Prediction of the breakthrough curves of VOC isothermal adsorption on hypercrosslinked polymeric adsorbents in a fixed bed
Jian Wuab,
Lijuan Jiaa,
Liuyan Wua,
Chao Long*a,
Weibing Dengc and
Quanxing Zhanga
aState Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University, 163 Xianlin Road, Nanjing 210023, China. E-mail: clong@nju.edu.cn; Tel: +86 25 89680380
bJiangsu Province Key Laboratory of Environmental Engineering, Jiangsu Provincial Academy of Environmental Science, 176 Jiangdong North Road, Nanjing, 210036, China
cDepartment of Mathematics, Nanjing University, 22 Hankou Road, Nanjing 210093, China
First published on 16th March 2016
Abstract
A mathematical model, which was based on the Linear Driving Force (LDF) model and Dubinin–Radushkevich (D–R) equation, was proposed to describe the removal of volatile organic compounds (VOCs) through a fixed bed packed with a hypercrosslinked polymeric resin (HPR) under isothermal conditions. The limiting volume adsorbed capacity (q0) and the adsorption characteristic energy (E) of the D–R equation were correlated well with the properties of the VOCs and HPR using multi-linear regression (MLR). Therefore, no experimental effort was required to predict equilibrium adsorption capacities of VOCs over a wide range of relative pressures. The model was validated for the breakthrough adsorption of hexane, heptane, and butanone on HPR. The experimental and predicted breakthrough time had a deviation within 10%. This result indicates that the model can be used to determine the breakthrough curves and time as long as the known properties of VOCs and HPR were found in advance, without requiring any adsorption experiments.
1. Introduction
The emission of volatile organic compound (VOC) vapors has caused not only severe air pollution but also a great loss of valuable chemicals. It is well known that adsorption is one of the most effective methods for separating and recovering VOCs from industrial waste gas. As a new kind adsorbent, hypercrosslinked polymeric resins (HPRs) represent a class of predominantly microporous organic materials with an extremely high specific surface area (>1000 m2 g−1), stable physical and chemical properties, as well as regeneration on site.1–5 Therefore, hypercrosslinked polymeric resins have received increasing attention in recent years as adsorbents for purifying air. Simpson et al.,6,7 Podlesnyuk et al.,8 Baya et al.9 and our research group10–14 have investigated the adsorption characteristics of hypercrosslinked polymeric resins for VOCs. These studies indicated that hypercrosslinked polymeric resin is an efficient and competitive adsorbent for VOCs recovery from polluted vapor streams.In practical applications, the fixed bed adsorption system has been widely used to control VOC emissions. To optimize design and operating conditions, the development of a mathematical model that can describe the dynamics of adsorption in a fixed bed with selected adsorbent is required. In general, a comprehensive mathematical model consists of coupled partial differential equations distributed over time and space representing material balances together with transport rates and equilibrium equations. It is worth noting that the adsorption equilibrium in a wide range of temperatures and concentrations is the most important process that controls the dynamics behavior of a packed column so that the general nature of a mass transfer zone is determined entirely by the equilibrium isotherm.15–20 To obtain the adsorption equilibrium equation, gas–solid equilibrium experiments must be performed, which may tend to be expensive and time consuming for different combinations of adsorbate–adsorbent systems. To the best of our knowledge, it has not been reported to develop an adsorption isotherm equation from commonly available adsorbent and adsorbate properties for predicting breakthrough curves of VOCs in a fixed bed.
Although Langmuir or Freundlich equation is commonly used to describe adsorption data, these equations cannot predict the adsorption equilibrium capacity of adsorbent from fundamental adsorbate properties. Dubinin–Radushkevich (D–R) equation, deduced from the potential theory, has been proved to be an effective pool to describe the adsorption capacity of VOCs on microporous adsorbents.21,22 According to D–R equation, the volumetric adsorption amount (qv, mL g−1) is related to the adsorption potential (ε) as given by eqn (1) and (2).
| qv = q0 exp[−(ε/E)2] | (1) |
ε = RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(Ps/P) ln(Ps/P)
| (2) |
To apply D–R equation to the prediction of adsorption capacity, the parameters q0 and E must be obtained in advance. Previous researchers considered that E for different substances was related to affinity coefficient (β):15
| E1/β1 = E2/β2 = Ei/βi = Ereference/βreference | (3) |
Benzene was usually taken as the reference compound with assumption βbenzene = 1, and Ei = βiEreference could be substituted into D–R equation. Affinity coefficients (β) can be approximated by ratio of molecular parachor, molar polarizability or molar volume of the specific adsorbate and reference compound (benzene).15 However, the selection of an appropriate reference adsorbate could be an arbitrary choice.23–25 The choice of a reference adsorbate has an important effect on the accuracy of the D–R equation for predicting the adsorption capacity of organic compounds on adsorbents. In addition, according to the theory of volume filling of micropores,26 there is a constants q0 for any chemical at a given value of Ei/βi. This method is suitable for carbonous adsorbent, and q0 is generally assumed to be equal to the volume of micropore. However, for VOCs–HPR adsorption system, our previous studies5,10–14,27 found that q0 was not equal to but greater than microporous volume of adsorbent. Possible reason is that hypercrosslinked polymeric resin can swell strongly after adsorbing organic compounds, which results in larger actual microporous volume compared with the measured value based on N2 adsorption experimental data. Therefore, in order to apply D–R equation to the prediction of the adsorption capacities of VOCs onto hypercrosslinked polymeric resin, it is important to obtain the parameters q0 and E accurately.
The aim of the present study is to establish a model to predict the breakthrough curves of VOCs adsorption on hypercrosslinked polymeric adsorbent in a fixed bed. Mass transfer was described by the Linear Driving Force (LDF) model. Some studies have given good results for modeling dynamic adsorption with LDF model.28–32 The adsorption equilibrium of VOCs on HPR was described by D–R equation; q0 and E were estimated using multi-linear regression (MLR) based on the physical-chemistry properties of adsorbents and adsorbates. The accuracy of the predictions was assessed by comparing them with experimental breakthrough curves.
2. Theoretical approach
The mathematical model of the dynamic adsorption breakthrough process in a fixed bed is based on transient material balance, gas phase and intrapellet mass transfer, adsorption equilibrium relationship, boundary conditions, and initial conditions. In this study, both adsorption equilibrium and kinetic aspect are taken into account. Adsorption equilibrium and mass-transfer rate are described by D–R equation and linear driving force (LDF) model respectively. The assumptions listed below are made to develop this model.(1) The pressure drop through bed is negligible.
(2) The plug flow model is used to describe the flow pattern. Adsorbate concentration, gas flow, porosity and temperature are uniform at any cross section of the bed.
(3) The physical properties of the adsorbent are constant, and accumulation of energy in the gas phase is negligible under constant temperature.
(4) The mass transfer rate described by the LDF model consists in a general expression that lumps together all diffusion mechanisms into one single parameter, k.33
(5) The flow rate is constant and the effect of axial dispersion is neglected.
2.1 Mass balance
The mass balance on a portion of the fixed bed is given by| {mass flux} + {mass accumulation in the gas phase} + {mass adsorbed in the solid phase} = 0 |
 | (4) |
2.2 Mass transfer rate
LDF model was used to describe the mass transfer:
 | (5) |
The k parameter is an adjustable parameter.14,26,32 It has been proven to be essential and can be described by following expression for the mass transfer rate:31,32
 | (6) |
2.3 Adsorption isotherm
D–R equation can be defined as the following equations by combining eqn (1) and (2):
 | (7) |
The parameters of q0 and E were estimated directly by using the multi-linear regression (MLR) based on the experiment data. MLR uses optimal combination of multiple independent variables to estimate the dependent variable, which is more effective than a single independent variable. MLR is a standard procedure that enables us to find the most significant variables (from the properties of VOCs and the hypercrosslinked polymeric adsorbent) on the measured response. Removing less significant variables leads to a more statistically reliable MLR model, which can be applied to a wide range of VOCs–resin systems. In this study, MLR models were established by using the statistical package SPSS19.
2.4 Initial and boundary conditions
The initial conditions are:| C = C0 for z = 0 and t = 0 |
| C = 0 for z > 0 and t = 0 |
| q = 0 for z ≥ 0 and t = 0 |
The boundary condition is:
| C = C0 for z = 0 and t > 0 | (8) |
2.5 Numerical simulations
The set of eqn (4)–(7) enabled us to compute the amount of VOCs adsorbed onto the particles of adsorbent (q) and the concentration of VOCs in the gas-phase (C) along the column. The gas concentration could be obtained by using the finite-difference method. Each discrete point represents a certain region, whose concentration is a measurement of the average concentration of this region at a certain time. Therefore, the numerical accuracy of calculations strongly depends on the number of designated discrete points. The calculation process was quite complicated, and MATLAB R2010a was applied to solve the differential equation set.3. Data set
3.1 Adsorbents and adsorbates
The adsorbents used in the experimental evaluation were the hypercrosslinked polymeric resins with different pore structure, which were prepared via a post-crosslinking step of low-crosslinked macroporous poly(styrene-divinylbenzene).14 The salient properties of hypercrosslinked polymeric resins are listed in Table 1.| Parameters | HY-1 | HPsorbent | ND-100 | Resin-1 | Resin-2 |
|---|---|---|---|---|---|
| a Synthesized by my colleagues in State Key Laboratory of Pollution Control and Resource Reuse, Nanjing University (NJU). | |||||
| SBET (m2 g−1) | 1244.42 | 1020.7 | 1325 | 811.9 | 944.35 |
| Micropore volume (Vmicro, cm3 g−1) | 0.541 | 0.394 | 0.423 | 0.400 | 0.430 |
| Average pore diameter (nm) | 2.35 | 2.52 | 2.54 | 2.92 | 2.73 |
| Bulk density (kg m−3) | 294.9 | 335.4 | 294.8 | 376.0 | 306.8 |
| Diameter of particles (mm) | 0.4–0.6 | 0.4–0.6 | 0.4–0.6 | 0.4–0.6 | 0.4–0.6 |
| Citation | 14 | 11 | 32 | NJU laba | NJU laba |
Fourteen common organic chemicals including hydrocarbons, halogenated hydrocarbons, ketones and esters were selected as adsorbates. Their physico-chemical property parameters used in modeling of adsorption capacities, shown in Table 2, were assembled from Lange's Handbook of Chemistry (Fifteenth Edition).
| Adsorbate | Density (kg m−3) | Molar volume (Vm, mL mol−1) | Molar polarizability (MP, cm3 mol−1) | Parachor (Pa, cm3 g1/4·s−1/2 mol−1) | Ionization potential (IP, eV) |
|---|---|---|---|---|---|
| a From Lange's Handbook of Chemistry (Fifteenth Edition). | |||||
| Pentane | 626 | 111.0 | 25.28 | 231.0 | 10.35 |
| Hexane | 659 | 127.5 | 29.90 | 270.8 | 10.13 |
| Heptane | 684 | 144.0 | 34.55 | 310.6 | 9.92 |
| Benzene | 879 | 89.4 | 26.27 | 207.2 | 9.25 |
| Chlorobenzene | 1106 | 101.3 | 31.15 | 243.1 | 9.06 |
| Dichloromethane | 1327 | 67.8 | 16.34 | 148.8 | 11.32 |
| Trichloromethane | 1500 | 79.5 | 21.46 | 184.6 | 11.37 |
| Dichloroethane | 1235 | 84.3 | 21.32 | 188.6 | 11.04 |
| Trichloroethene | 1460 | 89.1 | 25.76 | 210.4 | 9.47 |
| Acetone | 791 | 75.1 | 15.97 | 156.5 | 9.71 |
| Butanone | 805 | 91.6 | 20.60 | 196.3 | 9.51 |
| Methyl acetate | 934 | 81.5 | 17.72 | 176.2 | 10.27 |
| Ethyl acetate | 901 | 98.0 | 22.35 | 216.0 | 10.01 |
| Propyl acetate | 888 | 114.5 | 26.98 | 255.8 | 10.04 |
3.2 Adsorption equilibrium data
Here, adsorption equilibrium data of pentane, hexane, heptane, benzene and butanone on HY-1, benzene and chlorobenzene on HPsorbent, methyl acetate, ethyl acetate and propyl acetate on ND-100 were collected from our previous publications.11,13,27,34 Extra data measured by the authors for dichloromethane on HY-1, trichloromethane, dichloroethane and trichloroethene on Resin-1, and acetone and butanone on ND-100 were also used in this study. The measurements of the adsorption equilibrium of VOCs onto polymeric resins were conducted using a column adsorption method. The detailed experimental apparatus and adsorption procedure have been described previously.10 These data were used as the training set in building D–R model. Nonlinear regressions based on a least-squares criterion were performed on all of the data to obtain best fits for the parameters of E and q0 appearing in the D–R equation. The obtained values of q0 and E, shown in Table 3, will be correlated with properties parameters of adsorbates and adsorbents by using multi-linear regression.| Adsorbent | Adsorbate | Temperature range (K) | q0 (mL g−1) | E (kJ mol−1) | References |
|---|---|---|---|---|---|
| HY-1 | Pentane | 293–323 | 0.706 | 12.80 | 13 |
| Hexane | 293–323 | 0.685 | 12.89 | 13 | |
| Heptane | 293–323 | 0.678 | 15.00 | 13 | |
| Benzene | 303–328 | 0.544 | 11.98 | 29 | |
| Dichloromethane | 308–323 | 0.529 | 9.69 | This study | |
| Butanone | 303–328 | 0.558 | 12.17 | 29 | |
| HPsorbent | Benzene | 303–333 | 0.465 | 13.73 | 11 |
| Chlorobenzene | 303–333 | 0.505 | 13.95 | 11 | |
| ND-100 | Methyl acetate | 303–333 | 0.503 | 9.22 | 34 |
| Ethyl acetate | 303–333 | 0.476 | 10.26 | 34 | |
| Propyl acetate | 303–333 | 0.476 | 10.77 | 34 | |
| Resin-1 | Trichloromethane | 303–333 | 0.500 | 10.68 | This study |
| Dichloroethane | 303–333 | 0.486 | 10.53 | This study | |
| Trichloroethene | 303–333 | 0.407 | 10.47 | This study | |
| Resin-2 | Acetone | 308–328 | 0.509 | 8.58 | This study |
| Butanone | 308–328 | 0.485 | 9.43 | This study |
3.3 Breakthrough curve
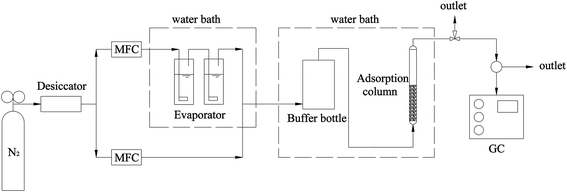
In order to validate the mathematical model of the dynamic adsorption breakthrough process, the breakthrough curves of hexane, heptane on HY-1, and butanone on Resin-2, which are plots of the concentration measured at the outlet in the column versus time, were measured by the column adsorption method, in which the detailed experimental apparatus and adsorption procedure have been described previously.10 Fig. 1 shows the schematic diagram of the experimental apparatus. Briefly, it consisted of a VOCs vapor generator, an adsorption column, and a gas analysis system. The N2 gas containing scheduled concentration of VOCs vapor passed through the adsorption column. The VOCs concentration at the outlet of the adsorption column was measured by using gas chromatography with a FID detector. The breakthrough curves were obtained by recording the concentration of VOCs consecutively at the outlet of adsorption column.4. Results and discussion
4.1 Multi-linear regression for q0 and E of DR model
The MLR models for the q0 and E of DR model are established as two functions of the properties of VOCs and hypercrosslinked polymeric adsorbents respectively. Two kinds of variables for the MLR model were considered in this case: porous parameters of adsorbents, such as micropore volume, specific surface area, average pore diameter, and physic-chemical properties of adsorbates.35,36 Dubinin and co-workers37,38 and others (see Wood15) suggested that the molar polarizability, molar volume, parachor, and ionization potential of adsorbates could be related to adsorption force. All these properties were chosen as preliminary parameters for MLR. Then practical model was established by removing less significant variables to achieve q0 and E.These observed q0 and E data in Table 3 were regressed against the properties of VOCs and hypercrosslinked polymeric adsorbents by SPSS 19 software. Equations are given as the following eqn (8) and (9):
| q0 = 0.361Vmicro + 0.0306IP + 0.0277MP + 0.0177Vm − 0.0103Pa − 0.109 | (8) |
R2 = 0.927, adjusted R2 = 0.891, s.d. = 0.028, F = 25.546, sig. = 0.000
| E = 0.275MP + 0.00318SBET + 1.509 | (9) |
R2 = 0.819, adjusted R2 = 0.791, s.d. = 0.862, F = 29.454, sig. = 0.000
Vmicro – micropore volume, IP – ionization potential, MP – molar polarizability, Vm – molar volume, Pa – parachor, SBET – specific surface area, where R2 is square of correlation coefficient, s.d. is the standard deviation of the regression, F is the Fisher F-statistic, sig. is the P value of significance. The equations are dimensionless, as they are obtained by statistical approach and have no specific physical meaning. The analysis of variance (ANOVA) list for the MLR is showed in Table 4.
The analysis of variance was performed for the regression significance test by F-test. If the significance level α is set as 0.05, F(q0) = 25.546 > Fα(k, n − k − 1) = F0.05(5, 10) = 3.33, F(E) = 29.454 > Fα(k, n − k − 1) = F0.05(2, 13) = 3.80. What is more, P value of significance (sig.) equal to zero indicates that regression equation is highly significant. The relatively higher R2 means satisfactory goodness of fit, demonstrating that the most difference of q0 and E (dependent variables) can be credibly ascribed to the distinction of the properties of VOCs and hypercrosslinked polymeric adsorbents (explicative variables).
4.2 Verifying the accuracy of MLR model
By eqn (8) and (9), q0 and E can be estimated for the adsorption of VOCs onto hypercrosslinked polymeric adsorbents without requiring any adsorption experiments conducted in advance. Fig. 2 shows the observed and calculated q0 and E of various VOCs onto hypercrosslinked polymeric adsorbent. The accuracy of the predictions is assessed by comparing them with experimental adsorption isotherms. The errors between observed and calculated q0 values range from −8.43% to 10.24%, and that of E values range from −12.75% to 10.78%. Relatively good consistency is shown between the calculated values and the experimental values of q0 and E.The calculated equilibrium adsorption amounts in the conditions of experiment are obtained by D–R equation with predicted q0 and E. The comparison between calculated and experimental equilibrium adsorption amounts reflects the accuracy of the MLR equation more clearly. Fig. 3 shows the comparison between experimental and numerical results. It seems that the experimental and predicted equilibrium adsorption amounts in different conditions have the deviation mostly within 10%, while better agreement can be observed at high pressure.
4.3 Prediction of breakthrough curves
In order to predict the breakthrough curves, the column was divided into N equal increments (Δz = L/N), and the greater value of N makes the prediction closer to the actual situation. Predicted breakthrough curves almost do not change when N is greater than 100. In order to save computing time, we used N = 100 to ensure that the prediction was accurate enough.For D–R equation, 0 is not acceptable for P, and complexed computation will occurred by minor increase of P value when P is very close to 0. Therefore, linear relation was used instead of D–R equation when P is between 0 and 0.01 kPa to make the equation could be solved efficiently.
During prediction process, we chose hexane and heptane adsorption on HY-1 (at 293 K, 308 K and 323 K), and butanone on Resin-2 (at 308 K, 318 K and 328 K) to produce results comparison. The prediction results of D–R equation estimated by the method in Section 4.1 were substituted into the prediction model of breakthrough curves.
The comparisons between experimental and numerical results of hexane and heptane adsorption on HY-1 and butanone on Resin-2 are shown in Fig. 4, respectively. Examples of the reasonably good agreement between the model and the experimental breakthrough curves can be observed. The variation of isotherm prediction can enlarge the deviation of breakthrough curve prediction when D–R equation being substituted into equation group. Nevertheless Fig. 5 shows that the experimental and predicted breakthrough time (tb), which is defined as the time when C/C0 = 0.01, has the deviation within 10%.
 | ||
| Fig. 4 Experimental and predicted breakthrough curves for hexane and heptane adsorption on HY-1 and butanone on Resin-2, respectively. | ||
 | ||
| Fig. 5 Observed and calculated breakthrough time of VOCs onto hypercrosslinked polymeric adsorbents. | ||
Because of the good agreement between the experimental and predicted data, it is possible to utilize the proposed model to analyze the relationship of breakthrough curve with time and bed thickness. During model application, these prediction models play an important role when there is no experimental data. As long as the related parameters of adsorbate and adsorbent are known, dimension of device and dosage of adsorbent can be calculated. In addition, enough hypercrosslinked polymeric resins may be filled into adsorption column to make the actual breakthrough time 10–20% longer than predicted in order to obtain the reliable treatment effect.
4.4 Parameter sensitivity
The sensitivity test is performed from the measurements of the breakthrough time tb shown in Fig. 6, which is significant to determine the parameter which may signally influence the adsorption process. The breakthrough time tb is predicted with the parameter values identical to those used in the computation except for the parameter to be tested for sensitivity. U, C0, L (the length of column), q0 and E appear to be the most sensitive parameters, and other factors are not very sensitive. The values of U, C0 and L are definite parameters and can be calculated accurately from the operating conditions. Therefore, the unknown parameters q0 and E in D–R equation become more important for predicting the breakthrough time accurately. In this study, the q0 and E were related well with the properties of polymeric resins and VOCs using multi-linear regression method, and then the reasonable D–R equation was obtained for predicting the adsorption capacities of VOCs on polymeric resins. Therefore, the good accurate prediction for the adsorption capacities makes the breakthrough times prediction also have good accuracy.5. Conclusions
A mathematical model was built to simulate the adsorption breakthrough curves of VOCs onto hypercrosslinked polymeric adsorbents in a fixed bed. First of all, the q0 and E value of D–R equation were related well with the structure properties of resin (micropore volume, specific surface area) and properties of VOCs (molar volume, molar polarizability, parachor, ionization potential) using multi-linear regression method. A reasonably good agreement was obtained between the predicted and experimental equilibrium data with the deviation lower than 10%. Secondly, based on predicted D–R equation and LDF model, the breakthrough curves of hexane, heptane, and butanone were successfully predicted. The breakthrough time had the deviation within 10% between experimental and theoretical results. This may indicate the potential applicability of the model. The main advantage of the approach developed in this work is that the model could be used to determine the breakthrough curves with time as long as the known properties of VOCs and hypercrosslinked polymeric resins without requiring any adsorption experiments conducted in advance.Acknowledgements
This research was financially supported by National Natural Science Foundation of China (Grant No. 51578281).References
- V. A. Davankov and M. P. Tsyurupa, React. Funct. Polym., 1990, 13, 27–42 CrossRef CAS.
- M. P. Tsyurupa and V. A. Davankov, React. Funct. Polym., 2006, 66, 768–779 CrossRef CAS.
- N. Fontanals, M. Galià, P. A. G. Cormack, R. M. Marcé, D. C. Sherrington and F. Borrull, J. Chromatogr. A, 2005, 1075, 51–56 CrossRef CAS PubMed.
- C. Valderrama, X. Gamisans, F. X. D. L. Heras, J. L. Cortina and A. Farrán, React. Funct. Polym., 2007, 67, 1515–1529 CrossRef CAS.
- C. Long, A. M. Li, H. S. Wu and Q. X. Zhang, Colloids Surf., A, 2009, 333, 150–155 CrossRef CAS.
- E. J. Simpson, W. J. Koros and R. S. Schechter, Ind. Eng. Chem. Res., 1996, 35, 1195–1205 CrossRef CAS.
- E. J. Simpson, W. J. Koros and R. S. Schechter, Ind. Eng. Chem. Res., 1996, 35, 4635–4645 CrossRef CAS.
- V. V. Podlesnyuk, J. Hradil and E. Králová, React. Funct. Polym., 1999, 42, 181–191 CrossRef CAS.
- M. P. Baya, P. A. Siskos and V. A. Davankov, J. AOAC Int., 2000, 83, 579–583 CAS.
- P. Liu, C. Long, Q. F. Li, H. M. Qian, A. M. Li and Q. X. Zhang, J. Hazard. Mater., 2009, 166, 46–51 CrossRef CAS PubMed.
- C. Long, Q. F. Li, Y. Li, Y. Liu, A. M. Li and Q. X. Zhang, Chem. Eng. J., 2010, 160, 723–728 CrossRef CAS.
- C. Long, P. Liu, Y. Li, A. M. Li and Q. X. Zhang, Environ. Sci. Technol., 2011, 45, 4506–4512 CrossRef CAS PubMed.
- J. Wu, L. Zhang, C. Long and Q. X. Zhang, J. Chem. Eng. Data, 2012, 57, 3426–3433 CrossRef CAS.
- C. Long, Y. Li, W. H. Yu and A. M. Li, Chem. Eng. J., 2012, 180, 106–112 CrossRef CAS.
- G. O. Wood, Carbon, 2001, 39, 343–356 CrossRef CAS.
- X. H. Ye, N. Qi, Y. Q. Ding and M. D. LeVan, Carbon, 2003, 41, 681–686 CrossRef CAS.
- S. L. Tian, L. Z. Zhu and Y. Shi, Environ. Sci. Technol., 2004, 38, 489–495 CrossRef CAS PubMed.
- L. Li, P. A. Quinlivan and D. R. U. Knappe, Environ. Sci. Technol., 2005, 39, 3393–3400 CrossRef CAS PubMed.
- H. W. Hung, T. F. Lin and J. Air, Waste Manag., 2007, 57, 497–506 CAS.
- J. Giraldo, N. N. Nassar, P. Benjumea, P. P. Almao and F. B. Cortés, Energy Fuels, 2013, 27, 2908–2914 CrossRef CAS.
- J. Wu, M. E. Stromqvist, O. Claesson, I. E. Fangmark and L. Hammarstrom, Carbon, 2002, 40, 2587–2596 CrossRef CAS.
- P. R. Duchowicz, H. Castaneta, E. A. Castro, F. M. Fernandez and J. L. Vicente, Atmos. Environ., 2006, 40, 2929–2934 CrossRef CAS.
- P. J. Reucroft, W. H. Simpson and L. A. Jonas, J. Phys. Chem., 1971, 75, 3526–3531 CrossRef CAS.
- K. Urano, S. Omori and E. Yamamoto, Environ. Sci. Technol., 1982, 16, 10–14 CrossRef CAS.
- M. J. Lashaki, M. Fayaz, S. Niknaddaf and Z. Hashisho, J. Hazard. Mater., 2012, 241–242, 154–163 CrossRef PubMed.
- M. M. Dubinin, E. D. Zaverina and L. V. Radushkevich, Zh. Fiz. Khim., 1947, 21, 1351–1362 CAS.
- C. Long, Y. Li, W. H. Yu and A. M. Li, J. Hazard. Mater., 2012, 203–204, 251–256 CrossRef CAS PubMed.
- S. Brosillon, M. H. Manero and J. N. Foussard, Environ. Sci. Technol., 2001, 35, 3571–3575 CrossRef CAS PubMed.
- T. B. Cheng, Y. Jiang, Y. P. Zhang and S. Q. Liu, Carbon, 2004, 42, 3081–3085 CrossRef CAS.
- R. Chauveau, G. Grévillot, S. Marsteau and C. Vallières, Chem. Eng. Res. Des., 2013, 91, 955–962 CrossRef CAS.
- F. Delage, P. Pré and P. L. Cloirec, Environ. Sci. Technol., 2000, 34, 4816–4821 CrossRef CAS.
- G. Sylvain, P. Pascaline and L. C. Pierre, Environ. Sci. Technol., 2009, 43, 1173–1179 CrossRef.
- E. Glueckauf, Theory of chromatography. Part 10, Trans. Faraday Soc., 1955, 51 Search PubMed.
- L. Y. Wu, L. J. Jia, X. H. Liu and C. Long, Front. Environ. Sci. Eng. DOI:10.1007/s11783-015-0826-6.
- L. B. Kier and L. H. Hall, Res. Stud., Wash. State Univ., 1986, 69–75 CAS.
- C. Brasquet, B. Bourges and P. L. Cloirec, Environ. Sci. Technol., 1999, 33, 4226–4231 CrossRef CAS.
- M. M. Dubinin and E. Sawerina, Acta Physicochim. URSS, 1936, 4, 647–674 CAS.
- M. M. Dubinin and P. Tomofeyev, Dokl. Akad. Nauk SSSR, 1946, 54, 701–704 CAS.
| This journal is © The Royal Society of Chemistry 2016 |