Structurally diverse stilbene dimers from Gnetum montanum Markgr.: studies on the 1H chemical shift differences between dimeric stilbene epimers correlating to the relative configurations†
Yi-Ming Zhaiab,
Kun Jianga,
Shi-Jin Qua,
Hong-Feng Luoa,
Jun-Jie Tana and
Chang-Heng Tan*a
aDepartment of Natural Medicinal Chemistry, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, P. R. China. E-mail: chtan@simm.ac.cn; Fax: +86-21-50806728; Tel: +86-21-50806728
bUniversity of Chinese Academy of Sciences, No. 19A Yuquan Road, Beijing 100049, P. R. China
First published on 11th May 2016
Abstract
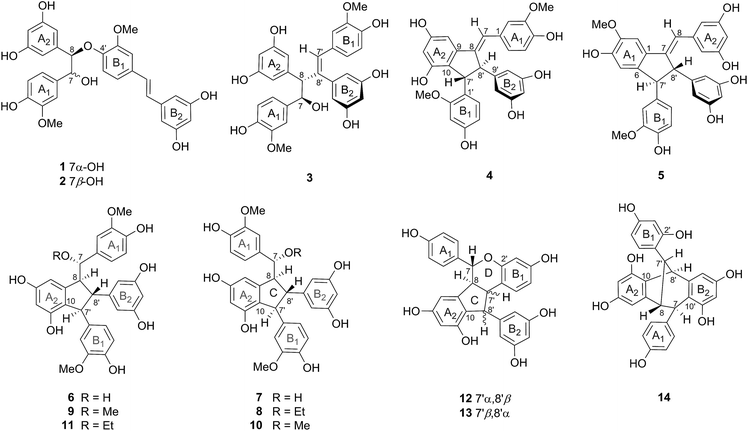
Twenty-seven structurally diverse stilbenoids, comprising nine new dimeric stilbenoids, gnemontanins A (1), B (2), C (4), D (5), E (7), F (8), and G (12), as well as (−)-gnetuhainin P (3) and (−)-gnetuhainin I (6), were isolated from the caulis of Gnetum montanum Markgr. The structures of those new compounds were elucidated by mean of extensive analysis of MS, 1D and 2D NMR spectroscopic data. Naturally occurring stilbene dimers polymerized through one bond of 8-O-4′ (1 and 2) as well as two bonds of 7-8′ and 6-7′ (5) are reported for the first time. The 1H chemical shift differences between dimeric stilbene epimers on C-7 were analysed and summarized, which could be used as a diagnostic to determine the relative configuration of C-7 of several types' dimeric stilbenes. Two misassigned structures of gnetuhainins D and E were revised as gnetuhainin S and 12, respectively.
Introduction
Stilbenoids are a class of plant polyphenols characteristic of monomeric or polymeric 1,2-diarylethylene, which have attracted intense interest for their intricate structures and diverse biological activities.1–4 Gnetum montanum Markgr. (Gnetaceae) is an evergreen vine indigenous to southern China and Southeast Asia.5 The leaves, stems and rhizomes of this plant contain abundant stilbenes and stilbene-related alkaloids, and are used as folk medicine for the treatment of lumbago, rheumatic arthralgia and chronic bronchitis.5–8 In our continuing search for bioactive and diverse ingredients from medicinal plants,9–11 twenty-seven stilbenoids, including 24 stilbene dimers polymerized via diverse modes, such as one bond of 8-O-4′ (1 and 2) or 8-8′ (3), two bonds forming an indane (4–11, 22) or a benzofuran (15–21), and three bonds forming an indano[1,2-c]-chromene (12 and 13), a dibenzobicyclo[3.2.1]octane (14), an indano[2,1-a]indane (23), or a benzo[6,7]cyclohepta[1,2,3-cd]benzofuran (24), were isolated from the caulis of the title plant. Among them, gnemontanins A (1), B (2), C (4), D (5), E (7), F (8), and G (12), as well as (−)-gnetuhainin P (3) and (−)-gnetuhainin I (6) (Chart 1) were structurally elucidated as new compounds by extensive analysis of MS, 1D and 2D NMR spectroscopic data. The 1H chemical shift differences between dimeric stilbene epimers on C-7 were analysed and summarized. Herein, the isolation, identification, and structural characterization of the new dimeric stilbenoids, as well as the key 1H NMR shifts correlating to the configuration of C-7 of several types' dimeric stilbenes are described.Results and discussion
8-O-4′ Stilbene dimers
Gnemontanin A (1) was obtained as a pale amorphous powder. Its molecular formula of C30H28O9 was determined by HRESIMS ion [M + Na]+ at m/z 555.1632 (calcd 555.1631). Its 1H and 13C NMR spectra (Tables 1 and 2), with the aid of 1H–1H COSY and HSQC spectra, showed 30 carbon and 22 carbon-bearing hydrogen signals for two 1,2,4-trisubstituted benzene rings (ring A1: δH 6.69, 6.66 and 6.66, each br s, correlated with δC 112.3, 115.5 and 121.2, respectively, in the HSQC spectrum; ring B1: δH 6.73, 6.87 and 7.15, ABX model, J = 8.4, 2.0 Hz), two symmetric 1,3,5-trisubstituted benzene rings (ring A2: δH 6.11 and 6.09, A2X system, J = 2.1 Hz; ring B2: δH 6.45 and 6.16, A2X system, J = 2.1 Hz), an E-double bond (δH 6.92 and 6.83, each d, J = 16.2 Hz), two vicinal oxygenated methines (δH 4.92 and 4.84, each d, J = 7.6 Hz; δC 79.1 and 88.0), and two methoxyls (δH 3.97 and 3.72). The observed four aromatic rings and one double bond combined with 17 unsaturated degrees in the molecule demonstrated 1 to be a single bond connective stilbene dimer. The HMBC interactions established the jointed mode of 8-O-4′, and the two methoxyls on C-3 and C-3′, respectively, based on observed cross-peaks of H-10(14)/C-8, H-8/C-4′, as well as 3-OMe/C-3, and 3′-OMe/C-3′ (Fig. 1). The relative configuration of 7,8-threo for 1 was determined by the NOESY correlations of H-7/H-10(14), H-7/H-5′, and H-8/H-2(6) (Fig. 1). Accordingly, the structure of gnemontanin A (1) was elucidated as threo-7,8-dihydroxydihydroisorhapontigenin(8-O-4′)isorhapontigenin.| No. | 1a | 2a | 3a | 4b | 5b | 6b | 7b |
|---|---|---|---|---|---|---|---|
| a Recorded in methanol-d4.b Recorded in acetone-d6. | |||||||
| 2 | 6.69 (br s) | 6.95 (d, 1.7) | 6.85 (d, 1.7) | 6.85 (br s) | 7.00 (br s) | 6.55 (d, 1.6) | 6.93 (d, 1.7) |
| 5 | 6.66 (br s) | 6.68 (d, 8.1) | 6.63 (d, 8.2) | 6.66 (d, 8.2) | 6.55 (br s) | 6.67 (d, 8.0) | 6.68 (d, 8.0) |
| 6 | 6.66 (br s) | 6.71 (dd, 8.1, 1.7) | 6.74 (dd, 8.2, 1.7) | 6.75 (dd, 8.2, 2.0) | — | 6.51 (dd, 8.0, 1.6) | 6.64 (dd, 8.0, 1.7) |
| 7 | 4.84 (d, 7.6) | 4.93 (covered) | 5.06 (d, 9.7) | 6.95 (br s) | — | 4.50 (d, 8.0) | 4.56 (d, 7.3) |
| 8 | 4.92 (d, 7.6) | 5.16 (d, 4.5) | 3.71 (d, 9.7) | — | 7.10 (br s) | 3.38 (dd, 8.0, 3.5) | 3.48 (dd, 7.3, 4.5) |
| 10 | 6.11 (d, 2.1) | 6.18 (d, 2.2) | 6.08 (d, 2.1) | — | 6.43 (d, 2.0) | — | — |
| 12 | 6.09 (t, 2.1) | 6.14 (t, 2.2) | 6.04 (t, 2.1) | 6.30 (d, 2.0) | 6.18 (d, 2.0) | 6.32 (d, 1.9) | 6.22 (d, 1.9) |
| 14 | 6.11 (d, 2.1) | 6.18 (d, 2.2) | 6.08 (d, 2.1) | 6.76 (d, 2.0) | 6.43 (d, 2.0) | 6.62 (d, 1.9) | 5.98 (br s) |
| 2′ | 7.15 (d, 2.0) | 7.11 (d, 2.0) | 6.58 (d, 1.6) | — | 6.93 (br s) | 6.64 (d, 1.6) | 6.61 (d, 1.8) |
| 3′ | — | — | — | 6.45 (d, 2.0) | — | — | — |
| 5′ | 6.73 (d, 8.4) | 6.68 (d, 8.4) | 6.61 (d, 8.2) | 6.13 (d, 8.2, 2.2) | 6.65 (d, 8.4) | 6.72 (d, 8.1) | 6.67 (d, 8.1) |
| 6′ | 6.87 (dd, 8.4, 2.0) | 6.85 (dd, 8.4, 2.0) | 6.66 (dd, 8.2, 1.6) | 6.25 (dd, 8.2) | 6.62 (br d, 8.4) | 6.46 (dd, 8.1, 1.6) | 6.46 (dd, 8.1, 1.8) |
| 7′ | 6.92 (d, 16.2) | 6.91 (d, 16.2) | 6.78 (s) | 4.55 (br s) | 4.06 (br s) | 4.23 (d, 2.9) | 4.22 (d, 4.2) |
| 8′ | 6.83 (d, 16.2) | 6.81 (d, 16.2) | — | 4.07 (br s) | 4.00 (br s) | 2.96 (dd, 3.5, 2.9) | 3.41 (dd, 4.5, 4.2) |
| 10′(14′) | 6.45 (d, 2.1) | 6.44 (d, 2.2) | 6.09 (d, 2.2) | 6.44 (d, 2.0) | 6.32 (d, 2.0) | 5.91 (d, 2.1) | 6.13 (d, 2.2) |
| 12′ | 6.16 (t, 2.1) | 6.16 (t, 2.2) | 6.25 (t, 2.2) | 6.18 (t, 2.0) | 6.14 (t, 2.0) | 6.12 (t, 2.1) | 6.16 (t, 2.2) |
| 3-OMe | 3.72 (s) | 3.79 (s) | 3.72 (s) | 3.57 (s) | 3.84 (s) | 3.64 (s) | 3.72 (s) |
| 3′-OMe | 3.97 (s) | 3.89 (s) | 3.48 (s) | 3.94 (s) | 3.73 (s) | 3.72 (s) | 3.70 (s) |
| No. | 1a | 2a | 3a | 4b | 5b | 6b | 7b |
|---|---|---|---|---|---|---|---|
| a Recorded in methanol-d4.b Recorded in acetone-d6. | |||||||
| 1 | 132.6 | 133.1 | 136.4 | 130.3 | 130.1 | 136.7 | 138.6 |
| 2 | 112.3 | 112.8 | 111.9 | 112.2 | 111.4 | 111.4 | 111.9 |
| 3 | 148.3 | 148.3 | 147.4 | 148.0 | 147.2 | 148.0 | 147.8 |
| 4 | 147.0 | 146.9 | 146.1 | 146.4 | 147.2 | 146.2 | 146.4 |
| 5 | 115.5 | 115.1 | 114.9 | 115.5 | 117.1 | 115.0 | 114.7 |
| 6 | 121.2 | 121.6 | 120.7 | 123.4 | 127.1 | 120.4 | 121.4 |
| 7 | 79.1 | 78.2 | 75.9 | 122.9 | 136.2 | 77.2 | 77.2 |
| 8 | 88.0 | 86.0 | 64.8 | 143.2 | 125.7 | 61.8 | 62.1 |
| 9 | 141.7 | 141.4 | 143.9 | 148.0 | 144.2 | 148.9 | 147.3 |
| 10 | 107.3 | 107.5 | 108.5 | 123.2 | 105.1 | 122.3 | 123.1 |
| 11 | 159.3 | 159.1 | 158.5 | 155.9 | 159.2 | 155.0 | 154.9 |
| 12 | 103.0 | 102.8 | 101.4 | 103.4 | 102.4 | 102.3 | 102.2 |
| 13 | 159.3 | 159.1 | 158.5 | 159.6 | 159.2 | 158.8 | 158.6 |
| 14 | 107.3 | 107.5 | 108.5 | 98.4 | 105.1 | 106.1 | 105.5 |
| 1′ | 132.7 | 132.3 | 130.4 | 125.2 | 138.6 | 138.1 | 136.0 |
| 2′ | 110.9 | 111.2 | 112.7 | 158.5 | 111.9 | 112.2 | 111.9 |
| 3′ | 151.1 | 151.1 | 147.5 | 99.5 | 148.0 | 147.7 | 148.0 |
| 4′ | 148.6 | 148.7 | 146.1 | 157.9 | 145.9 | 145.5 | 145.4 |
| 5′ | 117.2 | 116.5 | 115.1 | 107.1 | 115.5 | 115.2 | 115.2 |
| 6′ | 120.9 | 120.9 | 123.9 | 128.1 | 120.6 | 120.8 | 120.9 |
| 7′ | 129.2 | 129.2 | 128.1 | 50.5 | 53.3 | 55.9 | 56.7 |
| 8′ | 128.4 | 128.1 | 141.8 | 59.4 | 52.3 | 59.4 | 59.0 |
| 9′ | 141.0 | 141.0 | 145.1 | 149.0 | 146.7 | 150.6 | 151.0 |
| 10′ | 105.9 | 105.9 | 108.8 | 106.9 | 107.0 | 106.0 | 106.3 |
| 11′ | 159.7 | 159.6 | 159.5 | 159.3 | 159.3 | 159.1 | 159.2 |
| 12′ | 102.9 | 102.9 | 101.9 | 101.2 | 101.7 | 101.0 | 101.0 |
| 13′ | 159.7 | 159.6 | 159.5 | 159.3 | 159.3 | 159.1 | 159.2 |
| 14′ | 105.9 | 105.9 | 108.8 | 106.9 | 107.0 | 106.0 | 106.3 |
| 3-OMe | 56.3 | 56.2 | 56.2 | 55.5 | 56.2 | 56.0 | 56.2 |
| 3′-OMe | 56.7 | 56.9 | 55.5 | 56.0 | 56.4 | 56.3 | 56.1 |
Gnemontanin B (2) shared the same molecular formula of C30H28O9 with 1 based on the HRESIMS and NMR data. Its 1H and 13C NMR spectra (Tables 1 and 2) displayed signals for the same fragments with those of 1, such as four aromatic rings (two 1,2,4-trisubstituted and two 1,3,5-trisubstituted), a trans-double bond, two vicinal methines (3J = 4.5 Hz), and two methoxyls. Analysis of 2D NMR spectra (1H–1H COSY, HSQC and HMBC, ESI Fig. S12–S14†) disclosed 2 to have the same gross structure with 1.
According to the possible staggered conformers with intramolecular hydrogen bonding of those vicinal diols,12 the Newman projections along C7–C8 bond of 1 and 2 (Fig. 2) showed large/small J7,8 for threo-/erythro-form, and the different shielded effect from the neighbour aryls, which interpreted the upfield shifts of H-7 and H-8 of 1 in comparison with those of 2. The above evidence illustrated 2 to be the 7,8-erythro epimer of 1, i.e., erythro-7,8-dihydroxydihydro-isorhapontigenin(8-O-4′)isorhapontigenin.
Gnemontanins A (1) and B (2) are the first example of 8-O-4′ jointed stilbene dimers obtained as natural forms. Shi et al. reported 7-O-methyl ethers of 1 and 2 that were synthesized from isorhapontigenin under treated with silver acetate in methanol,12 which indicated isorhapontigenin to be the probable biogenetic precursor of 1 and 2.
8-8′ Stilbene dimer
Stilbene 3, [α]25D −8.3 (c 0.06, MeOH), showed the molecular formula of C30H28O9 as deduced from the HRESIMS ([M − H]− ion at m/z 531.1668, calcd 531.1655). The 1H NMR spectrum of 3 (Table 1) displayed signals for two sets of ABX aromatic protons (ring A1: δH 6.63, 6.74 and 6.85, J = 8.2, 1.7 Hz; ring B1: δH 6.61, 6.66 and 6.58, J = 8.2, 1.6 Hz), two sets of A2X aromatic protons (ring A2: δH 6.08 and 6.04, J = 2.1 Hz; ring B2: δH 6.25 and 6.09, J = 2.2 Hz), one olefinic proton (δH 6.78, s), two vicinal methines (δH 5.06 and 3.71, J = 9.7 Hz), and two methoxyls (δH 3.72 and 3.48). Compared the NMR data with those of 1, the important differences were the absence of an olefinic hydrogen and upfield 1H and 13C shifts of one methine (δH 3.71 and δC 64.8, CH-8) in 3. The above evidence suggested 3 to be an isorhapontigenin dimer jointed with single C–C bond. The HMBC correlations of H-10(14)/C-8, H-8/C-8′, and H-10′(14′)/C-8′ indicated the 8-8′ dimerization (Fig. 1). The relative configuration of 7,8-threo and 7′Z of 3 were determined by the large coupling constant between H-7 and H-8 (J = 9.7 Hz) and NOESY correlations of H-7/H-7′ and H-7/H-10(14), as well as H-2′/H-10′(14′), respectively (Fig. 1). Stilbene 3 had the same relative structure and opposite optical rotation with gnetuhainin P ([α]25D +6.6), an 8-8′ isorhapontigenin dimer from G. hainanense.13 Stilbenoid 3 was hence determined to be (−)-gnetuhainin P.The 7′Z-configuration of 3 was also in agreement with the upfield shifts of H-7′ and protons of rings B1 and B2, due to the broken conjugated system and enhancive shielded effects in contrast with those of E-form analogues such as 1 and 2.
1-Benzylidene-2,3-diaryl-indane-type stilbene dimers
Stilbene 4 was obtained as a brown amorphous powder with [α]25D +69.3 (c 0.04, MeOH). It had the molecular formula of C30H26O8 as judged by the HRESIMS [M − H]− ion at m/z 513.1548 (calcd 513.1549) in combination with the 13C NMR data (30 carbons and 20 carbon-bearing hydrogens). The 1H and 13C NMR spectra (Tables 1 and 2) showed signals for four aromatic rings (ring A1: δH 6.66, 6.75 and 6.85, ABX coupled; ring A2: δH 6.76 and 6.30, meta-coupled; ring B1: δH 6.25, 6.13 and 6.45, ABX model; ring B2: δH 6.44 and 6.18, A2X system), one olefinic proton (δH 6.95, br s; δC 122.9), two aryl-bearing methines (δH 4.55 and 4.07, each br s), and two methoxyls (δH 3.94 and 3.57). In the HMBC spectrum (Fig. 3), obvious long-range correlations of H-7′ with C-2′, C-11 and C-8, H-8′ with C-7, C-1′, and C-10′, and H-7 with C-2, C-9 and C-8′ were observed, indicating a dimeric stilbene linked through 8-8′ and 10-7′. Two methoxyls were assigned on C-3 (δ 148.0) and C-2′ (δ 158.5) based on the HMBC cross-peaks and 13C shifts. The 7E-double bond and trans-orientated H-7′ and H-8′ were established by NOESY cross-peaks of H-7/H-14, H-7′/H-10′(14′) and H-8′/H-6′, respectively (Fig. 3). The relative structure of gnemontanin C (4) was therefore elucidated to be 2′-O-methylgnetuhainin J.14Gnemontanin C (4) possesses the same carbon skeleton with that of (−)-ampelopsin D, whose absolute configuration was determined to be 7′S,8′S with negative optical rotation ([α]22D −5.0) and positive cotton effects (CEs) at 237 nm and 272 nm, as well as negative CE at 314 nm in the circular dichroism (CD) spectrum.15,16 Based on the opposite optical rotation ([α]25D +69.3) and CD signs at 235 nm (−6.55 mdeg) and 314 nm (+6.29 mdeg), the absolute configuration of 4 was elucidated to be 7′R,8′R.
Gnemontanin D (5) isolated as a pink amorphous powder with [α]25D −3.9 (c 0.05, MeOH), which was an isomer of 4 as judged by HRESIMS ion [M − H]− at m/z 513.1545. Analysis of the 1H and 13C NMR spectra (Tables 1 and 2) discovered the existences of four phenyls (two symmetric 1,3,5-trisubstituted, one 1,2,4,5-tetrasubstituted, and one 1,2,4-trisubstituted), a trisubstituted conjugated double bond (δH 7.10, br s), two aryl-bearing methines (δH 4.06 and 4.00, each br s), and two methoxyls (δH 3.84 and 3.73). Further analysis of HMBC and NOESY spectra (Fig. 4) indicated 5 to be an 8-7′ and 7-6′ connected isorhapontigenin dimer with relative configuration of 7E and 7′,8′-trans. However, the CD spectrum of 5 didn't offer significant information correlating to the absolute configuration. Gnemontanin D (5) is the first instance of 7-8′ and 6-7′ connected stilbene dimer, representing a new polymerized mode.
The 7E-configurations of 4 and 5 could be easily deduced from the distinct downfield shift of the olefinic proton (H-7 or H-8) in contrast with that of 7Z-isomer, e.g., parthenocissin A (δH 6.31, br s), due to the broken conjugated system in the latter.17
1-Hydroxybenzyl-2,3-diaryl-indane-type stilbene dimers
Stilbene 6 was obtained as a brown amorphous powder with [α]25D −8.5 (c 0.06, MeOH), which had the molecular formula of C30H28O9 as deduced from its HRESIMS [M − H]− ion at m/z at 531.1658 (calcd 531.1655). Its 1H and 13C NMR spectra (Tables 1 and 2) showed 30 C-atom and 21 carbon-bearing H-atom signals for four aromatic rings (ring A1: δH 6.67, 6.51, and 6.55, ABX, J = 8.0, 1.6 Hz; ring A2: δH 6.32, and 6.62, meta-coupled, J = 1.9 Hz; ring B1: δH 6.72, 6.46 and 6.64, ABX, J = 8.1, 1.6 Hz; ring B2: δH 5.91 and 6.12, A2X, J = 2.1 Hz), along with four methines whose protons coupled in the order (δH 4.50, 3.38, 2.96 and 4.23, J = 8.0, 3.5 and 2.9 Hz), and two methoxyls (δH 3.72 and 3.64). The above NMR data of 6 were identical with those of gnetuhainin I ([α]25D +18.5; C30H28O9),18 indicating 6 to be (−)-enantiomer of gnetuhainin I.Gnemontanin E (7), [α]25D −8.9 (c 0.05, MeOH), exhibited the same molecular formula of C30H28O9 with 6 (HRESIMS [M − H]− m/z 531.1658). The 1H and 13C NMR spectra (Tables 1 and 2) displayed signals for the same moieties with those of 6, such as four aromatic rings (ring A1: δH 6.68, 6.64 and 6.93, ABX, J = 8.0, 1.7 Hz; ring A2: δH 6.22, and 5.98, meta-coupled, J = 1.9 Hz; ring B1: δH 6.67, 6.46 and 6.61, ABX, J = 8.1, 1.8 Hz; ring B2: δH 6.13 and 6.16, A2X, J = 2.2 Hz), four methines arraying in sequence (δH 4.56, 3.48, 3.41 and 4.22, J = 7.3, 4.5 and 4.2 Hz), and two methoxyls (δH 3.72 and 3.70). The HMBC experiments established the same gross structure with that of 6 as judged by long-range correlations of H-7′/C-9, C-11, C-2′ and C-6′, H-8′/C-7, C-8, C-9 and C-10′(14′), as well as H-8/C-1, C-9 and C-8′ (Fig. 5). In contrast with the 1H NMR data of 6, 7 had upshifted protons of ring A2 and downfield shifts of rings A1 and B2, and H-8′. The finding indicated rings A1 and A2 being cis-oriented (H-14 and H-12 being shielded by ring A1) in 7 but not trans-form as in 6 (H-2′, H-6′ and H-8′ being shielded by ring A1, and H-2 and H-6 being shielded by ring B2) (Fig. 6. More examples see ESI Tables S1 and S2†). This consequence was also confirmed by the NOESY correlations of H-2/H-14, H-7′/H-10′(14′), H-8′/H-2′ and H-6′, as well as H-8/H-10′(14′) (Fig. 5). Accordingly, gnemontanin E (7) was determined to be 7-epimer of (−)-gnetuhainin I.
Gnemontanin F (8) possessed the molecular formula C32H32O9 as determined by the HRESIMS [M + Na]+ ion at m/z 583.1945 (calcd 583.1944). The 1H and 13C NMR data of 8 (Tables 3 and 4) were close similar to those of 7 except for an additional ethoxyl (δH 3.24 and 3.04, each 1H, dq, J = 9.3, 7.0 Hz; 0.99, 3H, t, J = 7.0 Hz) presence in the former, suggesting an ethoxylated derivative of 7. The ethoxyl was assigned on C-7 based on the HMBC cross-peaks of EtO (δH 3.24 and 3.04) with C-7 (δC 85.4), which was in consistence with the downfield shift of C-7 (Δδ +8.2) and upshifted C-8 (Δδ −1.2) in contrast with those of 7 due to the etherification effect and γ-gauche effect, respectively.
| No. | 8b | 12 | 14 |
|---|---|---|---|
| a Recorded in 500 MHz (8) or 400 MHz (12 and 14).b The remaining δH: 3.73 (3H, s, 3-OMe), 3.69 (3H, s, 3′-OMe), 3.24 and 3.04 (each 1H, dq, 9.3, 7.0, 7-OCH2CH3), 0.99 (3H, t, 7.0, 7-OCH2CH3). | |||
| 2 | 6.85 (d, 1.7) | 7.14 (d, 8.6) | 7.09 (d, 8.5) |
| 3 | — | 6.81 (d, 8.6) | 6.70 (d, 8.5) |
| 5 | 6.71 (d, 8.0) | 6.81 (d, 8.6) | 6.70 (d, 8.5) |
| 6 | 6.60 (dd, 8.0, 1.7) | 7.14 (d, 8.6) | 7.09 (d, 8.5) |
| 7 | 4.07 (d, 7.8) | 4.67 (d, 8.7) | 4.18 (d, 1.7) |
| 8 | 3.43 (dd, 7.8, 4.5) | 3.53 (dd, 8.7, 7.5) | 3.40 (br s) |
| 12 | 6.22 (d, 2.0) | 6.12 (d, 2.1) | 6.04 (d, 1.9) |
| 14 | 5.93 (br s) | 5.60 (d, 2.1) | 6.59 (d, 1.9) |
| 2′ | 6.54 (d, 1.8) | — | — |
| 3′ | — | 6.30 (br s) | 6.28 (d, 2.4) |
| 5′ | 6.68 (d, 8.1) | 6.29 (br d, 7.7) | 6.03 (dd, 8.4, 2.4) |
| 6′ | 6.44 (dd, 8.1, 1.8) | 6.64 (d, 7.7) | 6.57 (d, 8.4) |
| 7′ | 4.23 (d, 4.0) | 3.49 (covered) | 3.91 (br s) |
| 8′ | 3.36 (dd, 4.5, 4.0) | 4.10 (d, 7.5) | 4.14 (br s) |
| 10′(14′) | 6.16 (d, 2.0) | 6.26 (br s) | 6.44 (d, 2.4) |
| 12′ | 6.18 (t, 2.0) | 6.26 (br s) | 6.14 (d, 2.4) |
| No. | 8b | 12 | 14 | |||
|---|---|---|---|---|---|---|
| Unit A | Unit B | Unit A | Unit B | Unit A | Unit B | |
| a Recorded in 125 MHz (8) or 100 MHz (12 and 14).b The remaining δC 56.2 (q, 2C, 3 and 3′-OMe), 64.1 (t, 7-OCH2CH3), 15.5 (q, 7-OCH2CH3). | ||||||
| 1 | 132.9 | 138.5 | 131.5 | 116.5 | 138.4 | 121.6 |
| 2 | 112.2 | 111.8 | 129.9 | 156.1 | 130.0 | 157.1 |
| 3 | 148.1 | 148.1 | 115.6 | 103.6 | 115.4 | 102.8 |
| 4 | 147.0 | 145.6 | 158.1 | 157.5 | 156.0 | 157.1 |
| 5 | 114.9 | 115.3 | 115.6 | 109.1 | 115.4 | 106.5 |
| 6 | 122.5 | 121.0 | 129.9 | 130.8 | 130.0 | 129.1 |
| 7 | 85.4 | 56.2 | 78.9 | 49.9 | 47.3 | 45.2 |
| 8 | 60.9 | 59.4 | 49.0 | 57.2 | 55.7 | 49.3 |
| 9 | 146.8 | 150.5 | 145.8 | 147.4 | 148.3 | 148.6 |
| 10 | 123.1 | 106.5 | 122.5 | 107.5 | 127.9 | 113.4 |
| 11 | 155.1 | 159.4 | 155.0 | 159.4 | 158.3 | 157.7 |
| 12 | 102.4 | 101.3 | 102.7 | 101.6 | 101.6 | 101.5 |
| 13 | 158.7 | 159.4 | 158.2 | 159.4 | 153.2 | 156.9 |
| 14 | 106.0 | 106.5 | 105.1 | 107.5 | 103.7 | 105.7 |
Stilbenes 9–11 were identified to be 7-O-methylgnetuhainin I (9, [α]25D −8.3), 7-O-methylgnemontanin E (10, [α]25D −8.1), and 7-O-ethylgnetuhainin I (11, [α]25D −7.0), respectively, based on their MS and analysis of NMR data (ESI Table S1†), which were previous reported as lehmbachols A–C (9–11) with uncertain C-7 configuration.19 Notably, the 1H assignment of δH ca. 4.8 ppm for H-10′(14′) and H-12′ of 10 therein was apparently incorrect, which was actually the water residue in methanol-d4.19 Two pairs of C-7 epimers, 10/9 and 8/11, showed the similar 1H chemical shift differences with that 7/6 (ESI Table S1†).
Indeno[1,2-c]chromene-type stilbene dimer
Gnemontanin G (12) was obtained as a yellow amorphous powder. The HRESIMS spectrum indicated the molecular formula of C28H22O7 ([M + Na]+ m/z 493.1267, calcd 493.1263) with 18 unsaturated degrees. The 1H NMR spectrum (Table 3) exhibited the presence of four methines which coupled in sequence in the 1H–1H COSY spectrum (δH 4.67, 3.53, 3.49 and 4.10, J = 8.7, 7.5 and 7.5 Hz), and four benzene rings composing of 12 protons (ring A1: δH 7.14 and 6.81, A′ABB′ model, J = 8.6 Hz; ring A2: δH 6.12 and 5.60, meta-coupled, J = 2.1 Hz; ring B1: δH 6.64, 6.29 and 6.30, ABX model, J = 7.7 Hz and br s; ring B2: δH 6.26, 3H), suggesting a three bonds connective stilbene dimer. In the HMBC spectrum (Fig. 7), obvious 3JCH long range correlations were observed between H-2(6)/C-7, H-14/C-8, H-6′/C-7′, and H-10′(14′)/C-8′, indicating that rings A1, A2, B1 and B2 were attached on C-7, C-8, C-7′, and C-8′, respectively. The polymerized mode of 7-O-2′, 8-7′ and 10-8′ was established by HMBC correlations of H-7/C-2′ and C-7′, H-8/C-7′ and C-8′, as well as H-8′/C-10 and C-11. The relative configuration was established as rel-7R,8S,7′S,8′R by NOESY correlations of H-7/H-8′, H-7′/H-10′(14′), and H-8′/H-2′ (Fig. 7).Two stilbene dimers named as gnetuhainins E (12a) and D (13a) had almost superimposed NMR data with those of 12 and gnetuhainin S (13), respectively (Fig. 8).20,21 It is evidently that the reported structures of 12a and 13a were not exact and should be revised as 12 and 13, respectively, based on following reasons: (i) as above analysis on the 1H chemical shift differences between 6 and 7, H-14 at δ 5.59/5.47 in 12a/13a indicated the cis- but not trans-oriented rings A1 and A2; (ii) the 1H NMR differences between 13a and macrostachyol C (authors described the structure as 7-O-methyl of 13a)22 are obviously mismatched with the alteration of their structures (ESI Table S3†); (iii) the coupling constant J7′,8′ = 8.4/10.5 Hz of 12a/13a are far larger than the other analogues (J7′,8′ < 4.6 Hz, ESI Tables S1–S4†); finally, the mixture of 12a and 13a showed positive HRFABMS ion at m/z 471.1465 as authors' description, which was actually the ion of [M + H]+ but not misassigned [M + H − H2O]+.21
Diaryl-dibenzobicyclo[3,2,1]octadiene-type stilbene dimer
Stilbene 14, [α]25D +22.4 (c 0.05, MeOH), was isolated as a pale white amorphous powder. All differences in the 1H NMR spectra of 14 (C28H22O7) and ampelopsin F (14b, C28H22O6) could be ascribed to the additional presence of a hydroxyl at C-2′ of the former.23 Upon comparison of the spectra of both, significant shifts were observed only for those signals due to H-3′, H-5′, H-6′ and H-7′ (ΔδH −0.28, −0.53, −0.21 and +0.27, respectively, ESI Table S5†). This inference was firmly confirmed by analysis of 2D NMR spectra (1H–1H COSY, HSQC, HMBC and NOESY, ESI Fig. S74–S77†). It is puzzling that 14 possesses opposite optical rotation and similar CD curve with that of (−)-14b (+CEs at 240 nm and 250 nm, and −CEs at 220 nm and 280 nm),24 the absolute configuration of 14 is still uncertain.Tanaka et al. reported the same structure, named as 2b-hydroxy-ampelopsin F (14a, [α]25D +12), with 14.25 The 1H NMR spectrum of 14a showed the marked more upfield H-14 (δ 5.68) and H-3(5) (δ 6.17) than those of 14 and 14b (ESI Table S5†). The similar distinction was also observed between 14b and its C-7 epimer, isoampelopsin F.23 The latter showed downshifted H-5, H-6 and H-7, as well as upfield shifts of H-2, H-3 and H-14, due to the cis-oriented rings A1 and A2 being severely shielded/deshielded from each other, and loss of the shielded effect of H-7 from ring A2 in the case of 14 (ESI Table S5†). From this viewpoint, 14a is inclined to be an atropisomer of gnetuhainin C (=2′-hydroxyisoampelopsin F)26 rather than 14.
The other known stilbenoids (ESI Chart S1†) were identified as stilbene monomers: resveratrol,7 gnetol,7 and isorhapontigenin;7 dimmers: (−)-ε-viniferin (15, [α]25D −33.9),7 rac-bisisorhapontigenin A (16, [α]25D 0),6 rac-gnetuhainin A (17, [α]25D 0),21 (−)-cis-ε-vineferin (18, [α]25D −38.6),27 gnetin C (19, [α]25D −28.3),28 gnetin D (20, [α]25D −2.0, probable inequality mixture of enantiomers),28 shegansu B (21, [α]25D +4.6),7 (−)-gnetulin (22, [α]25D −16.7),7 gneafricanin F (23, [α]25D 0),29 and (−)-ampelopsin A (24, [α]25D −137.3).30
These isolates were evaluated the cytotoxicities against human breast cancer cell line MCF-7 and human pancreatic cell line PANC-1 in vitro by MTT method. However, all stilbenoids were inactive (IC50 > 10 μM) for both cell lines.
Experimental
General experimental procedures
Optical rotations were measured on a Perkin-Elmer 341 polarimeter. UV spectra were recorded on a Varian Cary 50 spectrophotometer and IR on a Thermo Nicolet JS5 spectrophotometer. CD spectra were measured by a JASCO J-810 spectropolarimeter. ESIMS and HRESIMS spectra were obtained via a Bruker Esquire 3000 plus and a Waters Q-TOF-Ultima spectrometer, respectively. NMR data were carried out on a Bruker Advance III 500 or a Varian Mercury Plus, and chemical shifts were referenced to the residual solvent peaks (δH 3.31 and δC 49.0 for methanol-d4, δH 2.05 and δC 29.8 for methyl of acetone-d6). Silica gel (200–300 mesh, Qingdao Marine Chemical Factory, Qingdao, P. R. China), Sephadex LH-20 (Pharmacia Biotech AB, Uppsala, Sweden), and MCI Gel Chp20p (Mitsubishi Chemical, Tokyo, Japan) were used for column chromatography (CC). Semi-preparative HPLC was run on a Waters HPLC system (Waters) with Waters-2545-HPLC pump, Waters-2489 detector, and Xbridge-C18 or YMC-C18 column (5 μm, i.d. 10 mm × 250 mm).Plant material
The chopped caulis of G. montanum were purchased from the Herbal Medicine Fair of Hechi in October, 2014 (collected in Hechi city, Guangxi Province, P. R. China), and were identified by Professor Da-Yuan Zhu of Shanghai Institute of Materia Medica. A voucher sample (no. 20141002) was deposited with the Herbarium of Shanghai Institute of Materia Medica, Chinese Academy of Sciences.Extraction and isolation
The dried caulis of G. montanum (10 kg) were powdered and extracted with 95% EtOH (30 L × 3) at room temperature to afford a crude extract (800 g), which was partitioned successively between petroleum ether (3 × 2.5 L) and EtOAc (3 × 2.5 L) with water. The EtOAc layer (138 g) was separated into fr.1 (92 g) and fr.2 (40 g) through a MCI Gel CHP-20P CC eluted with EtOH–H2O (40% and 60%, v/v). Fr. 1 was subjected to CC over silica gel using gradient CH2Cl2–MeOH (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–2
1–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to afford frs 1A–1E. Fr. 1A (26.0 g) was chromatographed with a Sephadex LH-20 column eluted with MeOH to yield isorhapontigenin (5 g). Resveratrol (200 mg), gnetol (50 mg) and 16 (50 mg) were obtained from fr. 1C (5.5 g) after purification through CC of Sephadex LH-20 (MeOH) and silica gel (CH2Cl2–MeOH, 10
1, v/v) to afford frs 1A–1E. Fr. 1A (26.0 g) was chromatographed with a Sephadex LH-20 column eluted with MeOH to yield isorhapontigenin (5 g). Resveratrol (200 mg), gnetol (50 mg) and 16 (50 mg) were obtained from fr. 1C (5.5 g) after purification through CC of Sephadex LH-20 (MeOH) and silica gel (CH2Cl2–MeOH, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Fr. 2 was divided into frs 2A–2F through CC of MCI gel (EtOH–H2O, 40–60%, v/v). Fr. 2B (4.0 g) was isolated by silica gel CC (CH2Cl2–MeOH, 20
1). Fr. 2 was divided into frs 2A–2F through CC of MCI gel (EtOH–H2O, 40–60%, v/v). Fr. 2B (4.0 g) was isolated by silica gel CC (CH2Cl2–MeOH, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 10
1 → 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) then semi-preparative HPLC to afford 3 (24 mg), 7 (28 mg), 6 (54 mg) and 14 (15 mg). Fr. 2C (17.0 g) was purified via repeatedly CCs of silica gel (CH2Cl2–MeOH) and Sephadex LH-20 (MeOH) finally semi-preparative HPLC to furnish 9 (3 mg), 10 (4 mg), 11 (6 mg), 8 (5 mg), 23 (21 mg), 5 (80 mg), 12 (13 mg), 22 (6 mg), 4 (4 mg), 24 (10 mg), 17 (20 mg), 20 (13 mg), and 13 (5 mg). By the same procedures, 1 (10 mg), 2 (10 mg), 15 (7 mg), 21 (26 mg), 19 (13 mg), and 18 (3 mg) were isolated from fr. 2D (7.0 g).
1) then semi-preparative HPLC to afford 3 (24 mg), 7 (28 mg), 6 (54 mg) and 14 (15 mg). Fr. 2C (17.0 g) was purified via repeatedly CCs of silica gel (CH2Cl2–MeOH) and Sephadex LH-20 (MeOH) finally semi-preparative HPLC to furnish 9 (3 mg), 10 (4 mg), 11 (6 mg), 8 (5 mg), 23 (21 mg), 5 (80 mg), 12 (13 mg), 22 (6 mg), 4 (4 mg), 24 (10 mg), 17 (20 mg), 20 (13 mg), and 13 (5 mg). By the same procedures, 1 (10 mg), 2 (10 mg), 15 (7 mg), 21 (26 mg), 19 (13 mg), and 18 (3 mg) were isolated from fr. 2D (7.0 g).
Characteristic data of compounds
Gnemontanin A (1), pale amorphous powders, [α]25D −3.7 (c 0.04, MeOH); UV (MeOH) λmax (log ε) 204 (4.21), 324 (3.56) nm; CD (MeOH) λmax (Δε) 204 (−20.8), 207 (+9.2), 210 (−19.0), 215 (−2.6), 217 (−8.4), 222 (+2.5), 238 (−3.1), 299 (+2.9) nm. IR (KBr) νmax 3351, 2918, 2850, 2360, 2342, 1598, 1512 cm−1; 1H and 13C NMR (Tables 1 and 2); ESIMS: m/z 555 ([M + Na]+); HRESIMS: m/z 555.1632 ([M + Na]+, calcd for C30H28O9Na, 555.1631).Gnemontanin B (2), pale amorphous powders, [α]25D −5.4 (c 0.06, MeOH); UV (MeOH) λmax (log ε) 204 (4.23), 324 (3.59) nm; CD (MeOH) λmax (Δε) 203 (+17.8), 207 (−17.4), 210 (+12.6), 214 (−3.9), 217 (+1.3), 220 (−3.3), 242 (+2.3) nm. IR (KBr) νmax 3374, 1601, 1512, 1263, 1155 cm−1; 1H and 13C NMR (Tables 1 and 2); ESIMS: m/z 531 ([M − H]−); HRESIMS: m/z 555.1621 ([M + Na]+, calcd for C30H28O9Na, 555.1631).
(−)-Gnetuhainin P (3), brown amorphous powders, [α]25D −8.3 (c 0.06, MeOH); UV (MeOH) λmax (log ε) 275 (4.81) nm; IR (KBr) νmax 3420, 1606, 1515, 1452, 1276, 1156 cm−1; 1H and 13C NMR (Tables 1 and 2); ESIMS: m/z 531 ([M − H]−); HRESIMS: m/z 531.1668 ([M − H]−, calcd for C30H27O9, 531.1655).
Gnemontanin C (4), brown amorphous powders, [α]25D +69.3 (c 0.04, MeOH); UV (MeOH) λmax (log ε) 204 nm (4.82), 330 (4.19); CD (MeOH) λmax (Δε) 205 (−17.8), 218 (+5.3), 235 (−6.6), 285 (+6.9), 314 (+6.3) nm. IR (KBr) νmax 3405, 1600, 1514, 1284, 1158 cm−1; 1H and 13C NMR (Tables 1 and 2); ESIMS: m/z 513 ([M − H]−); HRESIMS: m/z 513.1548 ([M − H]−, calcd for C30H25O8, 513.1549).
Gnemontanin D (5), pink amorphous powder, [α]25D −12.0 (c 0.05, MeOH); UV (MeOH) λmax (log ε) 205 nm (5.28), 220 nm (5.03), 330 (4.65). IR (KBr) νmax 3448, 1618, 1512, 1157 cm−1; 1H and 13C NMR (Tables 1 and 2); ESIMS: m/z 513 ([M − H]−); HRESIMS: m/z 513.1545 ([M − H]−, calcd for C30H25O8, 513.1549).
(−)-Gnetuhainin I (6), brown amorphous powders, [α]25D −8.5 (c 0.06, MeOH); UV: (MeOH) λmax (log ε) 204 (4.38), 282 (3.25) nm; CD (MeOH) λmax (Δε) 209 (+2.7), 212 (−31.8), 215 (+22.1), 220 (−4.7) nm; IR (KBr) νmax 3348, 1603, 1514, 1464, 1432, 1343, 1274, 1149, 1125, 1031 cm−1; 1H and 13C NMR (Tables 1 and 2); ESIMS: m/z [M − H]− 531 ([M − H]−), 1063 ([2M − H]−); HRESIMS: m/z 531.1668 ([M − H]−, calcd for C30H27O9, 531.1655).
Gnemontanin E (7), white amorphous powders, [α]25D −8.9 (c 0.05, MeOH); UV (MeOH) λmax (log ε) 204 (4.38), 283 (3.25) nm; CD (MeOH) λmax (Δε) 209 (−25.3), 212 (+11.2), 215 (−11.6), 220 (+1.7) nm; IR (KBr) νmax 3347, 2920, 2851, 1596, 1512, 1498, 1459, 1273 cm−1; 1H and 13C NMR (Tables 1 and 2); ESIMS: m/z 531 ([M − H]−); HRESIMS: m/z 531.1658 ([M − H]−, calcd for C30H27O9, 531.1655).
Gnemontanin F (8), brown amorphous powders, [α]25D −8.1 (c 0.04, MeOH); UV (MeOH) λmax (log ε) 204 (4.43), 282 (3.30) nm; CD (MeOH) λmax (Δε) 209 (−3.9), 210 (+4.5), 212 (−5.7), 215 (−6.3), 220 (−3.6) nm. IR (KBr) νmax 3364, 2920, 2850, 1956, 1498, 1461 cm−1; 1H and 13C NMR (Tables 3 and 4); ESIMS: m/z 559 ([M − H]−); HRESIMS: m/z 583.1945 ([M + Na]+, calcd for C32H32O9Na, 583.1944).
Gnemontanin G (12), yellow amorphous powders, [α]25D −19.6 (c 0.05, MeOH); UV (MeOH) λmax (log ε) 205 (4.46), 279 (3.32) nm; CD (MeOH) λmax (Δε) 207 (−5.7), 210 (+1.5), 215 (−37.7), 217 (2.9), 220 (−19.4), 232 (−27.7), 281 (−2.7) nm. IR (KBr) νmax 3352, 2920, 2850, 1598, 1461, 1158 cm−1; 1H and 13C NMR (Tables 3 and 4); ESIMS: m/z 469 ([M − H]−); HRESIMS: m/z 493.1267 ([M + Na]+, calcd for C28H22O7Na, 493.1263).
2′-Hydroxyampelopsin F (14), pale white amorphous powders, [α]25D +22.4 (c 0.05, MeOH); UV (MeOH) λmax (log ε) 205 (4.36), 281 (3.25) nm; CD (MeOH) λmax (Δε) 208 (+10.5), 211 (+11.1), 220 (−43.1), 240 (+17.9), 250 (+17.5), 281 (−4.1) nm; IR (KBr) νmax 3347, 2921, 2850, 2360, 1602, 1509, 1458 cm−1; 1H and 13C NMR (Tables 3 and 4); ESIMS: m/z 469 ([M − H]−); HRESIMS: m/z 493.1268 ([M + Na]+, calcd for C28H22O7Na, 493.1263).
Conclusions
Gnetum montanum contains abundant stilbenes with diverse structure types. Natural occurring dimeric stilbenes polymerized through one bond of 8-O-4′ as well as two bonds of 7-8′ and 6-7′ are reported for the first time, which enriched the structural diversity of stilbenoids. The 1H chemical shift differences between dimeric stilbene epimers on C-7 were analysed and summarized, which could be used as a diagnostic to determine the relative configuration of C-7. By mean of it, the misassigned NMR data (10), the uncertain configurations (9–11), and the erroneous structures (12a and 13a) of previous reported dimeric stilbenoids were resolved.Acknowledgements
This study was financially supported by National Natural Science Foundation of China (21202182).Notes and references
- M. H. Keylor, B. S. Matsuura and C. R. J. Stephenson, Chem. Rev., 2015, 115, 8976 CrossRef CAS PubMed.
- T. Shen, X.-N. Wang and H.-X. Lou, Nat. Prod. Rep., 2009, 26, 916 RSC.
- K. Xiao, H.-J. Zhang, L.-J. Xuan, J. Zhang, Y.-M. Xu and D.-L. Bai, in Stud. Nat. Prod. Chem., Elsevier, New York, 2008, vol. 34, p. 453 Search PubMed.
- D.-S. Yang, Z.-L. Li, X. Wang, H. Yan, Y.-P. Yang, H.-R. Luo, K.-C. Liu, W.-L. Xiao and X.-L. Li, RSC Adv., 2015, 5, 13886 RSC.
- L.-K. Fu, Y.-F. Yu and M. G. Gilbert, in Flora of China, Scientific Press, Beijing, 1999, vol. 4, p. 102 Search PubMed.
- F. Martin, T. Grkovic, M. L. Sykes, T. Shelper, V. M. Avery, D. Camp, R. J. Quinn and R. A. Davis, J. Nat. Prod., 2011, 74, 2425 CrossRef CAS PubMed.
- X.-M. Li, M. Lin, Y.-H. Wang and X. Liu, Planta Med., 2004, 70, 160 CrossRef CAS PubMed.
- L.-Q. Wang, Y.-X. Zhao, J.-M. Hu, A.-Q. Jia and J. Zhou, Helv. Chim. Acta, 2008, 91, 159 CrossRef CAS.
- C. Zhang, K. Jiang, S.-J. Qu, Y.-M. Zhai, J.-J. Tan and C.-H. Tan, J. Asian Nat. Prod. Res., 2015, 17, 996 CrossRef CAS PubMed.
- Y. Zhang, K. Jiang, Y.-M. Zhai, J.-J. Tan, D.-L. Meng, S.-L. Guo, S.-J. Qu and C.-H. Tan, Helv. Chim. Acta, 2014, 97, 369 CrossRef CAS.
- K. Jiang, J.-Q. Bai, J. Chang, J.-J. Tan, S.-J. Qu, H.-F. Luo, C.-H. Tan and D.-Y. Zhu, Helv. Chim. Acta, 2014, 97, 64 CrossRef CAS.
- X.-F. Wang, Y. Zhang, M.-B. Lin, Q. Hou, C.-S. Yao and J.-G. Shi, J. Asian Nat. Prod. Res., 2014, 16, 511 CrossRef CAS PubMed.
- Y.-H. Wang, K.-S. Huang and M. Lin, J. Asian Nat. Prod. Res., 2001, 3, 169 CrossRef CAS PubMed.
- K.-S. Huang, Y.-H. Wang, R.-L. Li and M. Lin, Phytochemistry, 2000, 54, 875 CrossRef CAS PubMed.
- Y. Takaya, K. X. Yan, K. Terashima, J. Ito and M. Niwa, Tetrahedron, 2002, 58, 7259 CrossRef CAS.
- S. He, B. Wu, Y. J. Pan and L. Y. Jiang, J. Org. Chem., 2008, 73, 5233 CrossRef CAS PubMed.
- T. Tanaka, M. Iinuma and H. Murata, Phytochemistry, 1998, 48, 1045 CrossRef CAS.
- K.-S. Huang, Y.-H. Wang, R.-L. Li and M. Lin, Phytochemistry, 2000, 54, 875 CrossRef CAS PubMed.
- K. Kawazoe, N. Shimogai, Y. Takaishi, K. S. Rao and Y. Imakura, Phytochemistry, 1997, 44, 1569 CrossRef CAS.
- K.-S. Huang, S. Zhou, M. Lin and Y.-H. Wang, Planta Med., 2002, 68, 916 CrossRef CAS PubMed.
- K.-S. Huang, Y.-H. Wang, R.-L. Li and M. Lin, J. Nat. Prod., 2000, 63, 86 CrossRef CAS.
- S.-I. Piyawit, S. Jirapast, S. Pongpun and T.-P. Santi, Fitoterapia, 2011, 82, 460 CrossRef PubMed.
- H.-F. Luo, L.-P. Zhang and C.-Q. Hu, Tetrahedron, 2001, 57, 4849 CrossRef CAS.
- T. Ito, K. Nishiya, M. Oyama, T. Tanaka, J. Murata, D. Darnaedi and M. Iinuma, Phytochem. Lett., 2013, 6, 667 CrossRef CAS.
- I. Iliya, T. Tanaka, M. Iinuma, Z. Ali, M. Furasawa, K.-I. Nakaya, Y. Shirataki, J. Murata and D. Darnaedi, Chem. Pharm. Bull., 2002, 50, 796 CrossRef CAS PubMed.
- K.-S. Huang, Y.-H. Wang, R.-L. Li and M. Lin, J. Nat. Prod., 2000, 63, 86 CrossRef CAS.
- M. Fulvio, V. Urska, M. Giulia, M. Domenico, Z. Luca, S. Marco, M. Claudio, V. Riccardo and G. Graziano, J. Agric. Food Chem., 2011, 59, 5364 CrossRef PubMed.
- A. P. Lins, M. N. D. S. Ribeiro and H. E. Gottlieb, J. Nat. Prod., 1982, 45, 754 CrossRef CAS.
- I. Iliya, T. Tanaka, M. Iinuma, Z. Ali, M. Furasawa, K.-I. Nakaya, N. Matsuura and M. Ubukata, Heterocycles, 2002, 57, 1507 CrossRef CAS.
- Y. Oshima, Y. Ueno, H. Hikino, L.-L. Yang and K.-Y. Yen, Tetrahedron, 1990, 46, 5121 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed analysis of shielded effect for cis-/trans-indane-based stilbene dimers, 1D and 2D NMR, IR and MS spectra of compounds 1–8, 12 and 14. See DOI: 10.1039/c6ra08238f |
| This journal is © The Royal Society of Chemistry 2016 |