Synthesis and characterization of novel imidazolium-functionalized polyimides for high temperature proton exchange membrane fuel cells
Abstract
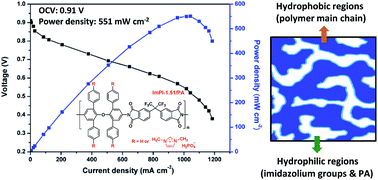
Novel imidazolium-functionalized polyimides (ImPI-x) were successfully synthesized from polyimides containing trifluoromethyl groups, ether linkages and four phenyl substituents (4PhODA/PI) via chloromethylation followed by quaternization with 1-methylimidazole. The cleavage of the ether linkage and crosslinking during chloromethylation can be circumvented by carrying out this reaction at 60 °C with suitable concentrations of chloromethylation reagents, catalyst and polyimides. The degree of substitution (DS) ranging from 0.15 to 2.17 per repeating unit can be achieved without polymer degradation and crosslinking. Phosphoric acid (PA) uptakes of ImPI-x ranged from 34 to 159% and increased with the increased DS values. The ImPI-x membranes also exhibited good thermal stability and mechanical properties in both their dry and PA doped states. The proton conductivity of the ImPI-x membranes with PA uptakes of 84–159% were from 0.008 to 0.057 S cm−1 at 160 °C under anhydrous conditions. ImPI-1.51 had a higher proton conductivity of 0.057 S cm−1 than m-PBI (0.046 S cm−1) even though it had a lower PA uptake (159%). A single fuel cell based on the ImPI-1.51 membrane with a PA uptake of 159% exhibited the peak power density of 551 mW cm−2 with H2/O2 under anhydrous conditions at 160 °C, which was higher than that of m-PBI (419 mW cm−2) with a PA uptake of 216%. From AFM phase images of ImPI-x, microphase separation which might have resulted from hydrophobic trifluoromethyl groups and hydrophilic imidazolium groups can be observed. The microphase separation might facilitate the formation of ionic channels and the transport of protons.

 Please wait while we load your content...
Please wait while we load your content...