Rational design of benzodithiophene based conjugated polymers for better solar cell performance
Abstract
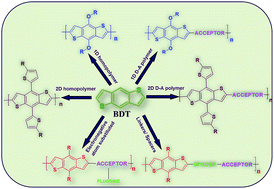
The rational design of conjugated polymers is crucial. As photovoltaic materials they need to be optimized for high-performance polymer solar cells (PSCs). We present a concise review of conjugated polymers based on benzodithiophenes (BDTs). In this account, we have discussed the conjugated polymeric designs of various architectures; consisting of electron rich donor and electron deficient acceptor moieties in the main chain, as well as in the side chains, to facilitate effective intra-molecular charge transfer. We summarize that the application of these polymeric materials drastically influences the power conversion efficiency (PCE) of polymeric solar cells. As of now, PCEs of over 10% are reported for these polymeric materials along with fullerene derivatives.

 Please wait while we load your content...
Please wait while we load your content...