Palladium nanoparticles modified electrospun CoFe2O4 nanotubes with enhanced peroxidase-like activity for colorimetric detection of hydrogen peroxide†
Abstract
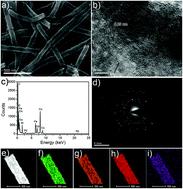
Herein, we report a simple procedure to decorate small palladium nanoparticles (Pd NPs) on the surface of CoFe2O4 nanotubes; the decorated nanotubes possess intrinsic peroxidase-like activity for the sensitive detection of H2O2. The CoFe2O4 nanotubes are prepared via an electrospinning technique, followed by a calcination process in air. The functionalization of Pd NPs on the surface of CoFe2O4 nanotubes was achieved through an in situ reduction process using ascorbic acid (AA) as a reducing agent. The synthesized Pd nanoparticles, which are small in size, are evenly dispersed on the surface of the CoFe2O4 nanotubes. The hollow structure of the CoFe2O4 nanotubes and the uniform distribution of the Pd nanoparticles enhance peroxidase-like activity toward the catalytic oxidation of the peroxidase substrate 3,3′,5,5′-tetramethylbenzidine (TMB) in the presence of H2O2. The peroxidase-like property of the Pd/CoFe2O4 composite nanotubes provides a facile approach for the colorimetric detection of H2O2 with a low detection limit. This work provides great potential for Pd/CoFe2O4 nanotubes as enzyme-like nanocatalysts to sensitively detect H2O2 in biological systems.

 Please wait while we load your content...
Please wait while we load your content...