Nanogold POxylation: towards always-on fluorescent lung cancer targeting†‡
A. Sofia Silvaab,
Marta C. Silvaa,
Sónia P. Miguelb,
Vasco D. B. Bonifácio*c,
Ilídio J. Correia b and
Ana Aguiar-Ricardo
b and
Ana Aguiar-Ricardo *a
*a
aLAQV-REQUIMTE, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, Campus de Caparica, 2829-516 Caparica, Portugal. E-mail: air@ftc.unl.pt
bCICS-UBI, Health Sciences Research Center, Faculdade de Ciências da Saúde, Universidade da Beira Interior, Av. Infante D. Henrique, 6200-506 Covilhã, Portugal
cCentro de Química-Física Molecular and Institute of Nanosciences and Nanotechnology, Instituto Superior Técnico, Av. Rovisco Pais, 1049-001 Lisboa, Portugal. E-mail: vasco.bonifacio@tecnico.ulisboa.pt
First published on 29th March 2016
Abstract
Gold nanoparticles (GNPs) are one of most investigated nanomaterials for lung cancer diagnosis and therapy (theragnosis). For imaging purposes, GNPs are often tagged with fluorescent probes, but unfortunately the associated plasmon resonance effect leads to fluorescence self-quenching, thus precluding accurate localization. In this study, biocompatible GNPs targeted with a laminin fragment were successfully engineered using fluorescent oligo-oxazolines produced in supercritical carbon dioxide. The architecture and properties of the POxylated constructs were fully characterized and confocal laser scanning microscopy measurements demonstrated a higher cellular uptake into A549 lung cancer cells through an active targeting mechanism.
GNPs can be engineered to exhibit diagnosis and therapy (theragnosis) features.1,2 Despite cargo and release functions, at both extracellular and intracellular levels, gold-based nanodevices are also able to penetrate into deep tissue and undergo cellular uptake. Surface design also provides a stealth surface to prevent opsonisation, increasing residence time and enhancing therapeutic delivery at specific sites. Usually, this goal is achieved by coating GNPs with hydrophilic poly(ethylene glycol) (PEG). However, some recent reports state that increasing amounts of PEG may lead to undesired hepatic accumulation leading to an inflammatory response triggered by the liver,3–5 a fact that led pharmaceutical companies to investigate alternatives to PEGylation. Poly(ethyleneimine) (PEI) is reported as an useful hydrophilic cationic polymer for transfection enhancement, however its use in biomedical applications is limited due to its known cytotoxicity.6–8 Related poly and oligo(2-alkyl-2-oxazolines) are another class of hydrophilic and biocompatible polymers regarded as versatile PEG alternatives. Importantly, they are easily eliminated by renal clearance and, opposing to PEG, their properties can be gradually fine-tuned over a broad range by side chain design enabling different biomedical applications. Therefore, coating with polyoxazolines (POxylation) is a promising strategy. In particular, poly(2-ethyl-2-oxazoline) is of special interest since is a FDA approved indirect food additive (adhesive, Aquazol®) and can be conjugated with low molecular weight drugs9 and model proteins.10 The synthesis of polyoxazolines is well established, either by microwave11 or supercritical carbon dioxide (scCO2)-assisted cationic ring-opening polymerization (CROP).12 In the last decade, our group fully explored the properties of oligo(2-alkyl-2-oxazolines) (OOxs) produced by a clean scCO2-assisted CROP, and enlarged the scope of its applications by producing biocompatible blue fluorescent low polydisperse oligomers.13–16 The luminescent properties of OOxs, absent in conventional PEG and PEI polymers, confer them unique properties for bioimaging purposes. Regarding the design of fluorescent GNP-based probes, it is known that the distance of the fluorophore to the GNP is crucial to avoid interactions of the electrons of the fluorophore with the plasmon field (typically >10 nm), being the fluorescence almost completely quenched on the particle surface.17,18 Herein, we report the optical features of stable fluorescent labelled GNPs, showing no plasmon-induced quenching, and its impact in the performance of cellular uptake into A549 lung cancer cells.
Since the surface chemistry is vital for cell–nanoparticles interactions we evaluated the coating effect of both fluorescent oligo(2-ethyl-2-oxazoline) (end-terminated with cysteamine for GNP conjugation) and an oligo(2-ethyl-2-oxazoline)–PEI co-oligomer having pendant chromylium salts, obtained by post-functionalization of oligo(2-ethyl-2-oxazoline). GNPs were further tagged with a specific peptide sequence, a laminin fragment (YIGSR) known to be recognized by the overexpress β1 integrins at A549 non-small cell lung cancer surface.19,20 Surprisingly, the engineered gold nanoconstructs exhibit fluorescence, thus meaning that the fluorescence of both investigated oligomers is not affected by the GNPs plasmon field. This observation points OOxs as exceptional polymer layers, able to ensure unprecedented optical properties to functional GNPs.
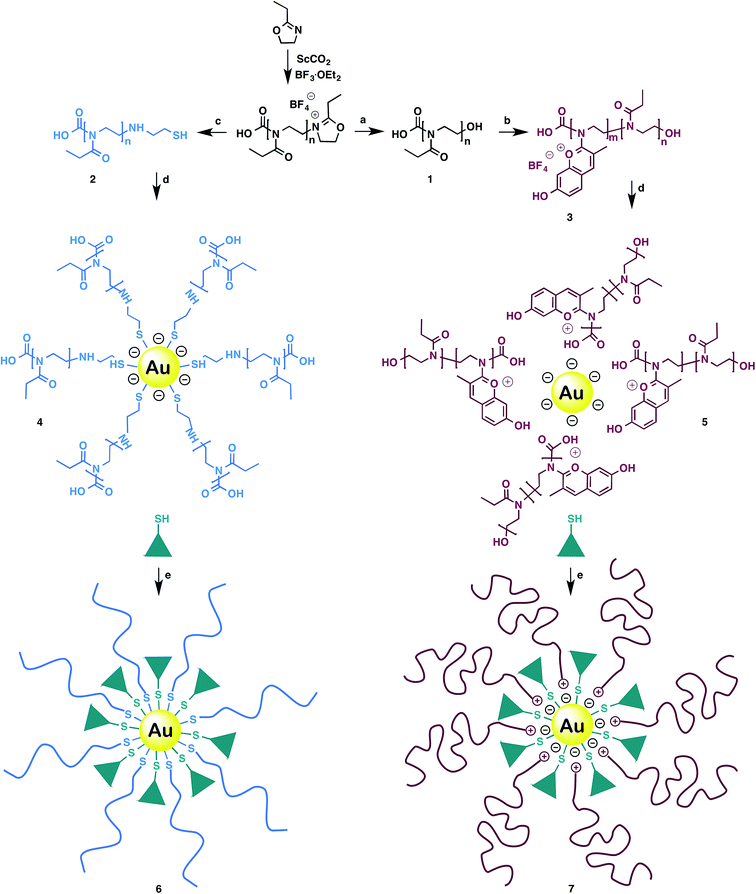
GNPs were synthetized according to Frens' method.21 Prior to the nanoparticles functionalization, OOxs were produced following a previously published protocol.22 At the end of the polymerization the living oligomer was end-capped either with water or cysteamine, generating OEtOx–OH (1) and OEtOx–SH (2), respectively (Scheme 1).
Post-functionalization of 1 with 2,4-dihydroxybenzaldehyde produced an oligo(2-ethyl-2-oxazoline) polycationic oligo(ethyleneimine-N-chromylium salt) co-oligomer, OEI–CS (3). The post-functionalization of an oligooxazoline to give oligo N-chromylium salts occurs under mild conditions and is reported for the first time. The synthesis of co-oligomer 3 results from partial reaction of 1 with 2,4-dihydroxybenzaldehyde. Next, the addition of gold nanoparticles to 2 and 3 generated POxylated intermediates 4 and 5, respectively. Finally, POxylated probes Au–OEtOx–SH–YIGSR (6) and Au–OEI–CS–YIGSR (7) were obtained upon conjugation with a laminin fragment (YIGSR).
The cellular uptake of the POxylated nanoprobes was then assessed by confocal fluorescence scanning microscopy (CLSM) analysis.22 The POxylated gold nanoprobes were easily visualised due to their blue fluorescence (Fig. 1). By comparing the results obtained for neutral Au–OEtOx–SH (Fig. 1A) and cationic Au–OEI–CS (Fig. 1B), a higher uptake was observed when a non-charged coating was used. Despite the charge effect, the data might be also explained by the higher concentration of OOxs chains attached to the GNP in the neutral system (AuNP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OOx molar ratio of 1
OOx molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000), thus leading to an extra stabilization of the nanoparticles and improved passive targeting. As expected, an enhanced cellular uptake was noticed when the peptide sequence YIGSR was conjugated to the nanoconstructs (Fig. 1C and D), leading to active targeting. Interestingly, using these probes an enriched nuclear location is observed (blue coloration). This higher internalization seems to be a result from the particles design: size, morphology, zeta potential and recognition active sites. Cell growth and adherence in the presence of the nanocarriers was also observed (see Fig. S1 in ESI‡).
5000), thus leading to an extra stabilization of the nanoparticles and improved passive targeting. As expected, an enhanced cellular uptake was noticed when the peptide sequence YIGSR was conjugated to the nanoconstructs (Fig. 1C and D), leading to active targeting. Interestingly, using these probes an enriched nuclear location is observed (blue coloration). This higher internalization seems to be a result from the particles design: size, morphology, zeta potential and recognition active sites. Cell growth and adherence in the presence of the nanocarriers was also observed (see Fig. S1 in ESI‡).
Transmission electron microscopy (TEM) analysis shows that the nanoconstructs possess a uniform shape (oval or nearly round) with a narrow size distribution of ca. 30 nm of diameter (Fig. 1E and F). In fact, dynamic light scattering (DLS) measurements are consistent with these data and the zeta potential increased after laminin fragment conjugation (probes 6 and 7) (Table 1).
| Nanoparticles | Particle size (nm) | Zeta potential (mV) |
|---|---|---|
| AuNPs | 28 ± 1 | −40 |
| Au–OEtOx–SH (4) | 28.7 ± 0.7 | −30 |
| Au–OEtOx–SH–YIGSR (6) | 29 ± 2 | −15 |
| Au–OEI–CS (5) | 29 ± 1 | −9 |
| Au–OEI–CS–YIGSR (7) | 31 ± 1 | −9 |
Successful coating with OOxs or YIGSR immobilization into the particles surface was also corroborated by a significant broadening of the characteristic UV-Vis band of GNPs (λmax = 530 nm) (Fig. 2A, top). Increased molar ratios and consequently increased number of molecules attached to GNPs lowers the intensity of this band. Still, our nanoparticles did not show signs of aggregation or flocculation (Fig. 2A, bottom).
The photophysical properties of the produced oligooxazolines 2 and 3 (Fig. 2B) and nanoconstructs 4 and 5 (Fig. 2C) were evaluated in aqueous media. After GNP conjugation oligomers 2 and 3 preserved their optical properties, and the typical fluorescence quenching, observed in reported systems, was absent.
The interactions between OOx's and the laminin fragment with the AuNPs were also proven by FTIR-ATR spectroscopy (see Fig. S2 in ESI‡). Specifically, a shift of the S–H stretch of the laminin fragment (2610 cm−1) was observed in 6 (2545 cm−1). The characteristic carbonyl band of 2 at 1628 cm−1 is also slightly shifted both in 4 (1598 cm−1) and in 6 (1620 cm−1). The 1H NMR and MALDI-TOF spectra of oligomer 3 were also obtained (see Fig. S3 and S4 in ESI‡).
The amount of organic components on the GNPs was assessed by thermal gravimetric analysis (TGA) (Fig. S5 in ESI‡). The total mass loss was 8.06% and 3.20% for 6 and 7, respectively. By combining the data from TGA analyses and HPLC peptide quantification,23 the amount of oligomers and YIGSR were quantified (see calculations in ESI‡).
Finally, particles biocompatibility (without drug) was accessed through the metabolic activity of the A549 cell line22,24 (Fig. 3). The nanoparticles and the oligomers showed almost no toxicity as cell viability was always above 80% or even higher after 72 hours. Besides, a significant difference in cell viability was obtained between the positive and negative control (p < 0.05), and cells exposed to the different precursors and the nanoparticles after 24 h (p* < 0.05) and 72 h (p# < 0.05) of incubation.
Conclusions
In summary, stable, non-toxic, laminin-conjugated gold nanoparticles were successfully engineered using fluorescent oligo-oxazolines (PEG alternatives) produced using a scCO2-assisted protocol. This POxylation approach circumvents targeting with expensive probes and avoids quenching by the strong surface plasmon resonance effect. The nanoconstructs have an ideal size for intracellular delivery and the additional conjugation with a laminin fragment strongly increased the particles uptake by the A549 cell line. Innovative particle design using nanogold POxylation will allow future tailoring of efficient, greener and low cost multifunctional nanocarriers for in vitro cancer imaging.Notes and references
- F. Caruso, T. Hyeon, V. Rotello, D. Lee, H. Koo, I. Sun, J. H. Ryu, K. Kim and I. C. Kwon, Chem. Soc. Rev., 2012, 41, 2656–2672 RSC.
- K. Kim, J. H. Kim, H. Park, Y.-S. Kim, K. Park, H. Nam, S. Lee, J. H. Park, R.-W. Park, I.-S. Kim, K. Choi, S. Y. Kim, K. Park and I. C. Kwon, J. Controlled Release, 2010, 146, 219–227 CrossRef CAS PubMed.
- T. X. Viegas, M. D. Bentley, J. M. Harris, Z. Fang, K. Yoon, B. Dizman, R. Weimer, A. Mero, G. Pasut and F. M. Veronese, Bioconjugate Chem., 2011, 22, 976–986 CrossRef CAS PubMed.
- Y. Akiyama, T. Mori, Y. Katayama and T. Niidome, J. Controlled Release, 2009, 139, 81–84 CrossRef CAS PubMed.
- A. Aguiar-Ricardo, V. D. B. Bonifácio, T. Casimiro and V. G. Correia, Philos. Trans. R. Soc., A, 2015, 373, 20150009 CrossRef PubMed.
- S. Nimesh, A. Goyal, V. Pawar, S. Jayaraman, P. Kumar, R. Chandra, Y. Singh and K. C. Gupta, J. Controlled Release, 2006, 110, 457–468 CrossRef CAS PubMed.
- F. M. Kievit, O. Veiseh, N. Bhattarai, C. Fang, J. W. Gunn, D. Lee, R. G. Ellenbogen, J. M. Olson and M. Zhang, Adv. Funct. Mater., 2009, 19, 2244–2251 CrossRef CAS PubMed.
- T. Ozeki, S. Beppu, T. Mizoe, Y. Takashima, H. Yuasa and H. Okada, Pharm. Res., 2006, 23, 177–183 CrossRef CAS PubMed.
- A. Mero, G. Pasut, L. Dalla, M. W. M. Fijten, U. S. Schubert, R. Hoogenboom and F. M. Veronese, J. Controlled Release, 2008, 125, 87–95 CrossRef CAS PubMed.
- K. Kempe, A. Vollrath, H. W. Schaefer, T. G. Poehlmann, C. Biskup, R. Hoogenboom, S. Hornig and U. S. Schubert, Macromol. Rapid Commun., 2010, 31, 1869–1873 CrossRef CAS PubMed.
- R. Hoogenboom, Angew. Chem., Int. Ed., 2009, 48, 7978–7994 CrossRef CAS PubMed.
- C. V. de Macedo, M. S. da Silva, T. Casimiro, E. J. Cabrita and A. Aguiar-Ricardo, Green Chem., 2007, 9, 948–953 RSC.
- V. G. Correia, V. D. B. Bonifácio, V. P. Raje, T. Casimiro, G. Moutinho, C. L. da Silva, M. G. Pinho and A. Aguiar-Ricardo, Macromol. Biosci., 2011, 11, 1128–1137 CrossRef CAS PubMed.
- V. D. B. Bonifácio, V. G. Correia, M. G. Pinho, J. C. Lima and A. Aguiar-Ricardo, Mater. Lett., 2012, 81, 205–208 CrossRef.
- V. G. Correia, M. Coelho, T. Barroso, V. P. Raje, D. B. Vasco, T. Casimiro, M. G. Pinho and A. Aguiar-Ricardo, Biofouling, 2013, 29, 273–282 CrossRef CAS PubMed.
- R. B. Restani, J. Conde, R. F. Pires, P. Martins, A. R. Fernandes, P. V. Baptista, V. D. B. Bonifácio and A. Aguiar-Ricardo, Macromol. Biosci., 2015, 15, 1045–1051 CrossRef CAS PubMed.
- K. A. Kang, J. Wang, J. B. Jasinski and S. Achilefu, J. Nanobiotechnol., 2011, 9, 16–25 CrossRef CAS PubMed.
- J. Solis David, W.-S. Chang, B. P. Khanal, K. Bao, P. Nordlander, E. R. Zubarev and S. Link, Nano Lett., 2010, 10, 3482–3485 CrossRef PubMed.
- S. Mukhopadhyay, P. Malik, S. K. Arora and T. K. Mukherjee, in Molecular Mechanisms of Tumor Cell Resistance to Chemotherapy, ed. B. Bonavida, Springer, New York, 2013, pp. 89–108 Search PubMed.
- U. M. Wewer, G. Taraboletti, M. E. Sobel, R. Albrechtsen and L. A. Liona, Cancer Res., 1987, 47, 5691–5698 CAS.
- G. Frens, Nature Phys. Sci., 1973, 241, 20–22 CrossRef CAS.
- A. S. Silva, V. D. B. Bonifácio, V. P. Raje, P. S. Branco, P. P. F. Machado, I. J. Correia and A. Aguiar-Ricardo, RSC Adv., 2015, 5, 10733–10738 RSC.
- P. K. Dubey, D. Singodia and S. P. Vyas, J. Drug Targeting, 2010, 18, 373–380 CrossRef CAS PubMed.
- V. P. Raje, P. I. Morgado, M. P. Ribeiro, I. J. Correia, V. D. B. Bonifácio, P. S. Branco and A. Aguiar-Ricardo, Biosens. Bioelectron., 2013, 39, 64–69 CrossRef CAS PubMed.
Footnotes |
| † Dedicated to Prof. Ana M. Lobo in the occasion of her 70th birthday. |
| ‡ Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra00532b |
| This journal is © The Royal Society of Chemistry 2016 |