and methanol, dried 150 °C under vacuum in 6 h, and reused if necessary. For the leaching test, upon completion of the reaction, the Cu-MOF-74 catalyst was separated from the reaction mixture by simple centrifugation. The liquid phase was transferred to a new reactor vessel, and fresh reagents were added to the solution. Reaction progress, if any, was monitored by GC as previously described.
3. Results and discussion
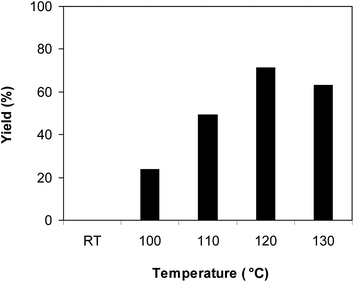
The metal–organic framework Cu-MOF-74 was synthesized according to a slightly modified literature procedure,72 and was characterized by many different techniques, including XRD, SEM, TEM, TGA, FT-IR, AAS, and nitrogen physisorption measurements (Fig. S1–S7†). The Cu-MOF was assessed for its catalytic activity in the condensation–cyclization reaction between 2-benzoyl pyridine and benzylamine to form 1,3-diphenylimidazo[1,5-a]pyridine as the principal product via oxidative amination of C(sp3)–H bond (Scheme 2). Initial studies addressed the effect of temperature on the yield of 1,3-diphenylimidazo[1,5-a]pyridine. In the first example of the direct transannulation of N-heteroaryl aldehydes or N-heteroaryl ketones with alkylamines via Csp3–H amination to produce imidazo[1,5-a]pyridines under air, Zeng and co-workers carried out the reaction at 80 °C.12 However, low yields were observed for the case of N-heteroaryl ketones. Xu and co-workers demonstrated that the yields of imidazo[1,5-a]pyridines could be improved at 110 °C.13 The condensation–cyclization reaction was then carried out under air in DMF for 8 h, using 3 equivalents of benzylamine, at 2-benzoyl pyridine concentration of 0.2 M, in the presence of 10 mol% Cu-MOF-74 catalyst, at room temperature, 100 °C, 110 °C, 120 °C, and 130 °C, respectively. It was observed that the transformation could not proceed at room temperature, with no trace amount of product being detected after 8 h. Increasing the temperature to 100 °C resulted in 23% yield of 1,3-diphenylimidazo[1,5-a]pyridine after 8 h. This value could be improved to 71% for the reaction carried out at 120 °C. Increasing the temperature to higher than 120 °C was found to be unnecessary as the formation of 1,3-diphenylimidazo[1,5-a]pyridine was not favored (Fig. 1). | ||
| Scheme 2 The synthesis of 1,3-diphenylimidazo[1,5-a]pyridine via oxidative amination of C(sp3)–H bond using Cu-MOF-74 catalyst. | ||
Another factor that should be addressed for the condensation–cyclization reaction between 2-benzoyl pyridine and benzylamine to form 1,3-diphenylimidazo[1,5-a]pyridine via oxidative amination of C(sp3)–H bond is the catalyst concentration. In the first example of the imidazo[1,5-a]pyridine synthesis via Csp3–H amination from N-heteroaryl aldehydes or N-heteroaryl ketones and alkylamines, up to 20 mol% CuBr was employed.12 Xu and co-workers also performed the condensation–cyclization between 2-benzoyl pyridine and benzylamine using 15 mol% Cu(OAc)2 as catalyst.13 The reaction was then carried out under air in DMF at 120 °C for 8 h, using 3 equivalents of benzylamine, at 2-benzoyl pyridine concentration of 0.2 M, in the presence of 2.5 mol%, 5 mol%, 7.5 mol%, 10 mol%, 12.5 mol%, and 15 mol% Cu-MOF-74 catalyst, respectively. It should be noted that only 14% yield of 1,3-diphenylimidazo[1,5-a]pyridine was formed in the absence of the Cu-MOF-74, indicating the necessity of using the Cu-MOF as catalyst for the condensation–cyclization between 2-benzoyl pyridine and benzylamine. As expected, the presence of Cu-MOF-74 as catalyst in the reaction mixture led to a significant enhancement in the yield of 1,3-diphenylimidazo[1,5-a]pyridine. The reaction using 5 mol% Cu-MOF-74 catalyst afforded 40% yield after 8 h, while 56% yield was observed for that using 7.5 mol% catalyst. It was found that the yield could be improved to 71% when 10 mol% Cu-MOF-74 catalyst was employed. However, using more than 10 mol% Cu-MOF-74 catalyst was found to be unnecessary for the condensation–cyclization reaction as the yield of 1,3-diphenylimidazo[1,5-a]pyridine was not increased significantly (Fig. 2).
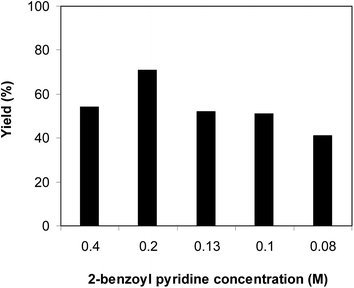
The rate of liquid phase organic transformations using solid catalysts might be significantly affected by the reactant concentration due to the mass transfer phenomenon. It was therefore decided to investigate the impact of reactant concentration on the formation of 1,3-diphenylimidazo[1,5-a]pyridine in the condensation–cyclization reaction between 2-benzoyl pyridine and benzylamine using Cu-MOF-74 catalyst. The reaction was then carried out in DMF under air at 120 °C for 8 h, using 3 equivalents of benzylamine, in the presence of 10 mol% Cu-MOF-74 catalyst, at 2-benzoyl pyridine concentration of 0.08 M, 0.1 M, 0.13 M, 0.2 M, and 0.4 M, respectively. It was observed that the reactant concentration exhibited a significant effect on the yield of 1,3-diphenylimidazo[1,5-a]pyridine. The condensation–cyclization reaction using 2-benzoyl pyridine concentration of 0.08 M proceeded with difficulty, affording only 41% yield after 8 h. Increasing the concentration of 2-benzoyl pyridine led to an enhancement in the yield of 1,3-diphenylimidazo[1,5-a]pyridine, and best yield was observed at 2-benzoyl pyridine concentration of 0.2 M. Lower yield was detected for the transformation using 2-benzoyl pyridine concentration of 0.4 M (Fig. 3). Furthermore, it was also found that the reagent molar ratio exhibited a significant impact on the yield of 1,3-diphenylimidazo[1,5-a]pyridine in the condensation–cyclization reaction between 2-benzoyl pyridine and benzylamine using Cu-MOF-74 catalyst. The condensation–cyclization reaction using 1 equivalent of benzylamine afforded only 21% yield of 1,3-diphenylimidazo[1,5-a]pyridine after 8 h, while this value could be improved to 71% for that using 3 equivalents of benzylamine. Using more than 3 equivalents of benzylamine led to a drop in the reaction yield (Fig. 4).
The solvent could exhibit a significant effect on the reaction rate of many organic transformations using solid catalysts, depending on the nature of the catalyst.73,74 In the first example of the CuBr-catalyzed direct transannulation of N-heteroaryl aldehydes or N-heteroaryl ketones with alkylamines via Csp3–H amination to produce imidazo[1,5-a]pyridines, Zeng and co-workers pointed out that acetonitrile should be the solvent of choice.12 Xu and co-workers performed the same transformation using Cu(OAc)2 as catalyst in different solvents, and demonstrated that DMF would be the best solvent for the transformation.13 It was therefore decided to investigate the effect of different solvents on the condensation–cyclization reaction between 2-benzoyl pyridine and benzylamine to form 1,3-diphenylimidazo[1,5-a]pyridine via oxidative amination of C(sp3)–H bond using Cu-MOF-74 catalyst. The reaction was then carried out under air at 120 °C for 8 h, using 3 equivalents of benzylamine, in the presence of 10 mol% Cu-MOF-74 catalyst, at 2-benzoyl pyridine concentration of 0.2 M, in NMA, NMP, toluene, chlorobenzene, DMF, DMSO, n-butanol, p-xylene, benzonitrile, and tert-butanol as solvent, respectively. It was observed that the yield of 1,3-diphenylimidazo[1,5-a]pyridine was significantly affected by the solvent. The condensation–cyclization reaction proceeded with difficulty in chlorobenzene, p-xylene, and tert-butanol, affording 45%, 37%, and 44% yields after 8 h. The reaction carried out in NMA offered 68% yield of 1,3-diphenylimidazo[1,5-a]pyridine after 8 h, while 69% yield was observed for that carried out in benzonitrile. Among these solvents, DMF exhibited the best performance, with 71% yield being achieved after 8 h (Fig. 5).
The catalytic activity of Cu-MOF-74 in the condensation–cyclization reaction between 2-benzoyl pyridine and benzylamine to form 1,3-diphenylimidazo[1,5-a]pyridine via oxidative amination of C(sp3)–H bond was also compared with that of other homogeneous catalysts, including Cu(NO3)2, CuCl2, CuSO4, Cu(OAc)2, Cu(acac)2, CuBr2, CuBr, CuI, CuCl, Co(OAc)2, Zn(OAc)2, Mn(OAc)2, Ni(OAc)2, AgOAc, and 2,5-dihydroxyterephthalic acid. The reaction was then carried out in DMF under air at 120 °C for 8 h, using 3 equivalents of benzylamine, in the presence of 10 mol% catalyst, at 2-benzoyl pyridine concentration of 0.2 M. It was found that copper species were more active for the transformation than other metals. Indeed, the condensation–cyclization reaction afforded 22%, 15%, 13%, 19%, and 9% yields of 1,3-diphenylimidazo[1,5-a]pyridine after 8 h for the case of Co(OAc)2, Zn(OAc)2, Mn(OAc)2, Ni(OAc)2, and AgOAc as catalyst, respectively. CuBr offered low activity for the reaction, with 29% yield being detected after 8 h. The reaction using Cu(NO3)2 as catalyst, the precursor of the Cu-MOF-74, proceeded to 40% yield after 8 h. Cu(acac)2 were found to be more catalytically active than Cu(NO3)2, producing 1,3-diphenylimidazo[1,5-a]pyridine in a yield of 48% after 8 h. Among these copper salts, Cu(OAc)2 exhibited the best performance as catalyst for the condensation–cyclization reaction, with 78% yield of 1,3-diphenylimidazo[1,5-a]pyridine being achieved after 8 h (Fig. 6). In the first example of the imidazo[1,5-a]pyridine synthesis via Csp3–H amination from N-heteroaryl aldehydes or N-heteroaryl ketones and alkylamines, Zeng and co-workers reported that CuBr was more active than other copper salts.12 However, Xu and co-workers demonstrated that Cu(OAc)2 was the best catalyst for the condensation–cyclization between 2-benzoyl pyridine and benzylamine.13
To highlight the advantage of using Cu-MOF-74 as catalyst for the condensation–cyclization reaction between 2-benzoyl pyridine and benzylamine to form 1,3-diphenylimidazo[1,5-a]pyridine via oxidative amination of C(sp3)–H bond, the catalytic activity of the Cu-MOF-74 was compared with that of other MOFs including Cu(BDC), Cu2(BDC)2(DABCO), Cu3(BTC)2, Cu2(BPDC)2(BPY), Cu(OBA), Cu2(OBA)2(BPY), and Cu(INA)2. These MOFs were synthesized by solvothermal method, and characterized according to literature procedures.46,71,72,75–80 The reaction was then carried out in DMF under air at 120 °C for 8 h, using 3 equivalents of benzylamine, in the presence of 10 mol% catalyst, at 2-benzoyl pyridine concentration of 0.2 M. The reaction using Cu(BDC) catalyst could proceed to 37% yield of 1,3-diphenylimidazo[1,5-a]pyridine after 8 h. Cu2(BDC)2(DABCO), Cu3(BTC)2, and Cu2(BPDC)2(BPY) were found to be less active for the condensation–cyclization reaction than Cu(BDC), affording 33%, 22%, and 33% yields of 1,3-diphenylimidazo[1,5-a]pyridine, respectively, after 8 h. The yield of the desired product could be improved to 46% and 48%, using Cu2(OBA)2(BPY) and Cu(OBA), respectively, as catalyst. Cu(INA)2 offered higher activity than Cu(OBA), producing 1,3-diphenylimidazo[1,5-a]pyridine in a yield of 56% after 8 h. Among these Cu-MOFs, Cu-MOF-74 should be the catalyst of choice, with 71% yield being achieved after 8 h. It should be noted that the reaction using the mixture of 2,5-dihydroxyterephthalic acid and Cu(NO3)2, two precursors of the Cu-MOF-74, afforded only 38% yield after 8 h (Fig. 7). These observations would indicate that the framework structure should be important for the condensation–cyclization transformation (see Tables S1 and S2 in ESI† for detailed data).
To investigate the possibility that some of active copper sites on Cu-MOF-74 could dissolve into the reaction solution during the course of the reaction, the leaching test was then carried out for the condensation–cyclization between 2-benzoyl pyridine and benzylamine to form 1,3-diphenylimidazo[1,5-a]pyridine via oxidative amination of C(sp3)–H bond. Indeed, in several cases, these leached species in the liquid phase could contribute significantly to the total yield of the reaction, thus implying that the transformation would not proceed under real heterogeneous catalysis conditions.74 In order to check if any 1,3-diphenylimidazo[1,5-a]pyridine could be formed via homogeneous catalysis, a control experiment was carried out. The condensation–cyclization reaction was then carried out at 120 °C under air in DMF for 10 h, using 3 equivalents of benzylamine, at 2-benzoyl pyridine concentration of 0.2 M, in the presence of 10 mol% Cu-MOF-74 catalyst. Upon completion of the reaction, the Cu-MOF-74 catalyst was separated from the reaction mixture by simple centrifugation. The liquid phase was transferred to a new reactor vessel, and fresh reagents were added to the solution. The resulting mixture was then stirred for 10 h at 120 °C. Experimental results showed that no further yield of 1,3-diphenylimidazo[1,5-a]pyridine was detected in the absence of the solid Cu-MOF-74 catalyst (Fig. 8). It should be noted that the transformation could proceed to 71% yield of 1,3-diphenylimidazo[1,5-a]pyridine in the presence of Cu-MOF-74 catalyst. These observations confirmed that the condensation–cyclization between 2-benzoyl pyridine and benzylamine to form 1,3-diphenylimidazo[1,5-a]pyridine could only occur in the presence of the solid Cu-MOF-74 catalyst, and no contribution from catalytically active species soluble in the solution, if any, was detected.
 | ||
| Fig. 8 Leaching test indicated no contribution from homogeneous catalysis of active species leaching into reaction solution. | ||
To gain insights into the reaction pathway of the condensation–cyclization between 2-benzoyl pyridine and benzylamine to form 1,3-diphenylimidazo[1,5-a]pyridine via oxidative amination of C(sp3)–H bond using Cu-MOF-74 catalyst, some mechanistic studies were carried out. In the first experiment, the condensation–cyclization reaction was then carried out at 120 °C under argon in DMF for 8 h, using 3 equivalents of benzylamine, at 2-benzoyl pyridine concentration of 0.2 M, in the presence of 10 mol% Cu-MOF-74 catalyst. It was observed that performing the reaction under air should be necessary, as the transformation carried out under argon afforded only 13% yield of 1,3-diphenylimidazo[1,5-a]pyridine after 8 h. It should be noted that up to 71% yield of the desired product was achieved for the condensation–cyclization reaction after 8 h under air. However, in the presence of di-tert-butyl peroxide as the oxidant, the transformation afforded only 7% yield of 1,3-diphenylimidazo[1,5-a]pyridine after 8 h. Indeed, homocoupling by-product of benzylamine was detected in the reaction mixture under this condition. In the second experiment, the condensation–cyclization reaction was carried out under air in the presence of water (5% by volume). It was observed that water exhibited a significant impact on the performance of the Cu-MOF-74 catalyst, with only 31% yield of the desired product being recorded after 8 h. This observation confirmed that the formation of 1,3-diphenylimidazo[1,5-a]pyridine via the condensation–cyclization between 2-benzoyl pyridine and benzylamine using Cu-MOF-74 catalyst was not favored by water residue in the solvent. In the third experiment, the Cu-MOF-74 catalyst was soaked in pyridine as catalyst poison prior to use in the condensation–cyclization between 2-benzoyl pyridine and benzylamine. Under this condition, only 31% yield of 1,3-diphenylimidazo[1,5-a]pyridine was detected after 8 h. This result indicated that the strong adsorption of pyridine on the copper sites in the Cu-MOF-74 would deactivate the Cu-MOF catalyst. However, further mechanistic studies would be necessary to clarify the reaction pathway of the condensation–cyclization between 2-benzoyl pyridine and benzylamine to form 1,3-diphenylimidazo[1,5-a]pyridine via oxidative amination of C(sp3)–H bond using Cu-MOF-74 catalyst.
Although several copper salts exhibited high activity in the condensation–cyclization between 2-benzoyl pyridine and benzylamine to form 1,3-diphenylimidazo[1,5-a]pyridine via oxidative amination of C(sp3)–H bond, these homogeneous catalysts could not be reused. Under the view of green chemistry, issues that should be addressed when using solid catalysts for organic transformations should be the ease of separation as well as the deactivation and reusability of the catalysts. It would be targeted that the solid catalyst can be recovered and reused several times before it eventually deactivates completely. The Cu-MOF-74 catalyst was therefore investigated for recoverability and reusability in the condensation–cyclization reaction between 2-benzoyl pyridine and benzylamine over 8 successive runs, by repeatedly separating the Cu-MOF-74 from the reaction mixture, washing it and then reusing it. The condensation–cyclization reaction was then carried out at 120 °C under air in DMF for 8 h, using 3 equivalents of benzylamine, at 2-benzoyl pyridine concentration of 0.2 M, in the presence of 10 mol% Cu-MOF-74 catalyst. After the first run, the Cu-MOF catalyst was separated from the reaction mixture by simple centrifugation, washed with copious amounts of DMF and dichloromethane to remove any physisorbed reagents, dried 150 °C under vacuum in 2 h. The recovered Cu-MOF-74 catalyst was then reused in further reaction under identical conditions to those of the first run. It was observed that the Cu-MOF-74 catalyst could be recovered and reused many times in the condensation–cyclization between 2-benzoyl pyridine and benzylamine to form 1,3-diphenylimidazo[1,5-a]pyridine via oxidative amination of C(sp3)–H bond without a significant degradation in catalytic activity. Indeed, 67% yield of 1,3-diphenylimidazo[1,5-a]pyridine was still observed in the 8th run (Fig. 9). Moreover, XRD (Fig. 10) and FT-IR (Fig. 11) results of the reused Cu-MOF-74 also confirmed that the Cu-MOF structure could be maintained during the course of the transformation.
To investigate whether reactions occurred at outside or inside the pores, experiments using grinded Cu-MOF-74 were performed under identical conditions. The results indicated that similar reaction yields were obtained in both cases (Table 1). It is known that catalysts with smaller size often provide better reaction efficiency if reaction occurs at the external catalyst surface. Though it is possible that reactions took place inside the catalyst cavities, further spectroscopic studies are still needed to gain more insights about this issue.
| MOFs | Median size (μm) | Mean size (μm) | Reaction yield |
|---|---|---|---|
| Non-grinded Cu-MOF-74 | 29.96 | 41.09 | 70 |
| Grinded Cu-MOF-74 | 5.13 | 9.99 | 71 |
The study was then extended to the synthesis of different imidazo[1,5-a]pyridines via oxidative amination of C(sp3)–H bond using Cu-MOF-74 catalyst. The condensation–cyclization reaction was then carried out at 120 °C under air in DMF for 8 h, using 3 equivalents of benzylamine, at 2-benzoyl pyridine concentration of 0.2 M, in the presence of 10 mol% Cu-MOF-74 catalyst. The desired imidazo[1,5-a]pyridine was purified by column chromatography. It was observed that 1,3-diphenylimidazo[1,5-a]pyridine was produced in 70% isolated yield via the condensation–cyclization reaction between 2-benzoyl pyridine and benzylamine using Cu-MOF-74 catalyst. However, the presence of a chloro group on the benzene ring in benzylamine resulted in a drop in the yield of the corresponding 1,3-diphenylimidazo[1,5-a]pyridine. Indeed, 3-(2-chlorophenyl)-1-phenylimidazo[1,5-a]pyridine was obtained in 53% yield for the condensation–cyclization reaction between 2-benzoyl pyridine and 2-chlorobenzylamine using Cu-MOF-74 catalyst. Similarly, 3-(3-chlorophenyl)-1-phenylimidazo[1,5-a]pyridine and 3-(4-chlorophenyl)-1-phenylimidazo[1,5-a]pyridine were produced in 54% and 53% yields for the reaction of 2-benzoyl pyridine with 3-chlorobenzylamine and 4-chlorobenzylamine, respectively. The presence of a methoxy group on the benzene ring in benzylamine was found to accelerate the transformation slightly. In the presence of 10 mol% Cu-MOF-74 catalyst, the reaction of 2-benzoyl pyridine with 2-methoxybenzylamine could offer 61% yield of 3-(2-methoxyphenyl)-1-phenylimidazo[1,5-a]pyridine, while 70% yield of 3-(3-methoxyphenyl)-1-phenylimidazo[1,5-a]pyridine was obtained for the case of 3-methoxybenzylamine. 4-Methoxybenzylamine was more reactive towards the condensation–cyclization reaction with 2-benzoyl pyridine, producing 3-(4-methoxyphenyl)-1-phenylimidazo[1,5-a]pyridine in 73% yield. It was also observed that the reaction of 2-benzoyl pyridine with 4-aminobenzylamine could proceed to 68% yield of 4-(1-phenylimidazo[1,5-a]pyridin-3-yl)benzenamine (Table 2).
4. Conclusions
In summary, the metal–organic framework Cu-MOF-74 was synthesized by a solvothermal method, and was characterized by a variety of different techniques including XRD, SEM, TEM, FT-IR, TGA, AAS, and nitrogen physisorption measurements. The Cu-MOF could be used as an efficient heterogeneous catalyst for the condensation–cyclization reaction between 2-benzoyl pyridine and different benzylamines via oxidative amination of C(sp3)–H bond to form 1,3-diarylated imidazo[1,5-a]pyridines using air as the oxidant. The Cu-MOF-74 offered better catalytic activity for the transformation than other MOFs including Cu(BDC), Cu2(BDC)2(DABCO), Cu3(BTC)2, Cu2(BPDC)2(BPY), Cu(OBA), Cu2(OBA)2(BPY), and Cu(INA)2. The condensation–cyclization transformation could only proceed in the presence of the solid Cu-MOF catalyst, and the contribution of leached active copper species to the formation of 1,3-diarylated imidazo[1,5-a]pyridines, if any, was negligible. The solid catalyst could be separated from the reaction mixture by centrifugation, and could be recovered and reused several times for the synthesis of 1,3-diarylated imidazo[1,5-a]pyridines without a significant degradation in catalytic activity. The fact that the condensation–cyclization reaction to produce 1,3-diarylated imidazo[1,5-a]pyridines could proceed under heterogeneous catalysis conditions should be of advantages, and might be interested to the chemical industry.Acknowledgements
The Vietnam National University – Ho Chi Minh City (VNU-HCM) is acknowledged for financial support through project No. B2015-20-03.References
- D. Kim, L. Wang, J. J. Hale, C. L. Lynch, R. J. Budhu, M. MacCoss, S. G. Mills, L. Malkowitz, S. L. Gould, J. A. DeMartino, M. S. Springer, D. Hazuda, M. Miller, J. Kessler, R. C. Hrin, G. Carver, A. Carella, K. Henry, J. Lineberger, W. A. Schleif and E. A. Emini, Bioorg. Med. Chem. Lett., 2005, 15, 2129–2134 CrossRef CAS PubMed.
- D. C. Mohan, S. N. Rao, C. Ravi and S. Adimurthy, Org. Biomol. Chem., 2015, 13, 5602–5607 Search PubMed.
- L. Salassa, C. Garino, A. Albertino, G. Volpi, C. Nervi, R. Gobetto and K. I. Hardcastle, Organometallics, 2008, 27, 1427–1435 CrossRef CAS.
- M. Alcarazo, S. J. Roseblade, A. R. Cowley, R. Fernández, J. M. Brown and J. M. Lassaletta, J. Am. Chem. Soc., 2005, 127, 3290–3291 CrossRef CAS PubMed.
- F. E. Hahn, Angew. Chem., Int. Ed., 2006, 45, 1348–1352 CrossRef CAS PubMed.
- F. Shibahara, A. Kitagawa, E. Yamaguchi and T. Murai, Org. Lett., 2006, 8, 5621–5624 CrossRef CAS PubMed.
- V. S. Arvapalli, G. Chen, S. Kosarev, M. E. Tan, D. Xie and L. Yet, Tetrahedron Lett., 2010, 51, 284–286 CrossRef CAS.
- F. Shibahara, R. Sugiura, E. Yamaguchi, A. Kitagawa and T. Murai, J. Org. Chem., 2009, 74, 3566–3568 CrossRef CAS PubMed.
- J. M. Crawforth and M. Paoletti, Tetrahedron Lett., 2009, 50, 4916–4918 CrossRef CAS.
- A. Zhang, X. Zheng, J. Fan and W. Shen, Tetrahedron Lett., 2010, 51, 828–831 CrossRef CAS.
- V. K. Fulwa and V. Manivannan, Tetrahedron, 2012, 68, 3927–3931 CrossRef CAS.
- M. Li, Y. Xie, Y. Ye, Y. Zou, H. Jiang and W. Zeng, Org. Lett., 2014, 16, 6232–6235 CrossRef CAS PubMed.
- (a) H. Wang, W. Xu, Z. Wang, L. Yu and K. Xu, J. Org. Chem., 2015, 80, 2431–2435 CrossRef CAS PubMed; (b) A. Joshi, D. C. Mohan and S. Adimurthy, Org. Lett., 2016, 18, 464–467 CrossRef CAS PubMed.
- H. K. Chae, D. Y. Siberio-Perez, J. Kim, Y. Go, M. Eddaoudi, A. J. Matzger, M. O'Keeffe and O. M. Yaghi, Nature, 2004, 427, 523–527 CrossRef CAS PubMed.
- D. J. Tranchemontagne, M. O. k. Z. Ni and O. M. Yaghi, Angew. Chem., Int. Ed., 2008, 47, 5136–5147 CrossRef CAS PubMed.
- S. S. Kaye, A. Dailly, O. M. Yaghi and J. R. Long, J. Am. Chem. Soc., 2007, 129, 14176–14177 CrossRef CAS PubMed.
- H. Furukawa, N. Ko, Y. B. Go, N. Aratani, S. B. Choi, E. Choi, A. O. Yazaydin, R. Q. Snurr, M. O'Keeffe, J. Kim and O. M. Yaghi, Science, 2010, 239, 424–428 CrossRef PubMed.
- P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J. S. Chang, Y. K. Hwang, V. Marsaud, P. N. Bories, L. Cynober, S. Gil, G. Férey, P. Couvreur and R. Gref, Nat. Mater., 2010, 9, 172–178 CrossRef CAS PubMed.
- R. J. Kuppler, D. J. Timmons, Q.-R. Fang, J.-R. Li, T. A. Makal, M. D. Young, D. Yuan, D. Zhao, W. Zhuang and H.-C. Zhou, Coord. Chem. Rev., 2009, 253, 3042–3066 CrossRef CAS.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276–279 CrossRef CAS.
- J. L. C. Rowsell and O. M. Yaghi, Microporous Mesoporous Mater., 2004, 73, 3–14 CrossRef CAS.
- Z.-Q. Li, L.-G. Qiu, T. Xu, Y. Wu, W. Wang, Z.-Y. Wu and X. Jiang, Mater. Lett., 2009, 63, 78–80 CrossRef CAS.
- B. Chen, S. Xiang and G. Qian, Acc. Chem. Res., 2010, 43, 1115–1124 CrossRef CAS PubMed.
- D. T. Genna, A. G. Wong-Foy, A. J. Matzger and M. S. Sanford, J. Am. Chem. Soc., 2013, 135, 10586–10589 CrossRef CAS PubMed.
- J. A. Mason, M. Veenstra and J. R. Long, Chem. Sci., 2014, 5, 32–51 RSC.
- M.-L. Ma, C. Ji and S.-Q. Zang, Dalton Trans., 2013, 42, 10579–10586 RSC.
- N. W. C. Jusoh, A. A. Jalil, S. Triwahyono and C. R. Mamat, Appl. Catal., A, 2015, 492, 169–176 CrossRef CAS.
- M. Babazadeh, R. Hosseinzadeh-Khanmiri, J. Abolhasani, E. Ghorbani-Kalhor and A. Hassanpour, RSC Adv., 2015, 5, 19884–19892 RSC.
- N. T. S. Phan, T. T. Nguyen, Q. H. Luu and L. T. L. Nguyen, J. Mol. Catal. A: Chem., 2012, 363–364, 178–185 CrossRef CAS.
- Z.-Y. Gu, J. Park, A. Raiff, Z. Wei and H.-C. Zhou, ChemCatChem, 2014, 6, 67–75 CrossRef CAS.
- P. Valvekens, F. Vermoortelea and D. D. Vos, Catal. Sci. Technol., 2013, 3, 1435–1445 CAS.
- J. Liu, L. Chen, H. Cui, J. Zhang, L. Zhang and C.-Y. Su, Chem. Soc. Rev., 2014, 43, 6011–6061 RSC.
- (a) T. D. Le, K. D. Nguyen, V. T. Nguyen, T. Truong and N. T. S. Phan, J. Catal., 2016, 333, 94–101 CrossRef CAS; (b) T. Truong, K. D. Nguyen, S. H. Doan and N. T. S. Phan, Appl. Catal., A, 2016, 510, 27–33 CrossRef CAS.
- X. Li, Z. Guo, C. Xiao, T. W. Goh, D. Tesfagaber and W. Huang, ACS Catal., 2014, 4, 3490–3497 CrossRef CAS.
- N. T. S. Phan, T. T. Nguyen, P. Ho and K. D. Nguyen, ChemCatChem, 2013, 5, 1822–1831 CrossRef CAS.
- N. T. S. Phan, T. T. Nguyen and P. H. L. Vu, ChemCatChem, 2013, 5, 3068–3077 CrossRef CAS.
- A. Corma, H. García and F. X. Llabrés i Xamena, Chem. Rev., 2010, 110, 4606–4655 CrossRef CAS PubMed.
- A. Dhakshinamoorthy and H. Garcia, Chem. Soc. Rev., 2012, 41, 5262–5284 RSC.
- M. Yoon, R. Srirambalaji and K. Kim, Chem. Rev., 2012, 112, 1196–1231 CrossRef CAS PubMed.
- F. Zadehahmadi, S. Tangestaninejad, M. Moghadam, V. Mirkhani, I. Mohammadpoor-Baltork, A. R. Khosropour and R. Kardanpour, Appl. Catal., A, 2014, 477, 34–41 CrossRef CAS.
- T. Otto, N. N. Jarenwattananon, S. Glöggler, J. W. Brown, A. Melkonian, Y. N. Ertas and L.-S. Bouchard, Appl. Catal., A, 2014, 488, 248–255 CrossRef CAS.
- Q.-x. Luo, X.-d. Song, M. Ji, S.-E. Park, C. Hao and Y.-q. Li, Appl. Catal., A, 2014, 478, 81–90 CrossRef CAS.
- K. Leus, Y.-Y. Liu, M. Meledina, S. Turner, G. V. Tendeloo and P. V. D. Voort, J. Catal., 2014, 316, 201–209 CrossRef CAS.
- P. Wu, C. He, J. Wang, X. Peng, X. Li, Y. An and C. Duan, J. Am. Chem. Soc., 2012, 134, 14991–14999 CrossRef CAS PubMed.
- A. S. Roy, J. Mondal, B. Banerjee, P. Mondal, A. Bhaumik and S. M. Islam, Appl. Catal., A, 2014, 469, 320–327 CrossRef CAS.
- T. Truong, V. T. Nguyen, H. T. X. Le and N. T. S. Phan, RSC Adv., 2014, 4, 52307–52315 RSC.
- Y. Luan, N. Zheng, Y. Qi, J. Tang and G. Wang, Catal. Sci. Technol., 2014, 4, 925–929 CAS.
- G. Yu, J. Sun, F. Muhammad, P. Wang and G. Zhu, RSC Adv., 2014, 4, 38804–38811 RSC.
- Y.-R. Lee, Y.-M. Chung and W.-S. Ahn, RSC Adv., 2014, 4, 23064–23067 RSC.
- A. S. Roy, J. Mondal, B. Banerjee, P. Mondal, A. Bhaumik and S. M. Islam, Appl. Catal., A, 2014, 469, 320–327 CrossRef CAS.
- G. H. Dang, D. T. Nguyen, D. T. Le, T. Truong and N. T. S. Phan, J. Mol. Catal. A: Chem., 2014, 395, 300–306 CrossRef CAS.
- G. H. Dang, T. T. Dang, D. T. Le, T. Truong and N. T. S. Phan, J. Catal., 2014, 319, 258–264 CrossRef CAS.
- P. Valvekens, M. Vandichel, M. Waroquier, V. V. Speybroeck and D. D. Vos, J. Catal., 2014, 317, 1–10 CrossRef CAS.
- M. Savonnet, S. Aguado, U. Ravon, D. Bazer-Bachi, V. Lecocq, N. Bats, C. Pinel and D. Farrusseng, Green Chem., 2009, 11, 1729–1732 RSC.
- E. Pérez-Mayoral, Z. Musilová, B. Gil, B. Marszalek, M. Položij, P. Nachtigall and J. Čejka, Dalton Trans., 2012, 41, 4036–4044 RSC.
- M. Opanasenko, M. Shamzhy and J. Čejka, ChemCatChem, 2013, 5, 1024–1031 CrossRef CAS.
- N. T. S. Phan, P. H. L. Vu and T. T. Nguyen, J. Catal., 2013, 306, 38–46 CrossRef CAS.
- O. V. Zalomaeva, A. M. Chibiryaev, K. A. Kovalenko, O. A. Kholdeeva, B. S. Balzhinimaev and V. P. Fedin, J. Catal., 2013, 298, 179–185 CrossRef CAS.
- H. T. N. Le, T. V. Tran, N. T. S. Phan and T. Truong, Catal. Sci. Technol., 2015, 5, 851–859 CAS.
- I. Luz, A. Corma and F. X. L. i. Xamena, Catal. Sci. Technol., 2014, 4, 1829–1836 CAS.
- T. Maity, D. Saha and S. Koner, ChemCatChem, 2014, 6, 2373–2383 CrossRef CAS.
- Y. Jiang, J. Huang, M. Hunger, M. Maciejewski and A. Baiker, Catal. Sci. Technol., 2015, 5, 897–902 CAS.
- A. Dhakshinamoorthy, A. M. Asiri and H. Garcia, Chem. Soc. Rev., 2015, 44, 1922–1947 RSC.
- A. H. Chughtai, N. Ahmad, H. A. Younus, A. Laypkov and F. Verpoort, Chem. Soc. Rev., 2015, 44, 6804–6849 RSC.
- A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Adv. Synth. Catal., 2009, 351, 2271–2276 CrossRef CAS.
- A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Adv. Synth. Catal., 2010, 352, 711–717 CrossRef CAS.
- A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Adv. Synth. Catal., 2010, 352, 3022–3030 CrossRef CAS.
- L. Alaerts, E. Séguin, H. Poelman, F. Thibault-Starzyk, P. A. Jacobs and D. E. De Vos, Chem.–Eur. J., 2006, 12, 7353–7363 CrossRef CAS PubMed.
- I. Luz, F. X. Llabrés i Xamena and A. Corma, J. Catal., 2012, 285, 285–291 CrossRef CAS.
- I. Luz, F. X. Llabrés i Xamena and A. Corma, J. Catal., 2010, 276, 134–140 CrossRef CAS.
- D. Ruano, M. Díaz-García, A. Alfayate and M. Sánchez-Sánchez, ChemCatChem, 2015, 7, 674–681 CrossRef CAS.
- R. Sanz, F. Martínez, G. Orcajo, L. Wojtas and D. Briones, Dalton Trans., 2013, 42, 2392–2398 RSC.
- G. Langhendries, D. E. D. Vos, G. V. Baron and P. A. Jacobs, J. Catal., 1997, 187, 453–463 CrossRef.
- N. T. S. Phan and C. W. Jones, J. Mol. Catal. A: Chem., 2006, 253, 123–131 CrossRef CAS.
- L. T. L. Nguyen, T. T. Nguyen, K. D. Nguyen and N. T. S. Phan, App. Catal., A, 2012, 425–426, 44–52 CrossRef CAS.
- H. Furukawa, J. Kim, N. W. Ockwig, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2008, 130, 11650–11661 CrossRef CAS PubMed.
- K. Tan, N. Nijem, P. Canepa, Q. Gong, J. Li, T. Thonhauser and Y. J. Chabal, Chem. Mater., 2012, 24, 3153–3167 CrossRef CAS.
- N. T. S. Phan, C. K. Nguyen, T. T. Nguyen and T. Truong, Catal. Sci. Technol., 2014, 4, 369–377 CAS.
- D. Britt, H. Furukawa, B. Wang, T. G. Glover and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 20637–20640 CrossRef CAS PubMed.
- G. H. Dang, T. D. Nguyen, D. T. Le, T. Truong and N. T. S. Phan, ChemPlusChem, 2014, 79, 1129–1137 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra00852f |
| This journal is © The Royal Society of Chemistry 2016 |