Recent developments on the structure–activity relationship studies of MAO inhibitors and their role in different neurological disorders
Bhupinder Kumar
a,
Sheetal
a,
Anil K. Mantha
bc and
Vinod Kumar
*a
aLaboratory of Organic and Medicinal Chemistry, Centre for Pharmaceutical Sciences and Natural Products, Central University of Punjab, Bathinda, Punjab, India-151001. E-mail: vpathania18@gmail.com; vinod.kumar@cup.ac.in; Tel: +911642864214
bCentre for Animal Sciences, School of Basic and Applied Sciences, Central University of Punjab, Bathinda, Punjab, India
cDepartment of Biochemistry and Molecular Biology, University of Texas Medical Branch, Galveston, TX-77555, USA
First published on 20th April 2016
Abstract
Monoamine oxidase (MAO) enzyme catalyzes the oxidative deamination of xenobiotic and endogenous amines including many neurotransmitters. The MAO enzyme exists in two isoforms; MAO-A and MAO-B and these isoforms display considerable sequence similarity but differ in tissue distribution, inhibitor selectivity and specificity towards ligands. The altered concentration of the neurotransmitters in the brain is linked with the biochemical pathology of various neurological disorders including depression, Alzheimer's disease and Parkinson's disease. MAO inhibitors were the first antidepressants discovered but their irreversible binding to the enzyme resulted in a number of side effects including fatal food–drug interactions. The new generation MAO inhibitors, especially reversible and selective inhibitors, were less toxic and found to be effective against various neurological disorders. Now the MAO enzyme has been recognised as an important drug target and MAO-A selective inhibitors are being developed as drug candidates for the management of depression and anxiety disorders, whereas MAO-B selective inhibitors are found to be effective for the treatment of Parkinson's disease and Alzheimer's disease with a better safety profile as compared to nonselective MAO inhibitors. The current review article describes recent developments on the design, synthesis and screening of MAO inhibitors, structure–activity relationship studies, and their role in the etiology and treatment of various neurological disorders.
1. Introduction
Monoamine oxidases (MAO) belong to a family of flavin adenine dinucleotide (FAD) dependent enzymes present on the outer membrane of the mitochondria.1 MAOs are responsible for the oxidative deamination of various xenobiotic and endogenous neurotransmitters and modulate their concentration in the brain and peripheral tissues.2 The enzyme is found to exist in two isoforms, MAO-A and MAO-B wherein both possess a sequence similarity of 73% but differ mainly in their substrate specificity, inhibitor selectivity and tissue distribution.3,4 The MAO-A isoform has substrate specificity for bulkier endogenous amines like serotonin, epinephrine and norepinephrine whereas MAO-B has substrate specificity for small amines such as benzylamine, β-phenyl ethylamine.5 Dopamine and tyramine are common substrates for both the enzyme isoforms. MAO enzyme is mostly found in the brain, however it is also reported in the gut, liver, placenta, skin, platelets and lymphocytes. The amine in the deprotonated form binds to the active site of the enzyme and is oxidised to imine with the reduction of 8α-S-cysteinyl FAD to its hydroquinone form.6 The reduced FAD cofactor reacts with oxygen (O2) to produce oxidised FAD and hydrogen peroxide which in turn generate highly toxic hydroxyl radicals responsible for the neuronal damage and death.7,8 The imbalance in the concentration of these neurotransmitters in the brain is linked with the biochemical pathology of various neurogenic disorders9 including depression, Alzheimer's disease (AD), and Parkinson's disease (PD). Various studies have confirmed that MAO-B is overexpressed in the brain of AD patients and is linked with the loss of cognitive functions.10 In similar reports it has been shown that there is a correlation between the low levels of monoamine neurotransmitters associated with severe depression cases and increased concentration of MAO-A in the cortex.11,12MAO inhibitors can block the catalytic activities of the enzyme and halt the catabolism process of monoamines. Inhibition of MAO enzyme, improve the concentration of the monoamine neurotransmitters (dopamine, serotonin etc.) stored in the nerve terminals. Thus MAO inhibitors can be developed as therapeutic agents in the disease state where MAO enzyme is overexpressed. In addition to this, MAO-catalysed metabolism of monoamine neurotransmitters generates hydrogen peroxide, toxic aldehydes and hydroxyl free radicals which are responsible for the neuronal damage and death. MAO inhibitors stop the production of these neurotoxic byproducts by halting monoamine oxidation process and hence prevent the resulting damage to the neurons. Hence, MAO inhibitors have also been proposed as potential neuroprotecting and neurorescue agents.
The X-ray crystal structures of human MAO-B and human MAO-A were reported in year 2002 and 2005 respectively (Fig. 1).13,14 Crystallographic studies of the enzyme showed that human MAO-B and rat MAO-A crystallises as dimer15,16 while human MAO-A crystallizes as monomeric unit. However, it has been observed that most of the membrane proteins are dimeric in the membrane form.17 Andres et al.18 through modeling studies and species-dependent genetic analysis concluded that the monomeric structure of human MAO-A could be due to Glu151Lys mutation near the dimer interface. The EPR data showed that rat and human MAO-A and MAO-B enzymes are dimeric in the membrane bound forms however human MAO-A crystallizes as monomer while MAO-B crystallizes as dimer.19 The volume of human MAO-A and MAO-B cavities are ∼400 Å3 and ∼700 Å3 respectively. The MAO-B cavity is divided into two separate spaces, the active site cavity for substrate binding (∼400 Å3) and the entrance cavity (∼300 Å3).20 Ile199 and Tyr326 side chains separate the two cavities in MAO-B isoform. Mutagenesis experiments on human MAO-B, Ile199Phe showed that bulky Phe side chain hampers conformational flexibility, reduces the space of the entrance cavity and interferes with the binding of MAO-B selective inhibitors.21 Thus Ile199 serves as a structural determinant for substrate and inhibitor recognition. Similarly, Tyr326 exhibit its conformational changes on the inhibitor binding to human MAO-B isoform. A single Ile199Ala mutation to the MAO-B permanently open the gate and double mutations Ile199Ala–Tyr326Ala exhibit inhibitor binding properties similar to those of MAO-A than to MAO-B. This shows that the bipartite cavity structure in the MAO-B plays an important role in substrate and inhibitor recognition to distinguish its specificities from those of MAO-A. The entrance cavity may act as an allosteric binding site for the MAO-B selective inhibitors and potentiation of ligand binding to the entrance cavity of MAO-B by the ligand occupancy in the substrate cavity opens up possibilities for the design of highly specific MAO-B inhibitors that specifically target the entrance cavity space.23 This information would be helpful in designing selective inhibitors for MAO-A and MAO-B isoforms.20
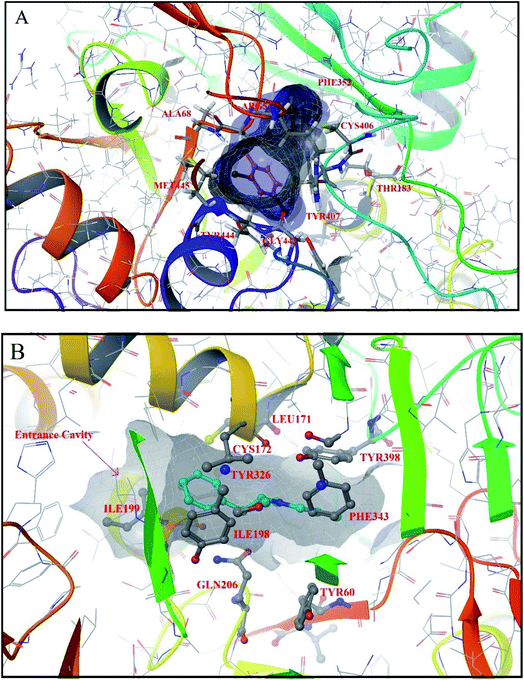 | ||
| Fig. 1 (A) The co-crystallized structure of clorgyline with human MAO-A PDB ID (2Z5X).22 The figure shows ligand interactions at the active site of MAO-A isoform with various amino acids; (B) the co-crystallized structure of human deprenyl with MAO-B (dimeric) PDB ID (2BYB).14 In the figure entrance cavity of the MAO-B isoform is shown along with the ligand's interactions with various amino acids at the active site cavity. | ||
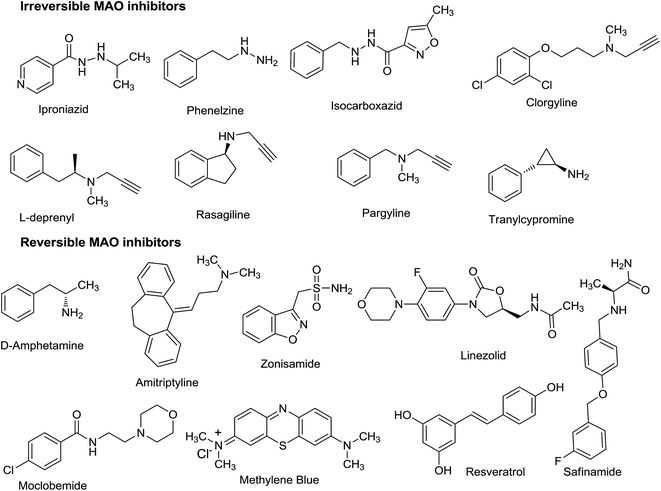
2. Problems with the irreversible MAO inhibitors
In the 1950's, irreversible MAO inhibitors were predominantly used for the treatment of depression but their use diminished in the following years due to their undesired side effects and fatal drug–food interactions. Various irreversible MAO inhibitors24 are shown in Fig. 2. One of the major problems associated with the use of irreversible MAO inhibitors was their interaction with the tyramine containing foods like old cheese and wine, as a consequence of inhibition of the isoenzyme MAO-A in the intestine (cheese-effect).25 Normally, the MAO enzyme removes excess tyramine out of the body, but in the presence of MAO inhibitors the catalytic action of MAO enzyme is halted which results in the increased level of tyramine in the blood.26 The excess amount of tyramine present in the blood causes the release of noradrenaline from the peripheral adrenergic neurons. This may leads to severe increase in the blood pressure,27 termed as hypertensive crisis.28,29 In addition, irreversible MAO inhibitors were also reported to exhibit various side effects like tachycardia, photophobia, palpitation, nausea and fatal drug–drug interactions. Irreversible MAO inhibitors like isoniazid and iproniazid have been withdrawn from the market in 1961 due to their high hepatotoxicity30,31 and research focus was shifted to new alternatives like selective serotonin reuptake inhibitors (SSRI), serotonin nor-epinephrine reuptake inhibitors and tricyclic antidepressant. However MAO inhibitors were the only effective medication available for the management of severe and treatment-resistant depression.32To address various problems associated with the first generation irreversible MAO inhibitors, new classes of compounds were developed which were not only selective for either of the MAO isoform but were also reversible in nature. Selective MAO-A inhibitors have potential to be developed as drugs for the treatment of depression and anxiety disorders,33 whereas MAO-B selective inhibitors are effective in several neurological disorders such as PD, AD, Huntington chorea and amyotrophic lateral sclerosis.34 The reversible and selective MAO-A inhibitors (Fig. 2) are devoid of ‘cheese-effect’35 and these ligands can inhibit the enzyme with decreased risk of tyramine reaction. The absorbed tyramine releases norepinephrine which competes with the reversible MAO-A inhibitors and reactivate the MAO-A enzyme in the intestine, liver and sympathomimetic neurons. In addition, a new delivery system has been developed in which MAO inhibitor like selegiline can be delivered directly to the blood circulation through skin patches.36,37 This inhibits both MAO-A and MAO-B in the brain but avoid drug interactions with the MAO enzyme in the gut. These new developments reduce the risk of hypertensive crisis making MAO inhibitors comparatively safer therapeutic agents.
Many of the side effects associated with the irreversible MAO inhibitors have been curtailed with the development of selective MAO inhibitors and now the MAO enzyme has been recognised as an important and attractive drug target for the treatment of various neurogenic disorders. In addition, recent reports on the neuroprotective and neurorescue potential38,39 of MAO inhibitors have generated enormous interests for exploring their role in the etiology of various neurogenic disorders. Since last few years, there is a surge in the number of research articles published on the synthesis and screening of selective and reversible MAO inhibitors. The PubMed data base shows more than 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 publications on the MAO enzyme. A variety of structurally different scaffolds have been screened for their selectivity and MAO inhibitory potential. Structure–activity relationship studies were conducted to identify important leads with an aim to develop them as potent and effective therapeutic agents for the treatment of various neurological disorders. A considerable progress has been made to understand the ligand interactions with the active site of the enzyme but it is difficult to generalize the results for MAO-A and MAO-B selectivity on the basis of chemical structure. However, through various molecular modeling studies and available crystal structures of the MAO enzymes it was concluded that the MAO-A isoform can accommodate bulkier ligands while the MAO-B isoform prefer smaller ligands due to its small entrance cavity. A number of review articles describe developments in the field of MAO inhibitors. For example, Norman et al.40 investigated the role of MAO inhibitors in panic disorders. Liebowitz et al.41 examined the role of reversible and irreversible MAO inhibitors in the psychiatric disorders. Similarly, Helguera et al.42 have reviewed various research articles on MAO-B inhibitors containing oxygen as heterocyclic atom. Al-Nuaimi et al.43 presented an overview of the neuroprotective/neurorescue properties of MAO inhibitors and their possible neuroprotective mechanisms. Recently role of the pyrazoline moiety44 has been explored as a promising scaffold for the inhibition of MAO enzyme. Similarly, a recent review on coumarin derivatives as MAO inhibitors highlighted the design and synthetic aspects of the coumarin based ligands and their role in the depression and AD. Patil et al.45 described synthetic aspects of nitrogen heterocycles as potential MAO inhibitors. Carradori et al.46 reviewed various selective MAO-B inhibitors derived from natural sources and belonging to different chemical classes. Most of these review articles are limited to discussion on a particular chemical class or describe MAO-A or MAO-B specific inhibitors and lack comprehensive discussion on compounds belonging to different chemical classes and their selectivity for either of the two MAO isoforms.
000 publications on the MAO enzyme. A variety of structurally different scaffolds have been screened for their selectivity and MAO inhibitory potential. Structure–activity relationship studies were conducted to identify important leads with an aim to develop them as potent and effective therapeutic agents for the treatment of various neurological disorders. A considerable progress has been made to understand the ligand interactions with the active site of the enzyme but it is difficult to generalize the results for MAO-A and MAO-B selectivity on the basis of chemical structure. However, through various molecular modeling studies and available crystal structures of the MAO enzymes it was concluded that the MAO-A isoform can accommodate bulkier ligands while the MAO-B isoform prefer smaller ligands due to its small entrance cavity. A number of review articles describe developments in the field of MAO inhibitors. For example, Norman et al.40 investigated the role of MAO inhibitors in panic disorders. Liebowitz et al.41 examined the role of reversible and irreversible MAO inhibitors in the psychiatric disorders. Similarly, Helguera et al.42 have reviewed various research articles on MAO-B inhibitors containing oxygen as heterocyclic atom. Al-Nuaimi et al.43 presented an overview of the neuroprotective/neurorescue properties of MAO inhibitors and their possible neuroprotective mechanisms. Recently role of the pyrazoline moiety44 has been explored as a promising scaffold for the inhibition of MAO enzyme. Similarly, a recent review on coumarin derivatives as MAO inhibitors highlighted the design and synthetic aspects of the coumarin based ligands and their role in the depression and AD. Patil et al.45 described synthetic aspects of nitrogen heterocycles as potential MAO inhibitors. Carradori et al.46 reviewed various selective MAO-B inhibitors derived from natural sources and belonging to different chemical classes. Most of these review articles are limited to discussion on a particular chemical class or describe MAO-A or MAO-B specific inhibitors and lack comprehensive discussion on compounds belonging to different chemical classes and their selectivity for either of the two MAO isoforms.
The scope of this review article is to highlight some recent developments in the field of MAO inhibitors. The current review article would focus on the synthesis, biological evaluation and relative selectivity of various ligands for the MAO-A and MAO-B enzyme isoforms and their potential role in different neurological disorders. Recent research articles on the MAO inhibitors have been reviewed in this manuscript and most of these were published in the last five years (from year 2010 onwards). The patents published on the subject were not covered in the present review article. The review article has been divided into three different sections describing selectivity of the ligands for MAO-A and MAO-B isoforms and their role in the etiology and treatment of PD, AD and depression. Although MAO inhibitors have been also linked with the etiology of many other disease states, however the scope of this review article is limited to their role in neurological disorders only, more specifically in PD, AD and depression.
3. Role of MAO inhibitors in Parkinson's disease
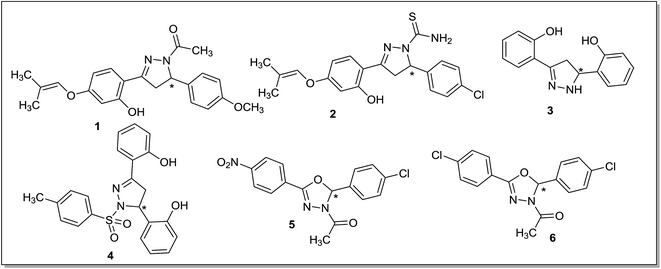
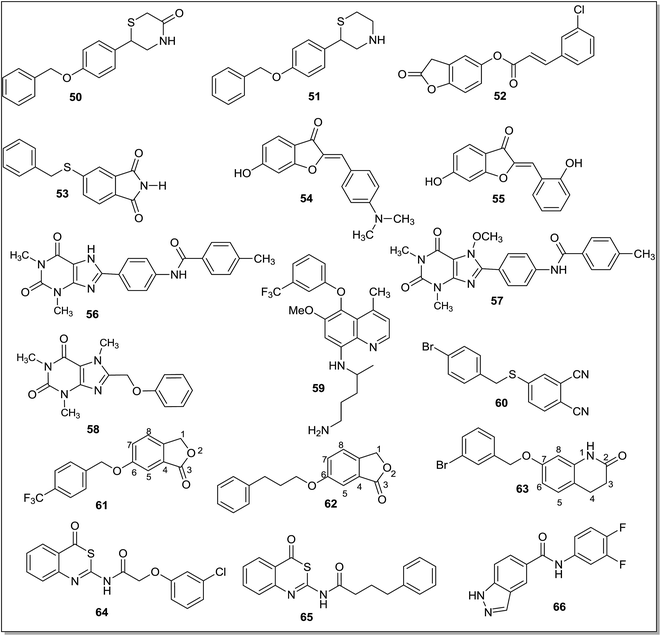
The MAO-B levels in the human increase four to five times on aging47,48 and hyperactivation of MAO-B is linked with the etiology of age related neurological disorders including PD.49 PD affects the basal ganglia region of the brain where MAO-B isoform seems to be significantly responsible for the metabolism of dopamine. Overactivation of the MAO-B isoform can diminish dopamine levels and increase the concentration of the catalytic reaction product H2O2 in the brain of patients suffering with PD. This promotes apoptotic signaling events resulting in the decreased levels of dopamine producing cells. Therefore inhibitors of the MAO-B isoform have been proposed as potential therapeutic agent for the management or treatment of PD.50 Levodopa, a metabolic precursor of dopamine, and MAO-B inhibitors are given to the PD patients as combination therapy to maintain dopamine levels. MAO-B inhibitors stop the catalytic metabolism of dopamine and raise the dopamine levels in the patient's brain and hence, a reduced dose of levodopa is required for getting a therapeutic effect. In addition, the MAO enzyme is responsible for the production of hydrogen peroxide and hydroxyl free radicals in the cells that causes neuronal damage and death. MAO-B inhibition reduces the generation of these reactive oxygen species and hence limits the neurodegenerative processes associated with the PD.51 In order to develop an effective therapeutic agent for the treatment of PD, a large number of compounds have been designed, synthesized and screened as reversible and selective MAO-B inhibitors and some of the recent reports are described below.Fioravanti et al.52 reported a series of N-substituted-3-[(2′-hydroxy-4′-prenyloxy)-phenyl]-5-phenyl-4,5-dihydro-(1H)-pyrazolines and the compounds were screened against the human MAO-A and MAO-B isoforms. In the reported series, compound 1 (IC50 = 4.22 μM) and 2 (IC50 = 6.08 μM) (Fig. 3) displayed good inhibitory activities and selectivity towards MAO-B isoform. Through structure–activity relationship studies it was found that the methoxy and chloro substitutions at the para position of the phenyl ring were essential for the activity. In addition, through molecular docking studies it was found that the isoprenyloxy moiety was linked with the FAD cofactor and the C5 pyrazoline substitution in the outer side of the hMAO-B cleft. The presence of different substituents like isoprenyloxy, benzyloxy, thioamide and phenolic hydroxyl group in the pyrazoline moiety improved interactions with the active site of hMAO-B. A series of pyrazoline derivatives with a 3,5-diaryl substitution was also reported53 and most of the compounds displayed reversible and selective inhibitor activities for the MAO-A or MAO-B isoform. Amongst the synthesized derivatives, compound 3 (IC50 = 780 nM) was found to be selective inhibitor of the MAO-B isoform while compound 4 (IC50 = 360 nM) was found to be selective for the MAO-A isoform. The molecular docking studies showed that compounds 3 and 4 (Fig. 3) were interacting with the enzyme through hydrogen bonding and a substitution at N1 position significantly increases the selectivity towards MAO-A isoform.
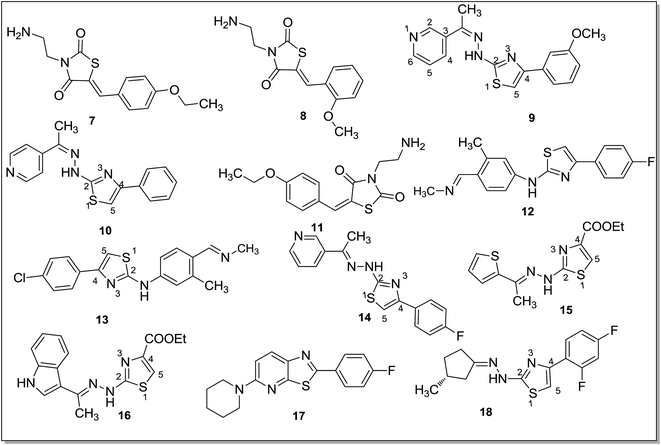
A series of 3-acetyl-2,5-diaryl-2,3-dihydro-1,3,4-oxadiazoles54 was tested and some of the compounds were selective towards MAO-B isoform displaying IC50 values in the nanomolar range. Compound 5 (IC50 = 121.6 nM) and 6 (IC50 = 115.3 nM) (Fig. 3) were the most potent MAO-B inhibitors. The docking studies revealed that these compounds were stabilized by interactions between 4-chlorophenyl group at the C5 and aromatic ring of Tyr435 near the FAD cofactor. The presence of an electron withdrawing group at the aromatic ring was crucial for the hydrophobic interactions within the binding pocket of the enzyme. The electron releasing substituents at the same position induces different orientation of the molecule and led to less stable derivatives. Structure-based virtual screening of pioglitazone55 was performed to identify novel and selective MAO-B inhibitors. The thiazolidinedione (TZD) moiety was important for binding to the MAO-B isoform. Two active compounds 7 and 8 (Fig. 4) were identified as potent MAO-B inhibitors with IC50 values of 82 nM and 195 nM respectively. Chimenti et al.56 reported a series of [4-(3-methoxyphenyl)-thiazol-2-yl]hydrazine derivatives as MAO inhibitors. Most of them showed IC50 values in the nanomolar range. The most active compound (9) (Fig. 4) obtained in this series displayed an IC50 value of 3.8 ± 0.1 nM, showed high selectivity (119 times) and five times more potency than the standard drug R-(−)-deprenyl for the human MAO-B isoform. From the molecular modeling studies, it was found that C4 of the thiazole ring was responsible for the interactions between the inhibitor and FAD cofactor which control the oxidative activity of the MAO enzyme. It was concluded that even a small modification of the substituent's dimension at the C4 position could attenuate the activity of the compound. Chimenti et al.57 also synthesized and evaluated 4-substituted-2-thiazolylhydrazone derivatives. Compound 10 (Fig. 4) with a C4 acetylpyridine moiety was found to be the most potent MAO-B inhibitor with IC50 value of 13.0 ± 1.2 nM. It was concluded that a thiazole ring as a substituent at the C4 position was required to obtain highly potent and selective hMAO-B inhibitors.
Carroll et al.58 performed structure–activity relationship studies and docking experiments on the thiazolidinedione type compounds and evaluated these for the MAO inhibition activity. These compounds selectively inhibited MAO-B isoform and compound 11 (Fig. 4) was found to be the most potent with an IC50 value of 82 nM (MAO-B). Compounds having an amino-ethyl group on the thiazolidinedione nitrogen caused the extension of the substrate cavity pushing it closer to the FAD moiety, which could be responsible for the increase in MAO-B inhibition activity.
Distinto et al.59 reported on the synthesis and screening of halogenated 1-arylidene-2-(4-phenylthiazol-2-yl)hydrazines. The objective of the study was to investigate the role of 4-chloro- or 4-fluorophenyl substituents on the MAO inhibitory activity of the ligands. The fluorine as a substituent played pivotal role both in imparting activity and selectivity to the ligands towards MAO-B isoform. In the computational studies it was observed that the fluorine substituent was interacting with the water molecule close to cofactor and information about these ligand–receptor interactions could help in designing compounds with improved selectivity and potency for the MAO-B isoform. The 4-fluorophenyl (12) and analogous 4-chlorophenyl (13) (Fig. 4) derivatives were the most potent MAO-B inhibitors in the series.
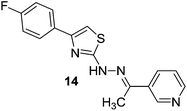
A series of arylidene-(4-substituted-thiazol-2-yl)hydrazines60 was reported as selective MAO-B inhibitors. They were mostly found to be selective for the hMAO-B isoform and displayed inhibition activity in the nanomolar range, higher than the reference drug. A pyridine ring with electron withdrawing substituents on the aryl ring at C4 of the thiazole nucleus and a substituent on the α-carbon to the N1-hydrazine moiety had a great influence on the activity and hMAO-B selectivity of the inhibitors. A methyl substituent in place of hydrogen on the α-carbon showed an improved hMAO-B inhibitory activity. Compound 14 (Fig. 4) was the most active and selective derivative with an IC50 value of 2.54 ± 0.22 nM and selectivity ratio more than 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 370 for the MAO-B isoform.
370 for the MAO-B isoform.
A series of hydrazothiazole derivatives61 was designed by joining the hydrazine part of iproniazid and thiazole moiety of glitazones. Most of them displayed selective hMAO-B inhibitory activity. SAR studies suggested that a small or less bulky substituent at the C4 (ethyl ester) position was essential for the inhibitory activity and lead to compounds 15 and 16 (Fig. 4) with IC50 values of 350.0 ± 26.1 nM and 851.3 ± 64.8 nM, respectively. These compounds were found to be more selective towards MAO-B isoform than the reference drugs iproniazid and isatin with SI of 285.7 and 117.5, respectively. Oxazolopyridine and thiazolopyridine derivatives62 were designed and evaluated against the MAO-B isoform. A series of 2-phenyloxazolopyridine derivatives was synthesized with various amino substituents and these compounds were tested against the hMAO-B isoform. Presence of a piperidino group was found essential for the activity. The phenyl ring was also substituted with a halogen atom which improved the inhibitory activity of the ligands. By replacing the basic structure from oxazolopyridine to thiazolopyridine, the activities were dramatically improved and compound 17 (Fig. 4) was the most potent in the series displaying IC50 value of 26.5 nM. D'Ascenzio et al.63 reported a series of 4-aryl-2-cycloalkylidenhydazinylthiazoles and studied the stereochemical properties of these compounds required for the MAO inhibitory activity. The 2- and 3-methylcyclohexylidene derivatives displayed good inhibitory activity and high selectivity towards MAO-B isoform. In the biological assay studies, compound 18 (Fig. 4) was reported as the most active and selective MAO-B inhibitor. The selectivity of compound 18 was further confirmed by docking studies where it showed better docking score and interaction energy with the MAO-B as compared to MAO-A isoform. In the MAO-A isoform, 18 has shown steric hindrance with four residues, Phe352, Lys305, Tyr444 and Tyr407.
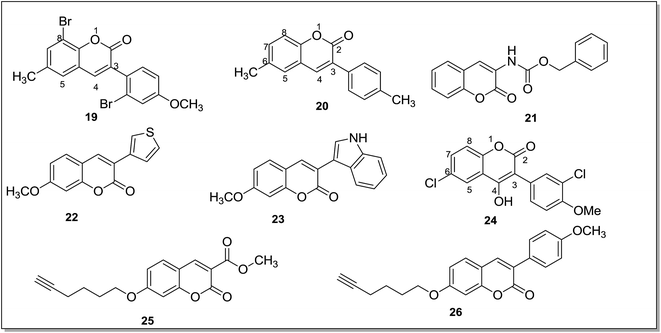
Matos et al.64 synthesized a series of bromo-6-methyl-3-phenylcoumarin derivatives and evaluated these against MAO enzyme. A halogen substituent on the coumarin moiety and a bromo-substituent at C8 position of the 3-phenyl coumarin improved inhibitory activity for the MAO-B isoform. A p-methoxy substitution on the phenyl ring (19) (Fig. 5) further improved the activity and it was found to be more selective and more potent (6 times) than the reference drug R-(−)-deprenyl. Compound 19 was the most potent and selective MAO-B inhibitor in the series with IC50 value in low nanomolar range. The same research group65 synthesized another series of coumarin derivatives, i.e., 6-substituted 3-arylcoumarins and evaluated them, in vitro, as human MAO-A and MAO-B inhibitors. In this series, compound 20 (Fig. 5) was found to be the most active and selective MAO-B inhibitor (IC50 = 0.31 ± 0.02 nM) with almost 64 times more potency than the standard inhibitor selegiline. A methyl or methoxy substituent at the C6 position of the coumarin and phenyl group at the C3 position were essential for the MAO-B inhibitory activity while presence of a bulkier group at the C6 position severely decreased the MAO-B inhibitory activity. The ADME properties of a series of (coumarin-3-yl)carbamates were also evaluated66 in order to determine the blood brain barrier permeation. In the reported series of compounds, benzyl(coumarin-3-yl)carbamate (21) (Fig. 5) was found to be the most active MAO-B inhibitor in the in vitro studies with an IC50 value of 45 nM which was very close to the standard inhibitor selegiline, (IC50 = 20 nM). In addition, in vivo assays and docking studies were also performed for the lead compound and administration of compound 21 (100 mg kg−1) in mice, pretreated with the reserpine improved the motor activity, velocity and movement as done by the standard inhibitor selegiline. A series of 3-indolyl and 3-thiophenylcoumarins67 was tested against human MAO enzyme.
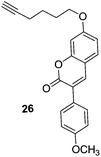
The compounds showed significant inhibitory activity towards hMAO-B isoform. The position of methoxy substituent on the 3-heteroarylcoumarin ring played crucial role in the activity of compounds. In the reported series, a methoxy substituent at the C7 position of compounds 22 (IC50 = 55.6 nM) and 23 (IC50 = 45.9 nM) (Fig. 5) increased the MAO-B inhibitory activity and selectivity. In the docking studies, compounds 22 and 23 displayed good binding affinity for MAO-B isoform. A series of 3-aryl coumarin derivatives68 was synthesized by introducing hydroxyl group at the C4 position of B ring of the coumarin skeleton. The compounds were evaluated for their affinity and selectivity for the MAO-B isoform. Structure–activity relationship studies revealed that the substitution of methoxy and chloro groups in the para and meta position of the 3-phenyl ring were crucial for the MAO-B inhibitory activities. Compound with a chloro substituent at the C6 position of coumarin ring (24) (Fig. 5) was the most potent inhibitor in the series with IC50 value of 2.79 μM and showed better selectivity (36 folds) towards MAO-B isoform. Mertens et al.69 synthesized thirty one alkynyl–coumarinyl ethers as MAO-B inhibitors and the 7-hex-5-ynyloxycoumarin derivative (25) (Fig. 5) was identified as dual inhibitor of both MAO-A and MAO-B isoforms with IC50 values of 9.6 ± 0.8 nM and 1.41 ± 0.15 nM respectively whereas the 3-(4-methoxy)phenyl derivative (26) was found to be a selective inhibitor of MAO-B isoform with IC50 value of 2.96 ± 0.10 nM. It exhibited more than 3400 folds selectivity over MAO-A isoform. The compounds were found to be reversible inhibitors and it was concluded that the triple bond in these compounds was not making them an irreversible inhibitor as done by propargyl group present in the irreversible inhibitor selegiline.
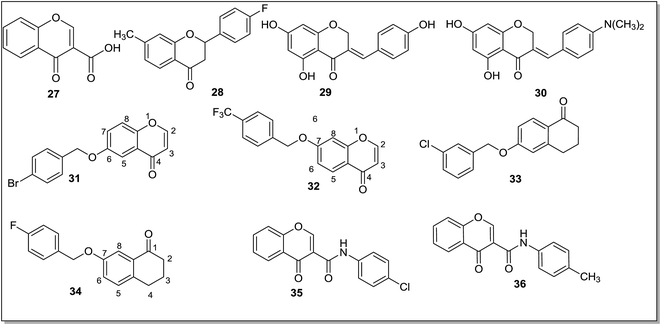
Chromone moiety plays a vital role in various medicinal and biological activities such as anti-inflammatory, antitumor and antimicrobial activities. The MAO inhibitory activity of the chromone moiety was also evaluated by number of research groups. Alcaro et al.70 screened two chromone isomers for their affinity towards MAO-A and MAO-B isoforms and it was found that the substitutions of an acidic group at C3 position of the heterocyclic scaffold (27) (Fig. 6) significantly increased the inhibitory activity towards MAO-B isoform (IC50 = 48 ± 2.6 nM) whereas an acid group at C2 position caused no inhibition. Chromone-3-carboxylic acid was found to be the most effective and selective MAO-B inhibitor. Molecular docking studies revealed that a hydrogen donor moiety should be placed at the position three of the pyrone ring (27) for favorable energy minimization and selectivity towards MAO-B isoform. A number of flavones, thioflavones and flavanones were evaluated71 as potential inhibitors of the MAO isoforms. The flavanone series was found to be most active against MAO-B isoform wherein the lead compound 28 (Fig. 6) displayed IC50 value of 130 ± 0.7 nM and selectivity index of about 769. The introduction of a double bond (flavones series) or of sulfur atom (thioflavones) to the flavanones led to substantial decrease in the MAO-B inhibitory activity. Desideri et al.72 synthesized different series of chromone derivatives and evaluated these for the MAO inhibitory activity. The (E)-3-benzylidenechroman-4-one derivatives exhibited activity in nanomolar range and higher selectivity towards hMAO-B isoform. The compounds (E)-3-(4-(dimethylamino)benzylidene)chroman-4-one (29) (IC50 = 8.61 ± 0.12 nM) and (E)-5,7-dihydroxy-3-(4-hydroxybenzylidene)chroman-4-one (30) (IC50 = 8.15 ± 0.32 nM) (Fig. 6) were reported as the most potent MAO-B inhibitors in the series, with even better activity profile than the reference drug selegiline. Legoabe et al.73 reported a series of chromone derivatives73 substituted with alkyloxy group at C6 position and evaluated them as inhibitors of recombinant human MAO-A and MAO-B isoforms. The C6 substituted chromones were found to be potent reversible MAO-B inhibitors with IC50 values in sub nanomolar range. These compounds bind reversibly to MAO-A, but with less affinities when compared with MAO-B isoform and hence it was concluded that the synthesized compounds were selective MAO-B inhibitors. In the docking experiment the lead compound (31) (Fig. 6) exhibited differing interactions and orientation within the two isoforms. The C6 substituted chromone derivatives possessed potency against MAO-B isoform and could be used as a promising lead for the development of potent drug candidate against PD. The same research group73 further carried out the modifications at the C7 position of the chromones and synthesized a series of C7 substituted chromone (1-benzopyran-4-one) derivatives. The compounds were evaluated for their potential to inhibit recombinant human MAO enzyme and exhibited IC50 values ranging from 8 nM to 370 nM for MAO-B isoform and IC50 values ranging from 495 nM to 803 nM for MAO-A isoform. 7-Benzyloxy substitution of chromone (32) (Fig. 6) was crucial for MAO-B inhibition activity. A series of fifteen α-tetralone (3,4-dihydro-2H-naphthalen-1-one) derivatives74 was evaluated for MAO inhibitory activity. In the synthesized series, 6-(3-iodobenzyloxy)-3,4-dihydro-2H-naphthalen-1-one (33) (Fig. 6) was found to be the most active MAO-B inhibitor exhibiting IC50 value 4.5 nM with 287 folds selectivity while the cyano-analogue 6-(3-cyanobenzyloxy)-3,4-dihydro-2H-naphthalen-1-one exhibited greater selectivity towards MAO-A isoform with an IC50 value of 24 nM and 3.25 folds selectivity. Structure–activity relationship studies showed that the substitution at the C6 position was essential for the MAO inhibitory activity. A benzyloxy substitution increased the MAO-A inhibitory activity and an alkyl and halogen substitution at the meta and para position of benzyloxy, promotes MAO-B inhibitory activity. Similarly, a series of fifteen C7 substituted α-tetralone derivatives75 was investigated for MAO inhibitory potential. Arylalkyloxy substitution on C7 position of the α-tetralone moiety improved potencies of the compounds towards hMAO-B isoform displaying IC50 values in submicromolar range. The synthesized α-tetralones were found to be selective for the MAO-B isoform over the MAO-A and all the compounds displayed IC50 values in submicromolar range (IC50 values < 0.47 nM). The most potent MAO-B inhibitor in the series was 7-(4-fluorobenzyloxy)-3,4-dihydro-2H-naphthalen-1-one (34), showing an IC50 value of 0.89 nM and SI of 13 over MAO-A. In the docking studies it was found that hydrogen bonding played relatively smaller role in inhibitor stabilization compared to the van der Waals interactions.
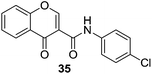
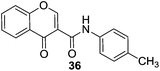
Gaspar et al.76 synthesized two different series of chromone derivatives (substituted at 2 and 3 position) and evaluated the compounds as MAO inhibitors. The chromones with a meta substituent on γ-pyrone nucleus showed selectivity towards the MAO-B isoform with IC50 values in the micromolar to nanomolar range. Most of the chromone 3-carboxamide derivatives exhibited selectivity towards the MAO-B isoform of the enzyme. In the reported series, compound 35 (Fig. 6) with a chloro substitution at the para position of the exocyclic aromatic ring was the most potent and selective inhibitor of the MAO-B isoform with an IC50 value of 63 nM and SI greater than 1500 towards MAO-B. Docking experiments of 35 showed better stabilization of the compound in the MAO-B cavity than in the MAO-A cavity. Two different series of compounds based on chromane-2,4-dione and chromone-3-carboxamide scaffolds77 were synthesized and most of the compounds displayed potent and selective MAO-B inhibitory activity in the micromolar to nanomolar range. The presence of C3 substituents and an amide spacer between the heterocyclic and the exocyclic ring was found to be crucial for MAO-B inhibitory activity. A para-chloro and a para-methyl group in the exocyclic ring favor activity and selectivity towards MAO-B. In this study compound 35 with an IC50 value of 2.9 nM and SI of more than 3400 and compound 36 with an IC50 value of 8.0 nM and SI of more than 1250 were found to be selective and potent MAO-B inhibitors, being more potent than the standard inhibitor deprenyl by 6.7 and 2.4-fold, respectively. Similarly, compound 36 was found to be the most potent inhibitor (hMAO-B) in another series76 with an IC50 values of 68 ± 3 nM.
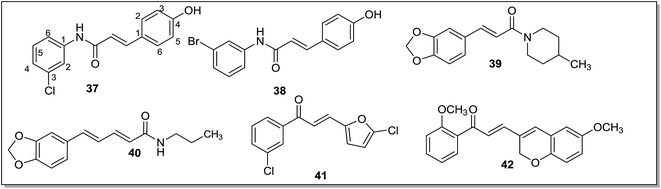
In a series of anilide derivatives,78 N,3-diphenylpropenamides were found to be promising MAO-B inhibitors. The screening of initially synthesized compounds led to the identification of two active compounds, (2E)-N-(3-chlorophenyl)-3-phenylprop-2-enamide and (2E)-N-(3-bromophenyl)-3-phenylprop-2-enamide as reversible and selective inhibitors of the MAO-B isoform with IC50 values of 0.5 μM and 0.4 μM, respectively. These inhibitors were further docked with the active site models of human MAO-A and MAO-B isoforms and docking studies led to the synthesis of phenolic derivatives 37 and 38 (Fig. 7). These derivatives were found to be selective and reversible MAO-B inhibitors with IC50 values of 32 nM and 26 nM respectively and were almost fourteen times more potent than their parent compounds. It was suggested that the substitution of the halogen atom at C3 position of the phenyl ring of the anilide moiety and a para hydroxyl substitution at the aromatic ring improved MAO-B binding affinity. Piperine and antiepilepsirine derivatives79 displayed selectivity for the MAO-B isoform and the most active compound (39) (Fig. 7) in the series exhibited an IC50 of 498 nM. The blood brain barrier permeability of the compounds was confirmed through PAMPA assays. It was noticed that the presence of a nitrogen atom in the ring reduces MAO-B inhibition potential of the compounds. The lead compound 39 was found to dock in a similar orientation to MAO-B as the parent compound, piperine. Mu et al.80 evaluated a series of piperine derivatives. The amine substituent on the piperidine ring improved the potency and selectivity of the inhibitors towards MAO-B isoform. 5-(3,4-Methylenedioxyphenyl)-2E,4E-pentadienoic acid n-propyl amide (40) (Fig. 7) has shown maximum MAO-B inhibitory activity (IC50 = 45 nM) and good selectivity (IC50 MAO-A = 3660 nM). A conjugated double bond and carbonyl group of the piperine ring were found to be essential functionalities for the MAO inhibitory activities. Docking studies of compound 40 with the MAO-B isoform showed that it interacts with Tyr60, Tyr326, Tyr398 forming an aromatic cage and the aromatic ring form π–π stacking interactions with Tyr407. It was also observed that compound 40 forms more hydrogen bonds with MAO-B rather than with the MAO-A isoform. In a series of furanochalcone derivatives81 {2E-3-(5-chlorofuran-2-yl)-1-(3-chlorophenyl)prop-2-en-1-one} (41) (Fig. 7) was identified as the most potent MAO-B inhibitor with an IC50 value of 174 nM. The kinetic studies revealed that the synthesized compounds were competitive and reversible inhibitors of the MAO-B isoform. It was found that furanochalcones with an electron withdrawing substituent at the phenyl ring were potent and selective MAO-B inhibitors. A series of chromenylchalcones82 was synthesized and MAO-B inhibition potential of the compounds was evaluated using an HPLC based method and an MAO-B enzyme assay kit. Among the several synthesized derivatives, (E)-3-(6-methoxy-2H-chromen-3-yl)-1-(2-methoxyphenyl)prop-2-en-1-one (42) (Fig. 7) with a half-maximal inhibitory concentration of 320 nM, was reported as the most promising MAO-B inhibitor. In silico docking experiments indicated that the compound 42 formed ligand–protein complex via same residue as in case of selegiline.
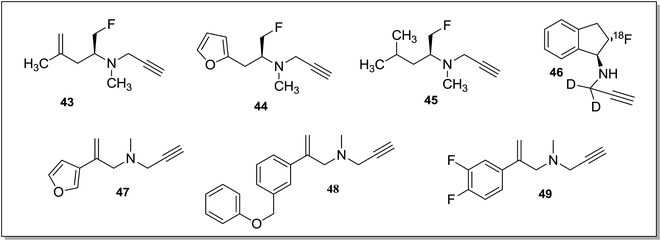
Nag et al.83 reported fluorine-18 labeled propargylamine derivatives as potential PET radio ligands to visualize MAO-B activity. Three ligands, (S)-1-fluoro-N,4-dimethyl-N-(prop-2-ynyl)-pent-4-en-2-amine (43), (S)-N-(1-fluoro-3-(furan-2-yl)propan-2-yl)-N-methylprop-2-yn-1-amine (44) and (S)-1-fluoro-N,4-dimethyl-N-(prop-2-ynyl)pentan-2-amine (45) (Fig. 8) were synthesized through multi-step procedures. The ligands were found to be selective for the MAO-B isoform displaying IC50 values of 664 ± 48.1 nM, 208.5 ± 13.4 nM and 131.5 ± 0.7 nM respectively and none of the ligand inhibited the MAO-A isoform.
Compound 45 demonstrated high selectivity for the MAO-B isoform and was selected for further investigation by PET. Compound 45 (Fig. 8) was a selective inhibitor of MAO-B isoform and in the in vivo studies it showed MAO-B specific binding pattern by PET in the monkeys. The compound was proposed as a candidate for further PET investigations in human. The same research group reported fluorine-18 labeled deuterium substituted analogues84 of rasagiline as potential PET radio ligand for the study of MAO-B isoform. The compound inhibited the MAO-B activity with an IC50 of 9.9 ± 1.1 μM. It was found that 46 (Fig. 8) bind to MAO-B rich regions in the monkey and was blocked by MAO-B selective compound L-deprenyl. The compound was proposed as an important lead for human PET studies. A library of arylalkenylpropargylamines85 was tested for the MAO inhibitory activities. The preliminary screening of the compounds were performed using rat MAO enzymes. Potent and selective inhibitors were subsequently screened against human MAO-A and MAO-B enzyme isoforms. Amongst the four different groups of compounds synthesized, compound 47 with a furanyl ring was the most potent hMAO-B inhibitor with IC50 value of 0.7 nM and SI of more than 2600. In addition, selected compounds were also tested, in vitro, for neuroprotection (PC-12 cells treated with 6-OHDA and rotenone) and cytotoxicity (TAMH liver cell line). Some of the compounds like 48 and 49 were identified as potent lead candidates that could be developed as drugs for the treatment of PD.
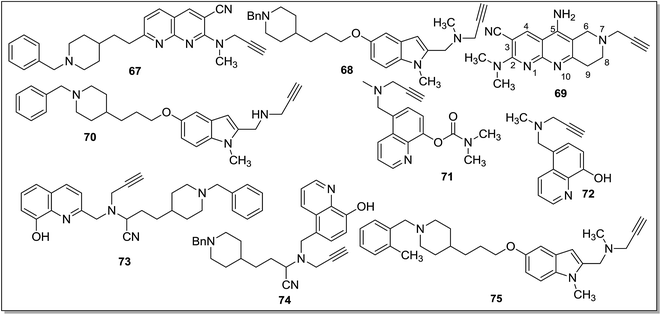
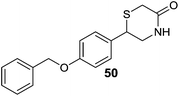
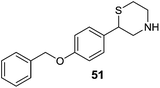
Luhr et al.86 designed and synthesized 2-arylthiomorpholine and 2-arylthiomorpholin-5-one derivatives as rat and human MAO inhibitors. It was found that a basic nitrogen atom in the ring was not necessary for the MAO inhibitory activity. In the docking studies it was concluded that three different interactions of ligands at the active site of receptor could modulate the bioactivity of MAO inhibitors, an aromatic ring that could participate in the π interactions with Tyr326, a heteroatom that could interact with Tyr398 and/or Tyr435 and a benzyl substituent at the opposite end of the molecule interacting at the entrance cavity which determines selectivity for the MAO-B isoform. The benzyloxy analogues 50 and 51 (Fig. 9) were found to be the most potent in the series with Ki values of 48 ± 30 nM and 38 ± 3 nM respectively for hMAO-B with SI of more than 2000 over MAO-A. Similarly for rat MAO-B compound 50 and 51 displayed Ki values of 74 ± 3 nM and 130 ± 4 nM respectively with selectivity of more than 2600 fold. Prins et al.87 synthesized a series of indole and benzofuran derivatives. They showed significant MAO-B inhibitory activity and SAR studies suggested that the chloro-substituent at the C5 phenyl side chain of the indoles and benzofuranes improved both MAO-A and MAO-B inhibitory potencies. Molecular modeling studies demonstrated that the compound 52 (Fig. 9) was stabilized in the active pocket of MAO-B and showed hydrogen bonding interactions with Tyr-435 and with the water molecule in the proximity of the FAD co-factor. Compound 52 was the most effective MAO-B inhibitor in the series. It was concluded that the indole and benzofuran derivatives were selective inhibitors of MAO-B and could be developed as promising drugs for the treatment of PD. A series of 5-sulphanylpthalimide analogues88 were screened for their affinity towards both the human MAO isoforms. Almost all the synthesized compounds exhibited MAO-B inhibitory activity with IC50 values in the nanomolar range. 5-(Benzylsulfanyl)phthalimide (53) (Fig. 9), with an IC50 value of 4.5 nM was the most effective MAO-B inhibitor with almost 400-fold selectivity as compared to MAO-A. Similarly, another series of thiazolidinedione (TZD) analogous89 has been synthesized in the same laboratory and ligand based virtual screening was performed. The most active compound (54) (Fig. 9) obtained in the series was reported as an inhibitor of both MAO-A (IC50 = 268 nM) and MAO-B (IC50 = 99 nM) isoforms. Compound 54 is a derivative of the naturally occurring and bioactive flavonoid, sulfuretin which possesses protective activity against mitochondrial toxins in a PD model. Structure–activity relationship studies revealed that the replacement of para tertiary dimethylamine group (54) with an ortho hydroxyl group at the phenyl ring improved the inhibitory activity of 54 from 99 nM to 11.5 nM (55). A number of 8-substituted benzamido–phenylxanthine derivatives90 were screened in vitro for their selectivity and MAO-B inhibition potential. Most of the compounds displayed promising activities and the para-substituted derivatives 56 (Ki value 0.3 μM) and 57 (Ki value 1.2 μM) (Fig. 9) were more potent than the meta-substituted derivatives due to steric interactions of the substituents at the binding pocket. It was concluded that caffeines can be explored as a favorable scaffold for designing potent MAO-B inhibitors. Similarly, with an aim to find out caffeine based potent MAO inhibitors, Okaecwe et al.91 investigated 8-phenoxymethylcaffeines and 8-[(phenylsulfanyl)methyl]caffeine derivatives against the MAO enzyme. The 8-phenoxymethylcaffeine derivatives were found to be reversible and potent MAO-B inhibitors with IC50 values ranging from 0.15 μM to 5.8 μM. The second series of compounds i.e., 8-[(phenylsulfanyl)methyl]caffeine derivatives were found to be weak MAO-B inhibitors with IC50 values ranging from 4 μM to 124 μM. In addition, both the series of compounds displayed low binding affinities for MAO-A isoform. The presence of a heteroatom at the C8 side chain of the caffeine scaffold improved the inhibitory activity and compound 58 (Fig. 9) was obtained as the most potent MAO-B inhibitor. 8-Phenoxymethylcaffeines have the potential to be developed as lead for the design of potent MAO-B selective inhibitors. Chaurasiya et al.92 synthesized and treated 5-phenoxyprimaquine analogous with MAO enzymes and evaluated their potency. Bioassays results showed that these compounds displayed better inhibitory activity towards both the isoforms when compared with the standard drug primaquine and the compound 5-(4-trifluoromethylphenoxy)-4-methylprimaquine (59) (Fig. 9) was the most active with selectivity towards MAO-B isoform and with an IC50 value of 150 nM. van der Walt et al.93 evaluated sulfanylphthalonitrile and sulfanylbenzonitriles analogous and most of the compounds were found to be potent and selective for MAO-B isoform with IC50 values in the nanomolar range. The sulfanylphthalonitriles displayed better binding affinities for MAO-B than the corresponding sulfanylbenzonitrile analogues. In the reported series, 4-[(4-bromobenzyl)sulfanyl]phthalonitrile (60) (Fig. 9) was the most promising MAO-B inhibitor (IC50 = 25 nM) with very high selectivity (8700 fold) for MAO-B over MAO-A isoform.
Strydom et al.94 designed a series of C6-substituted phthalide-[2-benzofuran-1(3H)-one] derivatives. All the phthalide derivatives showed good binding affinities to human MAO-A and MAO-B isoforms however C6 substituted phthalide exhibited MAO-B specific inhibition. A number of para-phenyl substituted derivatives of 6-benzyloxyphthalide were screened and the general order of potency, i.e., CF3 > I > Br > Cl > F > CH3 > H was observed. The most potent MAO-B inhibitor obtained in the series was a trifluoro substituted benzyloxyphthalide homologue (61) (Fig. 9) with an IC50 value of 1.4 nM while compound 62 with a phenylpropyl chain was the most active MAO-A inhibitor. The synthesized phthalides were reversible and competitive inhibitors at both isoforms and these compounds can be explored as leads for the development of new and effective drugs for the treatment of PD. A series of 3,4-dihydro-2(1H)-quinolinone derivatives95 was explored for human MAO inhibition activities. They were structurally related to the coumarin-(1-benzopyran-2-one) derivatives and were found to be potent and selective MAO-B inhibitors. 7-(3-Bromobenzyloxy)-3,4-dihydro-2(1H)-quinolinone (63) (Fig. 9) was the most potent MAO-B inhibitor with an IC50 value of 2.9 nM and more than 2700 folds selectivity over MAO-A. Structure–activity relationship studies suggested that a substituent at the C7 position of 3,4-dihydro-2(1H)-quinolinone was crucial for the MAO-B inhibitory activity. It was concluded that the C7 substituted 3,4-dihydro-2(1H)-quinolinone derivatives could be used as a lead for the development of drug candidate for the treatment of Parkinson's disease.
Stößel et al.96 synthesized and screened benzothiazinone derivatives as dual targeting agents of adenosine A2A receptors and MAO-B enzyme. In the screening studies, 2-(3-chlorophenoxy)-N-(4-oxo-4H-3,1-benzothiazin-2-yl)acetamide (64) (Fig. 9) has been identified as the most potent MAO-B inhibitor with an IC50 value of 1.6 nM but it displayed no affinity for adenosine A2A receptors. However, N-(4-oxo-4H-3,1-benzothiazin-2-yl)-4-phenylbutanamide (65) (Fig. 9) was found to be a competitive and reversible inhibitor of the human MAO-B (IC50 = 34.9 nM) and human A2A (Ki = 39.5 nM) receptors. These dual targeting compounds were proposed as potential candidates for the development of drugs for PD. Tzvetkov et al.97 designed and synthesized indazol-5-carboxamides, indol-5-carboxamide, and (1H-indazol-5-yl)methenamine derivatives as new classes of MAO-B inhibitors. Structure–activity relationship studies helped in the identification of some potent and reversible MAO-B inhibitors displaying sub nanomolar potency. Molecular modelling studies assisted in the design of ligands showing high affinity and selectivity towards the MAO-B isoform. N-(3,4-Difluorophenyl)-1H-indazole-5-carboxamide (66) (Fig. 9) showed remarkable in vitro MAO-B inhibitory potential (IC50 = 1.6 nM and SI more than 6000 fold) and well-balanced physicochemical profile for the CNS bioavailability. The promising compounds need to be evaluated for water-solubility, bioavailability, metabolism and toxicity so that a potent drug candidate could be developed for the treatment of PD.
4. Role of MAO inhibitors in the Alzheimer's disease
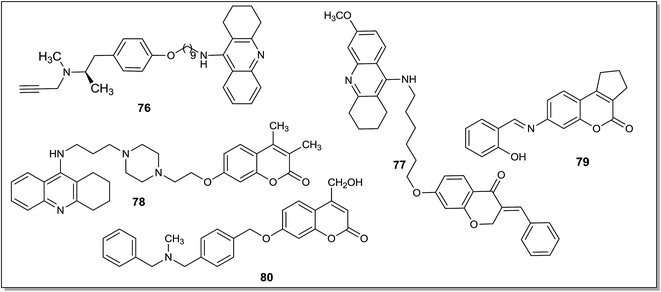
Alzheimer's disease (AD) is measured as extreme loss in memory and intellectual ability convoyed by behavioral disturbances and declining ability to perform basic activities of daily life.98 Depressive symptoms occur in patients suffering with AD and these may be associated with decrease in serotonergic and noradrenergic transmission in the limbic system.99,100 Although the exact cause of progressive neuronal degeneration in AD is not known, evidences like oxidative stress caused by increased levels of iron, nitration of tyrosine residues, MAO-B hyperactivity in gliosis, have been reported in the literature.101–103 The MAO inhibitor deprenyl is an anti-Parkinson drug and it has also been used to slow the progress of AD. Some clinical trials have shown that deprenyl alleviate the symptoms of AD.104 The patients suffering with AD were also found to have low levels of cholinergic neurons causing decreased concentration of acetylcholine in the brain which in turn affects the learning and memory functions. Therefore, various acetylcholinesterase inhibitors (AChEIs) like tacrine, rivastigmine, donepezil etc. have been developed but these compounds exhibited partial therapeutic benefits.105 On the other hand, the MAO enzyme is considered as an important target for the treatment of AD, as the enzyme catalyzes the oxidative deamination of a number of xenobiotic and endogenous amines with the formation of neurotoxic hydrogen peroxide and hydroxyl free radicals. Hence, the dual targeting ligands which can simultaneously inhibit MAO-B and cholinesterases are being developed as drugs for the management of AD. A number of dual targeting ligands have been reported as potential therapeutic agents for the treatment of AD. Samadi et al.106 reported synthesis, biological screening and docking studies of two different series of compounds which simultaneously inhibit MAO as well as acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE). The screening of compounds led to the identification of a lead that can act as multi-potent drug exhibiting high and selective AChE inhibitory activity (IC50 = 37 ± 4 nM) and moderate, but selective MAO-A inhibition activity (IC50 = 41 ± 7 μM). Molecular modeling studies of the most active compound confirmed its dual AChE inhibition activity, binding simultaneously at the catalytic active site and at the peripheric anionic site. The compound 67 (Fig. 10) was reported as the most active and selective dual AChE inhibitor, displaying a moderate and selective MAO-A inhibition activity. Compound 67 was proposed as a multi-potent lead that could be developed as a drug that simultaneously binds to two pharmacological targets and could play a role in the treatment of AD. Bolea et al.107 synthesized a series of compounds using a hybrid molecular approach by combining the benzylpiperidine scaffold of the donepezil (AChE inhibitor) and indolylpropargylamino part of the MAO inhibitor N-[(5-benzyloxy-1-methyl-1H-indol-2-yl)methyl]-N-methylprop-2-yn-1-amine. The two parts of the hybrid molecule were linked through an oligomethylene linker. Molecular docking and kinetic studies showed that the most promising hybrid 68 (Fig. 10) was the inhibitor of both MAO-A (IC50 = 5.2 ± 1.1 nM) and MAO-B (IC50 = 43 ± 8 nM) isoforms that also exhibited potency towards AChE and BuChE. The same research group108 designed heterocyclic substituted alkyl and cycloalkylpropargylamines as multi-targeting agents that simultaneously bind to the monoamine oxidases as well as cholinesterases. Indole derivatives reported in the series I type of compounds were well known MAO inhibitors and were investigated for their capacity to inhibit AChE and BuChE. In the series I, N-[(5-(benzyloxy)-1H-indol-2-yl)methyl]-N-methylbut-2-yn-1-amine has been found to be multi-potent compound that could selectively inhibit MAO-B (IC50 = 31 ± 2 nM) and eqBuChE (IC50 = 7 ± 1 μM) enzymes. The molecular modeling studies of this compound showed that high MAO-B inhibitory activity might be due to the presence of methyl propargyl group in the proximity of N5 of FAD in MAO-B. The series II compounds, i.e., 5-amino-7-(prop-2-yn-1-yl)-6,7,8,9-tetrahydropyrido[2,3-b][1,6]naphthyridines were reported as weak MAO inhibitors, but displayed selective AChE inhibitory activity. 5-Amino-7-(2-propyn-1-yl)-2-(1-pyrrolidinyl)-6,7,8,9-tetrahydropyrido[2,3-b]-1,6-naphthyridine-3-carbonitrile (69) (Fig. 10) exhibited a multi-potent pharmacological profile simultaneously binding to BuChE and MAO-B and it was proposed as a lead for the design of novel and potent multi-targeting cholinesterase and MAO inhibitors. Bautista-Aguilera et al.109 further designed and synthesized a series of donepezil-indolyl based amines, amides and carboxylic acid derivatives derived from N-((5-(3-(1-benzylpiperidin-4-yl)propoxy)-1-methyl-1H-indol-2-yl)methyl)-N-methylprop-2-yn-1-amine (68). These were found to be multipotent inhibitors, targeting cholinesterase and MAO enzymes. The amines were more potent ChE inhibitors than the corresponding amides and carboxylic acids, showing a clear selectivity for EeChE inhibition. The most potent inhibitor obtained in the series, i.e. [N-((5-(3-(1-benzylpiperidin-4-yl)propoxy)-1-methyl-1H-indol-2-yl)methyl)prop-2-yn-1-amine] (70) (Fig. 10) showed multi-receptor affinity profile for MAO-A (IC50 = 5.5 ± 1.4 nM), MAO-B (IC50 = 150 ± 31 nM), EeAChE (IC50 = 190 ± 10 nM) and eqBuChE (IC50 = 830 ± 160 nM). Compound 70 displayed good drug-like characteristics and ADMET properties and it could be developed as a drug candidate for the prevention and treatment of AD. Zheng et al.110 designed site-activated chelators targeting both AChE and MAO. The most active compound (71) had only little affinity for metal (Fe, Cu, and Zn) ions. The interesting characteristic of this compound is that the iron chelation capacity becomes activated after inhibition of AChE to release the active chelators M30 (72) (Fig. 10). Compound 71 showed high potency, in vitro, against MAO-A with an IC50 value of 7.0 ± 0.7 nM and weak inhibition of MAO-B. It displayed lower cytotoxicity and more lipophilicity than its activated brain permeable chelator 72. The compound 72 was able to modulate amyloid precursor protein regulation and β-amyloid reduction, suppress oxidative stress, and passivate excess metal ions (Fe, Cu, and Zn), which showed that chelators have potential to treat the underlying cause of AD.A number of donepezil derivatives111 were designed, synthesized and found to be potent metal chelators that target different enzyme systems related to AD (ChEs and MAO-A). Compound 73 exhibited excellent ChEs and a selective MAO-A inhibition potency (vs. MAO-B) along with the strong complexing affinities for zinc and copper ions (involved in the progression of AD). Compound 73 has shown significant MAO-A, hrAChE and hrBuChE inhibitory activities with IC50 values of 10.1 ± 1.1 μM, 0.029 ± 0.003 μM and 0.039 ± 0.003 μM respectively. Compound 73 as a donepezil–hydroxyquinoline hybrid was found to be a lead with the disease modifying anti-Alzheimer's drugs (DMAAD) profile. Wang et al.112 synthesized and evaluated multi target directed donepezil, propargylamine and 8-hydroxyquinoline hybrids for the treatment of Alzheimer's disease. The most active derivative obtained in the series was racemic α-aminotrile 4-(1-benzylpiperidin-4-yl)-2-(((8-hydroxyquinolin-5-yl)methyl)(prop-2-yn-1-yl)amino)butanenitrile (74) (Fig. 10) exhibiting affinity to MAO-A (IC50 = 6.2 ± 0.7 μM), MAO-B (IC50 = 10.2 ± 0.9 μM), AChE (IC50 = 1.8 ± 0.1 μM) and BuChE (IC50 = 1.6 ± 0.2 μM). In the docking studies compound 74 was found to be interacting simultaneously with the catalytic and peripheral site of eeAChE. Most of the compounds showed good ADMET profile and brain penetration capacity for the CNS activity. In the passive avoidance task, compound 74 significantly decreased scopolamine-induced learning deficits when tested on the mice with experimentally induced amnesia. Nineteen donepezil–indolyl hybrids113 were designed, synthesized and evaluated as multifunctional agents binding to the MAO and ChE enzymes. Compound 75 was the most potent inhibitor in the series exhibiting irreversible hMAO-A inhibition activity with an IC50 value of 6.3 ± 0.4 nM and was nine times more potent than the reference drug clorgyline and 29 fold more selective towards MAO-A over MAO-B. Compound 75 also showed hAChE and hBuChE inhibition potential with IC50 values of 2.8 ± 0.1 μM and 4.9 ± 0.2 μM respectively. Molecular docking studies with 75 showed that ortho methyl group improved the ligand recognition and increased the ligand–enzyme hydrophobic interaction in the hBuChE and π–π stacking in hMAO-A, hMAO-B, and hAChE enzyme. Virtual ADMET analysis of compound 75 showed good drug like characteristics with better brain penetration ability as compared to the standard drug selegiline.
Lu et al.114 synthesized and evaluated a series of tacrine–selegiline based hybrids as multi-potent agents with cholinesterase and MAO inhibitory activities for the treatment of AD. Compound 76 (Fig. 11) displayed an optimum inhibition profile for AChE, BuChE, hMAO-A and hMAO-B with IC50 values of 22.6 nM, 9.37 nM, 372.4 nM and 181 nM respectively. These types of hybrid compounds could improve patient's cognition by elevating levels of acetylcholine and protect neurons by retaining the activities of selegiline. The compounds were proposed as “one compound multi-target” drugs for the treatment of AD. Sun et al.115 designed, synthesized and screened a series of tacrine–homoisoflavonoid hybrids as multifunctional compounds and most of these were found to be promising inhibitors of the cholinesterases (ChEs) and human MAOs in nanomolar range. Amongst the reported series, a compound with linker chain of six carbons between tacrine and (E)-7-hydroxy-3-(4-methoxybenzylidene)chroman-4-one (77) (Fig. 11) was found to be a potent inhibitor of AChE and MAO-B with IC50 values of 67.9 nM and 401 nM respectively. In the artificial membrane permeation assay, compound 77 efficiently crossed the blood–brain barrier (BBB). A series of tacrine–coumarin hybrids116 were evaluated as multi-target ligands against AD. Most of the compounds exhibited potent inhibitory activities towards AChE, BuChE and were selective for the MAO-B isoform. The linker length connecting tacrine and coumarin fragments played a crucial role in AChE inhibition. Presence of meta and/or para substituents on the coumarins ring influenced the selectivity and inhibitory activity of ligands towards MAO enzyme. In the reported series, compound 78 (Fig. 11) was the most promising inhibitor with IC50 values of 0.1 μM, 16.1 μM, 112.7 μM for hMAO-B, hAChE and BuChE respectively. Compound 78 also showed good BBB permeability and exhibited low toxicity to SH-SY5Y cells. A series of coumarin derivatives117 were evaluated as multifunctional agents for the treatment of AD and most of the compounds displayed potent hMAO-B inhibitory activity with IC50 values in submicromolar range and high selectivity over hMAO-A isoform. This series of compounds also showed moderate to good potencies toward inhibition of self-mediated Aβ1–42 aggregation. In the series, compound 79 (Fig. 11) was the most potent hMAO-B inhibitor with an IC50 value of 81 nM and SI greater than 1200. In addition, compound 79 displayed metal chelation potential, effective blood–brain barrier (BBB) penetration and showed low cell toxicity in the rat pheochromocytoma (PC12) and SH-SY5Y cells. Farina et al.118 reported structure-based design and optimization of multitarget-directed 2H-chromen-2-one derivatives as inhibitors of the MAO-B and cholinesterases. Flexible N-benzyl-N-alkyloxy coumarins displayed good inhibitory activities at rMAO-B, AChE and BChE, but low selectivity. However rigid inhibitors, containing meta- and para-xylyl linkers, exhibited good inhibitory activities and high rMAO-B selectivity. Similarly, polar and hydrophilic 4-hydroxymethyl derivatives showed low submicromolar activity at the three target enzymes rMAO-B, AChE, BuChE, and low or no activity at rMAO-A. Some of the promising inhibitors were screened against human MAOs and AChE and in comparison to non-human enzymes, a significant increase of inhibitory activities was observed for hMAOs with relatively high selectivity index for MAO-B isoform. The 4-hydroxymethyl coumarin derivative 80 (Fig. 11), with IC50 values of 10 nM, 120 nM and 930 nM for hMAO-B, AChE and BuChE respectively, was found to be a lead for further pre-clinic studies on the basis of toxicity, neuroprotection and transport data. The compound was devoid of neurotoxicity, showed moderate neuroprotective effects and good BBB permeability profile with limited P-gp affinity.
5. Role of MAO inhibitors in depression
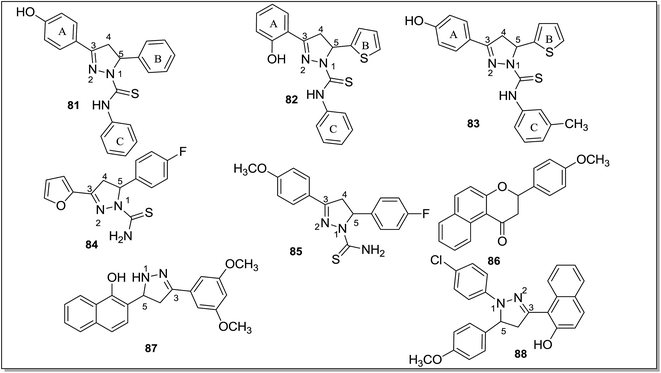
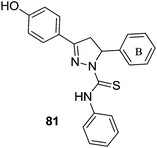
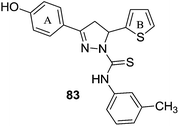
The antidepressant effect of MAO inhibitors was a serendipitous discovery.119 The antituberculosis drug iproniazid was found to help depression when it was administered to the tuberculosis patients who were also suffering with depression.120 The antidepression action of the drug was due to its inhibition of mono amine oxidase enzyme. However, in addition to monoaminergic systems, many neurotransmitter systems such as noradrenergic, serotonergic, dopaminergic, neuroendocrinergic and neuropeptide systems have been reported as being involved in the pathophysiology of depression.121–123 The etiology of the depression is linked with the suboptimal concentrations of these neurotransmitters in the central nervous system (CNS).9 Depression can be recognized with symptoms like panic-agoraphobia syndrome, severe phobias, social anxiety disorder, and obsessive-compulsive disorder.124 Recent advancements in the antidepressant therapeutics include use of reversible inhibitors of MAO-A including tricyclic antidepressants (TCAs), Selective Serotonin Reuptake Inhibitors (SSRI) and Serotonin Reuptake Transporter (SERT) inhibitors. These inhibitors increase the synaptic concentration of three neurotransmitters; serotonin, norepinephrine and dopamine in the brain. SSRI and SERT inhibitors were found to be less toxic when compared with the first generation irreversible and non-selective MAO inhibitors. However, in case of severe and treatment-resistant depression, MAO inhibitors are the only available most effective drugs. The MAO enzyme can metabolize serotonin, norepinephrine and dopamine and low levels of these three neurotransmitters and the over expressed MAO enzyme is linked with the etiology of depression. In order to develop an effective and potent antidepressant, the brain MAO-A must be inhibited. One of the major limitations associated with the use of irreversible MAO inhibitors was risk of hypertensive crisis. However, with the development of reversible and selective MAO-A inhibitors, this problem has been minimized or eliminated. Thus, selective MAO-A inhibitors are being explored for the development of effective drug candidates for the treatment of severe depression.Karuppasamy et al.125 reported a series of 3,5-diarylpyrazoline derivatives and evaluated their MAO inhibitory activities. Many of the reported compounds were found to be reversible and selective inhibitors of the MAO-A isoform with selectivity index of 103 to 105. A 4-hydroxy substitution on ring A (81) (Fig. 12) improved the potency towards MAO-A isoform. The thiophen-2-yl group as ring B (82) (Fig. 12) caused better MOA-A inhibition than the furan-2-yl group. A methyl substituent on the ring C (83) was preferred for the selectivity and potency towards MAO-A isoform. Compound 83 was reported as the most potent in the series with an IC50 value of 150 ± 10 nM. A series of N1-thiocarbamoyl-3,5-di(hetero)aryl-4,5-dihydro-(1H)-pyrazoles126 was synthesized and assayed for MAO-A and MAO-B inhibitory activities. SAR studies showed that a fluorine atom at the para position of the 5-phenyl substituent was essential for the activity. The presence of a hetero-aromatic substituent at 3- and 5-position of the pyrazoline ring was responsible for the decreased potency and selectivity towards MAO-B while the presence of 4′-methylphenyl and 4′-fluorophenyl at the same position of the ring retained the activity and selectivity. The compounds containing 4′-chlorophenyl substituent at 3-position had increased the activity.
N1-Thiocarbamoyl-3-(fur-2′-yl)-5-(4′-fluoro-phenyl)-4,5-dihydro-(1H)-pyrazole (84) (Fig. 12) was the most active candidate with an IC50 value of 2.7 ± 0.8 μM and selectivity ratio of 25 over MAO-B isoform. A series of 3-aryl-5-(4-fluorophenyl)-N-substituted-4,5-dihydro-1H-pyrazole-1-carbothioamide derivatives127 was screened, in vitro, for the MAO inhibitory activities and selectivity towards MAO-A and MAO-B isoforms. 5-(4-Fluorophenyl)-3-(4-methoxyphenyl)-N-methyl-4,5-dihydro-1H-pyrazole-1-carbothioamide (85) (Fig. 12) was the most potent MAO-A inhibitor with Ki value of 1 nM. Docking results suggested that 85 binds efficiently with the MAO-A enzyme, and a methoxy or chloro substituents at 3-phenyl ring improved the potency and selectivity towards MAO-A isoform. Jo et al.128 designed, in silico, three MAO-A inhibitors 3-(4-methoxyphenyl)-2,3-dihydro-1H-benzo[f]chromen-1-one (86), 2-(5-(3,5-dimethoxyphenyl)-4,5-dihydro-1H-pyrazol-3-yl)naphthalen-1-ol (87) and 1-(1-(4-chlorophenyl)-5-(4-methoxyphenyl)-4,5-dihydro-1H-pyrazol-3-yl)naphthalen-2-ol (88) (Fig. 12). In the docking experiments, 86 showed stronger interactions with the MAO-A isoform as compared to the standard inhibitor clorgyline. Further, these compounds were evaluated for the MAO-A inhibition activity and compound 86 showed a similar inhibitory effect like the standard clorgyline.
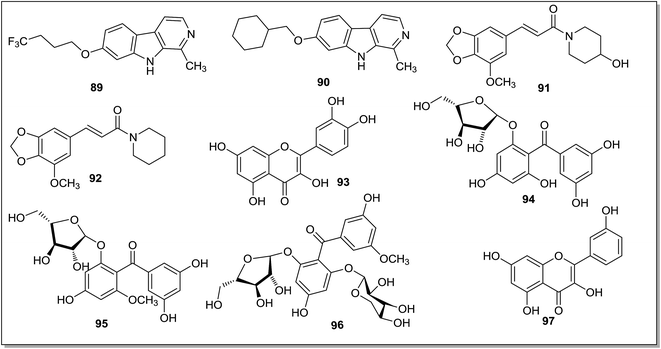
A series of β-carboline derivatives129 was synthesized and screened for MAO-A and MAO-B inhibition activities and compared with the harmine as reference drug. The presence of ortho-alkylated lipophilic groups like cyclohexyl, phenyl and aliphatic chains increase the MAO-A inhibitory activity but decreases activity towards MAO-B enzyme. The lead compound containing trifluorobutyloxy group (89) (Fig. 13) displayed maximum MAO-A inhibition with Ki of 3.6 nM. The computational studies revealed that the trifluorobutyloxy group occupies the hydrophobic pocket of the enzyme which was left vacant by harmine. The cyclohexyl bearing analogue (90), was also reported as the potent MAO-A inhibitor with Ki value of 4.3 nM. A series of piperamide derivatives130 was synthesized and compounds were explored for their MAO inhibitory and antioxidant activities. The antidepressant activity of the compounds was assessed using tail suspension test and forced swim test. Compounds 91 and 92 (Fig. 13) were active in both the tests. The antioxidant activities of the compounds were determined through DPPH and superoxide radical scavenging method. Compound 92 was found to be the most potent antioxidant. Bandaruk et al.131 evaluated the MAO-A inhibitory effects of quercetin and tea catechins in the mouse brain mitochondria. In different biological assays, quercetin (93) (Fig. 13) was less active when compared with the other antidepressant drugs but was comparatively safer as it did not show any interaction with the intestine MAO-A. Demirkiran et al.132 purified and isolated four different compounds (94–97) (Fig. 13) from the n-butanol fraction of 80% ethanol extract of Hypericum thasium Griseb. The isolated compounds, along with kaempferol (97) and quercetin (93) were screened for MAO-A inhibitory activities. These compounds were evaluated with clorgyline as a standard inhibitor and kaempferol (97) was found to be the most potent compound with IC50 value of 17.5 μM.
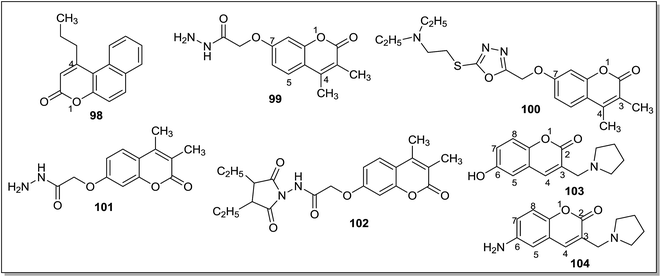
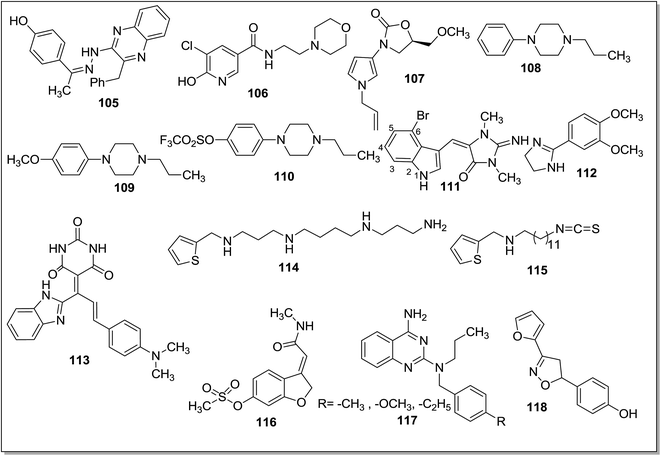
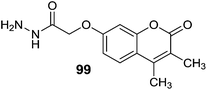
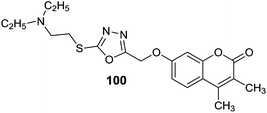
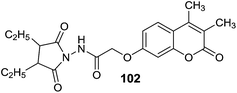
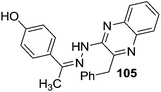
Masand et al.133 reported the interactions pattern of 4-propyl-2H-benzo[h]-chromen-2-one (98) (Fig. 14) with MAO enzyme using molecular docking studies and it was found that most of the interactions of 98 with the MAO-A crystal structure were hydrophobic and steric in nature. However, the traditional MAO-A inhibitors like clorgyline displayed hydrophobic as well as polar interactions. In the structure–activity relationship studies it was concluded that the presence of a hydrophobic group at the C4 position of 98 was favorable for the activity. Abdelhafez et al.134 designed and synthesized a number of 4-methyl and 3,4-dimethyl-7-oxycoumarins which include oxadiazole, thiadiazole, triazole and thiazolidinone derivatives. Most of the reported compounds have displayed high affinity and selectivity towards MAO-A isoform with Ki values in the picomolar range. In addition, the reported series of compounds also exhibited MAO inhibitory activity when tested in vivo. The molecular modeling studies of the ligands provided new information about the enzyme–inhibitor interactions and potential therapeutic application of 7-oxycoumarin scaffold. The acetohydrazide derivative, 99 with a Ki of 5 pM and SI of 9.66 × 104 (MAO-A) and the diethylaminoethylthio-1,3,4-thiadiazole (100), with Ki of 5.18 pM, and SI of 9.58 × 104 (MAO-A) (Fig. 14) were reported as the most potent and selective MAO-A inhibitors. The same research group135 reported a series of 7-oxycoumarin derivatives and in vitro studies showed that most of the compounds were potent and selective MAO-A inhibitors. In the in vivo studies it was found that the synthesized compounds were more active than the standard inhibitor iproniazid. The most promising inhibitors obtained in the series were 2-(3,4-dimethylcoumarin-7-yloxy)acetohydrazide (101) (Fig. 14) with an in vitro Ki i values of 5.01 pM for MAO-A and 4.84 × 105 pM for MAO-B and in vivo ED50 of 9143 nM and 2-(3,4-dimethylcoumarin-7-yloxy)-N-(2,5-dioxopyrrolidin-1-yl)acetamide (102) (Fig. 14) with an in vitro Ki values of 6.34 pM for MAO-A and 5.75 × 105 pM for MAO-B and in vivo ED50 of 9.15 nM. The molecular docking studies showed a direct correlation between the binding affinities of the ligands and percentage inhibition of the MAO-A and MAO-B isoforms. A series of 6-substituted 3-(pyrrolidin-1-ylmethyl)chromen-2-ones136 (coumarin) were evaluated for their inhibition potential against the MAO-A and MAO-B isoforms. A substituent at the C6 position of the coumarin ring and a basic amine group at C3 position produced selective inhibitors for MAO-A isoform. In the series of reported compounds, 103 (IC50 = 1.46 μM) and 104 (IC50 = 3.77 μM) (Fig. 14) were the most potent and selective MAO-A inhibitors exhibiting good aqueous solubility. In the rat brain, 104 decreased the level of 3,4-dihydroxyphenylacetic acid (DOPAC) and 5-hydroxyindoleacetic acid (5-HIAA) while increases the level of 3-methoxytyramine (5 MT). Khattab et al.137 evaluated a series of 2-benzyl-3-(2-arylidenehydrazinyl)quinoxalines, 4-benzyl-1-aryl-[1,2,4]triazolo[4,3-a]quinoxalines and phenyl(1-aryl[1,2,4]triazolo[4,3-a]quinoxalin-4-yl)methanone derivatives and evaluated them for MAO-A and MAO-B inhibitory activities. They showed more activity towards MAO-A than the standard inhibitor clorgyline. The molecular modeling studies revealed that the proposed compounds showed hydrophobic interactions with Phe208 and hydrogen bonds to Ser209, Pro72, Tyr69, Tyr444, Glu74, Glu216. Compound 105 (For MAO-A IC50 = 2.8 ± 0.13 nM) (Fig. 15) was reported as the most active analogue and found to be selective against MAO-A isoform with SI of 3 × 106. Shi et al.138 evaluated N-(2-morpholinoethyl)nicotinamide and N-(2-morpholinopropyl)nicotinamide derivatives against MAO-A and MAO-B isoforms. Most of the synthesized compounds were found to be potent and MAO-A selective. The compound 5-chloro-6-hydroxy-N-(2-morpholinoethyl)nicotinamide (106) (Fig. 15) was the most potent for MAO-A isoform with an IC50 value of 0.04 μM.
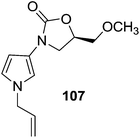
The molecular docking studies of 106 showed that pyridine ring inside the MAO-A binding site was interacting with the ‘aromatic cage’ formed by Tyr197, Tyr407, Tyr444 and the FAD aromatic ring. The pyridine ring also showed π–π stacking interactions with Tyr407. The morpholine ring of 106 was implanted in a large lipophilic pocket formed by Ile180, Phe208, Asn181, Gln215 and Ile335. It was concluded that the better binding interactions of 106 with MAO-A isoform might be responsible for the higher MAO-A inhibitory potency of the compound. Valente et al.139 synthesized a series of 3-(1H-pyrrol-3-yl)-2-oxazolidinone derivatives as reversible, potent and selective MAO-A inhibitors. The MAO-A inhibitory activity depended on the substituent at the pyrrole N1 position and replacement of methyl- and allyl- substituent with an ethyl group increased the activity from micromolar to nanomolar level. One of the potent compounds (107) (Fig. 15) obtained in the series displayed 2 × 105 times more selectivity towards MAO-A isoform and the compounds were proposed as promising antidepressant agents. Pettersson et al.140 synthesized para-substituted 4-phenylpiperidines/piperazines (108–110) (Fig. 15) and evaluated their affinities to recombinant rat cerebral cortex MAO-A and MAO-B. The para substituent on 4-phenylpiperidines/piperazines with low dipole moment increased the affinity towards MAO-A isoform while with a high dipole moment displayed no or poor affinity for the MAO-A isoform. Similarly polarity and bulk of the para substituents affected the MAO-B affinities and it was found that presence of a large and hydrophobic substituent increases MAO-B affinity. The compounds were examined in the freely moving rats and it was found that affinity of ligands for MAO-A was directly linked with the levels of DOPAC and 3-MT in the striatum. A series of fifty aplysinopsin analogs141 was screened for MAO-A and MAO-B inhibitory activities. Aplysinopsins are reported to possess number of bioactivities including modulation of neurotransmission. In the reported series, three compounds exhibited significant MAO inhibition profile and (E)-5-[(6-bromo-1H-indol-3-yl)methylene]-2-imino-1,3-dimethylimidazolidin-4-one (111) (Fig. 15) was the most potent one with an IC50 value of 5.6 nM at MAO-A and with selectivity index of 80.24. SAR studies showed that N-methyl substituent especially at position N-2′ and bromine substituent at C5 or C6 were essential for the MAO-A potency and selectivity. Villarinho et al.142 examined the MAO inhibitory activity and antidepressant potential of 2-(3,4-dimethoxyphenyl)-4,5-dihydro-1H-imidazole (2-DMPI) (112) (Fig. 15) in the mice. 2-DMPI exhibited inhibitory activity against both the MAO isoforms but was found more selective towards MAO-A (30 times). This reversible MAO-A inhibitor showed its antidepressant-like activity by decreasing 5-HT and DA turnover without affecting the motor performance, even at high doses.
Mathew et al.143 synthesized some (1H)benzimidazoles bearing pyrimidine-triones and screened them for selective MAO-A inhibitory activities. Molecular docking studies suggested that the lipophilic group on ring C could experience an extra hydrophobic binding region at the active site of the enzyme and could be contributing towards the CNS depressant activity. Amongst the various synthesized derivatives, compound 113 (Fig. 15) showed good antidepressant activity when compared to the standard clomipramine. The computational values obtained after the docking calculation were closely related to the experimental values. Bonaiuto et al.144 reported on polyamine derivatives related to N1,N12-dibenzyldodecane-1,12-diamine (Bis-Bza-Diado) and N1-benzyl-spermine (BD6) scaffolds. In the series, compound 114 (Fig. 15) was reported as a reversible mixed inhibitor with selectivity for the MAO-B (KIE = 23 μM) isoform. Compound 115 (Fig. 15), containing 12-methylene carbon chain with an isothiocyanate (ITC) group was found as the most potent in the series acting as competitive inhibitor of MAO-B and as irreversible inhibitor of MAO-A. The amino group of Lys305 was interacting covalently with ITC group of the inhibitor resulting in irreversible inhibition of MAO-A isoform. Pisani et al.145 synthesized a series of 6′-substituted-(E)-2-(benzofuran-3(2H)-ylidene)-N-alkylacetamides and the 6′-sulfonyloxy derivatives (116) (Fig. 15) showed affinities towards MAO-A, whereas 6′-benzyloxy derivatives showed potency towards MAO-B. Structure–activity relationship studies revealed the importance of rigidity in planar conformation of exocyclic double bond and indicated its role in binding with the receptor. The ligand with an exocyclic double bond in E-configuration exhibited more effective binding as compared to the more flexible and less active 2-(1-benzofuran-3-yl)-N-methylacetamide isomers and 4-N-methylcarboxamidomethylcoumarin analogous. The electronic and steric environment of the compound played a decisive role and influenced the activity. It was proposed that 6′-sulfonyloxy derivatives might be further developed as drug candidates for the treatment of depression, PD or other neurodegenerative diseases. Srivastav et al.146 designed and synthesized 6,7-dimethoxy-N2-(substituted benzyl)-N2-propylquinazoline-2,4-diamine derivatives (117) (Fig. 15) and screened these for MAO inhibitory activities. They showed excellent antidepressant activity when compared with the imipramine (standard drug). A substitution on the phenyl ring linked with a tertiary amine group attached to the 2nd position of the quinazoline ring was essential for the MAO inhibitory activity. The presence of electron withdrawing groups like –Cl, –F and –NO2 on the phenyl ring at ortho, meta or para position of 117 decreased the activity whereas the presence of a hydroxyl group (OH) at ortho position improved its antidepressant activity while hydroxyl substitution at meta or para position decreases the potency. Similarly, it was concluded that the presence of electron releasing groups like –CH3, –OCH3, –C2H5 and –OC2H5 at para position improved the antidepressant activity of the inhibitors. Kumar et al.147 synthesized a series of 3-(furan-2-yl)-5-(substituted phenyl)-4,5-dihydro-1,2-oxazole derivatives and assessed their antidepressant and antianxiety activity by using force swimming test (FST) and elevated plus maze method respectively. The compounds displayed significant activity when compared with the imipramine and diazepam as standard drugs. 4-[3-(Furan-2-yl)-4,5-dihydro-1,2-oxazol-5-yl]phenol (118) (Fig. 15) was the most potent antidepressant agent acting through MAO inhibition. The molecular docking studies confirmed that the lead compound showed greater binding affinity with the enzyme and formed hydrogen bonding and hydrophobic interactions with the active residues.
Thus, a large number of structurally different compounds can interact with the MAO-A and MAO-B isoforms with varying degree of selectivity and potency. It is difficult to generalize a chemical scaffold for its preference for MAO-A or MAO-B isoform; however some of the compounds reported in this review article with very high selectivity index are summarized below. The compounds number 81, 83, 99 to 102, 105 and 107 displayed very high selectivity for the MAO-A isoform while compounds number 14, 26, 35, 36, 50, 51, 60 and 66 exhibited high selectivity for the MAO-B isoform (Table 1).
6. Conclusion and future prospective
The MAO enzyme has been recognized as an important drug target for the treatment of various neurogenerative disorders. The first generation irreversible MAO inhibitors were predominantly used for the treatment of depression but now most of these were withdrawn from the clinical practice due to severe side effects and introduction of other classes of therapeutic agents. Fatal drug–drug interactions and dietary restrictions were the main reasons cited for not prescribing first generation MAO inhibitors. The new generation of MAO inhibitors were developed which were reversible in nature and selective for either of the two isoforms making these comparatively safer as compared to irreversible inhibitors. Recently developed transdermal drug delivery systems are devoid of cheese reaction as these avoid drug–MAO-A interactions in the intestinal mucosa and hence eliminate the need of dietary tyramine restrictions. In addition, selegiline transdermal delivery system was found to be safe along with many clinically prescribed decongestants. The discovery of crystal structure of MAO-A and MAO-B isoforms contributed enormously to understand drug–receptor interactions at the molecular level. Target based rational designing of isoform selective MAO inhibitors has expedited the discovery of novel leads. A variety of structurally different scaffolds have been synthesized and investigated for their role in MAO inhibitory activities and for establishing the etiology of the neurological diseases. The structure based drug design and synthesis of novel MAO inhibitors may help in better understanding of drug–receptor interactions and in the development of effective therapeutic agents for the treatment of various neurogenic disorders. The neuroprotective potential of MAO inhibitors has also attracted interest of the researchers and neuro-scientists. However, the underlying mechanism of MAO inhibitors as neurorescue agents is not clear yet. Due to the complexity of various neurogenic disorders, now the attention is being shifted towards the development of single drug as multi-target agent. These multi-target drugs are expected to simultaneously bind to different targets and hence help in the management of complex neurological disorders. The synthetic and computational chemistry are the tools to design ligands with features for simultaneous inhibition of MAO enzyme and other receptors/enzymes such as cholinesterases. A lot of research work still needs to be done in order to establish the role of MAO inhibitors in the etiology of other disorders like ageing, cerebral ischaemia, neuroprotection/neurorescue and substance-abuse risk.Abbreviations
| MAO | Monoamine oxidase |
| FAD | Flavin adenine nucleotide |
| MAO-A | Monoamine oxidase A |
| MAO-B | Monoamine oxidase B |
| AD | Alzheimer's disease |
| PC | Pheochromocytoma |
| PD | Parkinson's disease |
| Glu | Glutamine |
| Ile | Isoleucine |
| Phe | Phenylalanine |
| TAMH | TGF-α transfected mouse hepatocyte |
| Tyr | Tyrosine |
| TGF-α | Transforming grown factor alpha |
| SAR | Structure activity relationship |
| SI | Selectivity index |
| μM | Micromolar |
| nM | Nanomolar |
| EPR | Electron paramagnetic resonance |
| KIE | Enzyme–inhibitor dissociation constant/inhibition constant |
| pgp | P-glycoprotein |
Acknowledgements
V. K. is thankful to the University Grant Commission, New Delhi for providing financial assistance (BSR Grant No. F.20-17(12)/2012); and A. K. M. acknowledges financial support from Alzheimer's Association, USA (NIRG-11-203527) to carry out this work.References
- K. Tipton, Cell Biochem. Funct., 1986, 4, 79–87 CrossRef CAS PubMed.
- J. Shih and R. Thompson, Am. J. Hum. Genet., 1999, 65, 593 CrossRef CAS PubMed.
- C. W. Abell and S.-W. Kwan, Prog. Nucleic Acid Res. Mol. Biol., 2000, 65, 129–132 Search PubMed.
- J. C. Shih, Neuropsychopharmacology, 1991, 4, 1–7 CAS.
- R. F. Squires, Adv. Biochem. Psychopharmacol., 1972, 5, 355 CAS.
- D. E. Edmondson, C. Binda and A. Mattevi, Arch. Biochem. Biophys., 2007, 464, 269–276 CrossRef CAS PubMed.
- D. E. Edmondson, A. K. Bhattacharyya and M. C. Walker, Biochemistry, 1993, 32, 5196–5202 CrossRef CAS PubMed.
- K. Inaba-Hasegawa, Y. Akao, W. Maruyama and M. Naoi, J. Neural Transm., 2013, 120, 435–444 CrossRef CAS PubMed.
- M. B. Youdim, D. Edmondson and K. F. Tipton, Nat. Rev. Neurosci., 2006, 7, 295–309 CrossRef CAS PubMed.
- B. Kennedy, M. Ziegler, M. Alford, L. Hansen, L. Thal and E. Masliah, J. Neural Transm., 2003, 110, 789–801 CAS.
- M. S. Benedetti and P. Dostert, Biochem. Pharmacol., 1989, 38, 555–561 CrossRef.
- L. Emilsson, P. Saetre, J. Balciuniene, A. Castensson, N. Cairns and E. E. Jazin, Neurosci. Lett., 2002, 326, 56–60 CrossRef CAS PubMed.
- C. Binda, P. Newton-Vinson, F. Hubálek, D. E. Edmondson and A. Mattevi, Nat. Struct. Mol. Biol., 2002, 9, 22–26 CAS.
- L. De Colibus, M. Li, C. Binda, A. Lustig, D. E. Edmondson and A. Mattevi, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 12684–12689 CrossRef CAS PubMed.
- C. Binda, J. Wang, L. Pisani, C. Caccia, A. Carotti, P. Salvati, D. E. Edmondson and A. Mattevi, J. Med. Chem., 2007, 50, 5848–5852 CrossRef CAS PubMed.
- R. M. Geha, K. Chen, J. Wouters, F. Ooms and J. C. Shih, J. Biol. Chem., 2002, 277, 17209–17216 CrossRef CAS PubMed.
- J. N. Sachs and D. M. Engelman, Annu. Rev. Biochem., 2006, 75, 707–712 CrossRef CAS PubMed.
- A. M. Andres, M. Soldevila, A. Navarro, K. K. Kidd, B. Oliva and J. Bertranpetit, Hum. Genet., 2004, 115, 377–386 CrossRef CAS PubMed.
- C. Binda, A. Mattevi and D. E. Edmondson, Int. Rev. Neurobiol., 2011, 100, 1–11 CrossRef CAS PubMed.
- E. M. Milczek, C. Binda, S. Rovida, A. Mattevi and D. E. Edmondson, FEBS J., 2011, 278, 4860–4869 CrossRef CAS PubMed.
- F. Hubálek, C. Binda, A. Khalil, M. Li, A. Mattevi, N. Castagnoli and D. E. Edmondson, J. Biol. Chem., 2005, 280, 15761–15766 CrossRef PubMed.
- S.-Y. Son, J. Ma, Y. Kondou, M. Yoshimura, E. Yamashita and T. Tsukihara, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 5739–5744 CrossRef CAS PubMed.
- D. Bonivento, E. M. Milczek, G. R. McDonald, C. Binda, A. Holt, D. E. Edmondson and A. Mattevi, J. Biol. Chem., 2010, 285, 36849–36856 CrossRef CAS PubMed.
- R. Ramsay, Curr. Pharm. Des., 2012, 19, 2529–2539 CrossRef.
- J. Finberg and K. Gillman, Int. Rev. Neurobiol., 2011, 100, 169–190 CrossRef CAS PubMed.
- B. Blackwell and L. Mabbitt, Lancet, 1965, 285, 938–940 CrossRef.
- M. Bianchetti, I. Minder, C. Beretta-Piccoli, A. Meier and P. Weidmann, Klin. Wochenschr., 1982, 60, 465–470 CrossRef CAS PubMed.
- T. S. Rao and V. K. Yeragani, Indian J. Psychiatry, 2009, 51, 65 CrossRef PubMed.
- M. B. Youdim and M. Weinstock, Neurotoxicology, 2004, 25, 243–250 CrossRef CAS PubMed.
- J. R. Mitchell, H. J. Zimmerman, K. G. Ishak, U. P. Thorgeirsson, J. A. Timbrell, W. R. SnodGrass and S. D. Nelson, Ann. Intern. Med., 1976, 84, 181–192 CrossRef CAS PubMed.
- S. Nelson, J. Timbrell, W. Snodgrass and G. Corcoran, Science, 1976, 193, 901–903 CAS.
- J. D. Amsterdam and J. Shults, J. Affective Disord., 2005, 89, 183–188 CrossRef CAS PubMed.
- R. W. Fuller, Prog. Neuro-Psychopharmacol., 1978, 2, 303–311 CrossRef CAS.
- M. Yamada and H. Yasuhara, Neurotoxicology, 2004, 25, 215–221 CrossRef CAS PubMed.
- M. Da Prada, G. Zürcher, I. Wüthrich and W. Haefely, J. Neural Transm., Suppl., 1987, 26, 31–56 Search PubMed.
- L. Wecker, S. James, N. Copeland and M. A. Pacheco, Biol. Psychiatry, 2003, 54, 1099–1104 CrossRef CAS PubMed.
- M. Mawhinney, D. Cole and A. J. Azzaro, J. Pharm. Pharmacol., 2003, 55, 27–34 CrossRef CAS PubMed.
- M. Naoi and W. Maruyama, Expert Rev. Neurother., 2009, 9, 1233–1250 CrossRef CAS PubMed.
- O. Bar-Am, T. Amit and M. B. Youdim, J. Neurochem., 2007, 103, 500–508 CrossRef CAS PubMed.
- T. Norman and G. Burrows, J. Neural Transm., Suppl., 1988, 28, 53–63 Search PubMed.
- M. R. Liebowitz, E. Hollander, F. Schneier, R. Campeas, L. Welkowitz, J. Hatterer and B. Fallon, Acta Psychiatr. Scand., 1990, 82, 29–34 CrossRef.
- A. M. Helguera, G. Pérez-Machado, M. D. S. Cordeiro and F. Borges, Mini-Rev. Med. Chem., 2012, 12, 907–919 CrossRef CAS PubMed.
- S. K. Al-Nuaimi, E. M. MacKenzie and G. B. Baker, Am. J. Ther., 2012, 19, 436–448 CrossRef PubMed.
- B. Mathew, J. Suresh, S. Anbazhagan and G. Elizabeth Mathew, Cent. Nerv. Syst. Agents Med. Chem., 2013, 13, 195–206 CrossRef CAS PubMed.
- P. O. Patil, S. B. Bari, S. D. Firke, P. K. Deshmukh, S. T. Donda and D. A. Patil, Bioorg. Med. Chem., 2013, 21, 2434–2450 CrossRef CAS PubMed.
- S. Carradori, M. D'Ascenzio, P. Chimenti, D. Secci and A. Bolasco, Mol. Diversity, 2014, 18, 219–243 CrossRef CAS PubMed.
- C. Fowler, Å. Wiberg, L. Oreland, J. Marcusson and B. Winblad, J. Neural Transm., 1980, 49, 1–20 CrossRef CAS PubMed.
- J. Fowler, N. Volkow, G.-J. Wang, J. Logan, N. Pappas, C. Shea and R. MacGregor, Neurobiol. Aging, 1997, 18, 431–435 CrossRef CAS PubMed.
- J. K. Mallajosyula, D. Kaur, S. J. Chinta, S. Rajagopalan, A. Rane, D. G. Nicholls, D. A. Di Monte, H. Macarthur and J. K. Andersen, PLoS One, 2008, 3, e1616 Search PubMed.
- P. Jenner and C. W. Olanow, Neurology, 1996, 47, 161S–170S CrossRef.
- H. H. Fernandez and J. J. Chen, Pharmacotherapy, 2007, 27, 174S–185S CrossRef CAS PubMed.
- R. Fioravanti, A. Bolasco, F. Manna, F. Rossi, F. Orallo, M. Yanez, A. Vitali, F. Ortuso and S. Alcaro, Bioorg. Med. Chem. Lett., 2010, 20, 6479–6482 CrossRef CAS PubMed.
- A. Sahoo, S. Yabanoglu, B. N. Sinha, G. Ucar, A. Basu and V. Jayaprakash, Bioorg. Med. Chem. Lett., 2010, 20, 132–136 CrossRef CAS PubMed.
- E. Maccioni, S. Alcaro, R. Cirilli, S. Vigo, M. C. Cardia, M. L. Sanna, R. Meleddu, M. Yanez, G. Costa and L. Casu, J. Med. Chem., 2011, 54, 6394–6398 CrossRef CAS PubMed.
- W. J. Geldenhuys, A. S. Darvesh, M. O. Funk, C. J. Van der Schyf and R. T. Carroll, Bioorg. Med. Chem. Lett., 2010, 20, 5295–5298 CrossRef CAS PubMed.
- F. Chimenti, A. Bolasco, D. Secci, P. Chimenti, A. Granese, S. Carradori, M. Yáñez, F. Orallo, F. Ortuso and S. Alcaro, Bioorg. Med. Chem., 2010, 18, 5715–5723 CrossRef CAS PubMed.
- P. Chimenti, A. Petzer, S. Carradori, M. D'Ascenzio, R. Silvestri, S. Alcaro, F. Ortuso, J. P. Petzer and D. Secci, Eur. J. Med. Chem., 2013, 66, 221–227 CrossRef CAS PubMed.
- R. T. Carroll, D. E. Dluzen, H. Stinnett, P. S. Awale, M. O. Funk and W. J. Geldenhuys, Bioorg. Med. Chem. Lett., 2011, 21, 4798–4803 CrossRef CAS PubMed.
- S. Distinto, M. Yanez, S. Alcaro, M. C. Cardia, M. Gaspari, M. L. Sanna, R. Meleddu, F. Ortuso, J. Kirchmair and P. Markt, Eur. J. Med. Chem., 2012, 48, 284–295 CrossRef CAS PubMed.
- D. Secci, A. Bolasco, S. Carradori, M. D'Ascenzio, R. Nescatelli and M. Yanez, Eur. J. Med. Chem., 2012, 58, 405–417 CrossRef CAS PubMed.
- S. Carradori, M. D'Ascenzio, C. De Monte, D. Secci and M. Yanez, Arch. Pharm., 2013, 346, 17–22 CrossRef CAS PubMed.
- H. R. Park, J. Kim, T. Kim, S. Jo, M. Yeom, B. Moon, I. H. Choo, J. Lee, E. J. Lim and K. D. Park, Bioorg. Med. Chem., 2013, 21, 5480–5487 CrossRef CAS PubMed.
- M. D'Ascenzio, S. Carradori, D. Secci, L. Mannina, A. P. Sobolev, C. De Monte, R. Cirilli, M. Yanez, S. Alcaro and F. Ortuso, Bioorg. Med. Chem., 2014, 22, 2887–2895 CrossRef PubMed.
- M. J. Matos, D. Viña, P. Janeiro, F. Borges, L. Santana and E. Uriarte, Bioorg. Med. Chem. Lett., 2010, 20, 5157–5160 CrossRef CAS PubMed.
- M. J. Matos, C. Terán, Y. Pérez-Castillo, E. Uriarte, L. Santana and D. Vina, J. Med. Chem., 2011, 54, 7127–7137 CrossRef CAS PubMed.
- M. J. Matos, S. Vilar, R. M. Gonzalez-Franco, E. Uriarte, L. Santana, C. Friedman, N. P. Tatonetti, D. Viña and J. A. Fontenla, Eur. J. Med. Chem., 2013, 63, 151–161 CrossRef CAS PubMed.
- G. Delogu, C. Picciau, G. Ferino, E. Quezada, G. Podda, E. Uriarte and D. Vina, Eur. J. Med. Chem., 2011, 46, 1147–1152 CrossRef CAS PubMed.
- S. Serra, G. Ferino, M. J. Matos, S. Vazquez-Rodriguez, G. Delogu, D. Vina, E. Cadoni, L. Santana and E. Uriarte, Bioorg. Med. Chem. Lett., 2012, 22, 258–261 CrossRef CAS PubMed.
- M. D. Mertens, S. Hinz, C. E. Müller and M. Gütschow, Bioorg. Med. Chem., 2014, 22, 1916–1928 CrossRef CAS PubMed.
- S. Alcaro, A. Gaspar, F. Ortuso, N. Milhazes, F. Orallo, E. Uriarte, M. Yanez and F. Borges, Bioorg. Med. Chem. Lett., 2010, 20, 2709–2712 CrossRef CAS PubMed.
- F. Chimenti, R. Fioravanti, A. Bolasco, P. Chimenti, D. Secci, F. Rossi, M. Yáñez, F. Orallo, F. Ortuso and S. Alcaro, Bioorg. Med. Chem. Lett., 2010, 18, 1273–1279 CrossRef CAS PubMed.
- N. Desideri, A. Bolasco, R. Fioravanti, L. Proietti Monaco, F. Orallo, M. Yanez, F. Ortuso and S. Alcaro, J. Med. Chem., 2011, 54, 2155–2164 CrossRef CAS PubMed.
- L. Legoabe, A. Petzer and J. P. Petzer, Eur. J. Med. Chem., 2012, 49, 343–353 CrossRef CAS PubMed.
- L. Legoabe, A. Petzer and J. P. Petzer, Bioorg. Med. Chem. Lett., 2014, 24, 2758–2763 CrossRef CAS PubMed.
- L. J. Legoabe, A. Petzer and J. P. Petzer, Chem. Biol. Drug Des., 2015, 86, 895–904 CAS.
- A. Gaspar, J. Reis, A. Fonseca, N. Milhazes, D. Viña, E. Uriarte and F. Borges, Bioorg. Med. Chem. Lett., 2011, 21, 707–709 CrossRef CAS PubMed.
- F. Cagide, T. Silva, J. Reis, A. Gaspar, F. Borges, L. Gomes and J. Low, Chem. Commun., 2015, 51, 2832–2835 RSC.
- L. Legoabe, J. Kruger, A. Petzer, J. J. Bergh and J. P. Petzer, Eur. J. Med. Chem., 2011, 46, 5162–5174 CrossRef CAS PubMed.
- O. Al-Baghdadi, N. I. Prater, C. J. Van der Schyf and W. J. Geldenhuys, Bioorg. Med. Chem. Lett., 2012, 22, 7183–7188 CrossRef CAS PubMed.
- L.-H. Mu, B. Wang, H.-Y. Ren, P. Liu, D.-H. Guo, F.-M. Wang, L. Bai and Y.-S. Guo, Bioorg. Med. Chem. Lett., 2012, 22, 3343–3348 CrossRef CAS PubMed.
- S. J. Robinson, J. P. Petzer, A. Petzer, J. J. Bergh and A. C. Lourens, Bioorg. Med. Chem. Lett., 2013, 23, 4985–4989 CrossRef CAS PubMed.
- G. Jo, S. Ahn, B.-G. Kim, H. R. Park, Y. H. Kim, H. A. Choo, D. Koh, Y. Chong, J.-H. Ahn and Y. Lim, Bioorg. Med. Chem., 2013, 21, 7890–7897 CrossRef CAS PubMed.
- S. Nag, G. Kettschau, T. Heinrich, A. Varrone, L. Lehmann, B. Gulyas, A. Thiele, É. Keller and C. Halldin, Bioorg. Med. Chem., 2013, 21, 186–195 CrossRef CAS PubMed.
- S. Nag, L. Lehmann, G. Kettschau, M. Toth, T. Heinrich, A. Thiele, A. Varrone and C. Halldin, Bioorg. Med. Chem., 2013, 21, 6634–6641 CrossRef CAS PubMed.
- P. B. Huleatt, M. L. Khoo, Y. Y. Chua, T. W. Tan, R. S. Liew, B. Z. Balogh, R. Deme, F. R. Gölöncsér, K. Magyar and D. P. Sheela, J. Med. Chem., 2015, 58, 1400–1419 CrossRef CAS PubMed.
- S. Luhr, M. Vilches-Herrera, A. Fierro, R. R. Ramsay, D. E. Edmondson, M. Reyes-Parada, B. K. Cassels and P. Iturriaga-Vasquez, Bioorg. Med. Chem., 2010, 18, 1388–1395 CrossRef PubMed.
- L. H. Prins, J. P. Petzer and S. F. Malan, Eur. J. Med. Chem., 2010, 45, 4458–4466 CrossRef CAS PubMed.
- M. M. Van der Walt, G. Terre'Blanche, A. Petzer and J. P. Petzer, Bioorg. Med. Chem. Lett., 2012, 22, 6632–6635 CrossRef CAS PubMed.
- W. J. Geldenhuys, M. O. Funk, C. J. Van der Schyf and R. T. Carroll, Bioorg. Med. Chem. Lett., 2012, 22, 1380–1383 CrossRef CAS PubMed.
- B. Song, T. Xiao, X. Qi, L.-N. Li, K. Qin, S. Nian, G.-X. Hu, Y. Yu, G. Liang and F. Ye, Bioorg. Med. Chem. Lett., 2012, 22, 1739–1742 CrossRef CAS PubMed.
- T. A. Dorcas Okaecwe, Inhibition of monoamine oxidase by selected 8-[(phenylsulfanyl)methyl] caffeine derivatives/Thokozile Okaecwe, Diss, North-West University, 2012, pp. 4–78 Search PubMed.
- N. D. Chaurasiya, S. Ganesan, N. Nanayakkara, L. R. Dias, L. A. Walker and B. L. Tekwani, Bioorg. Med. Chem. Lett., 2012, 22, 1701–1704 CrossRef CAS PubMed.
- M. M. Van der Walt, G. Terre'Blanche, A. C. Lourens, A. Petzer and J. P. Petzer, Bioorg. Med. Chem. Lett., 2012, 22, 7367–7370 CrossRef CAS PubMed.
- B. Strydom, J. J. Bergh and J. P. Petzer, Bioorg. Med. Chem. Lett., 2013, 23, 1269–1273 CrossRef CAS PubMed.
- L. Meiring, J. P. Petzer and A. Petzer, Bioorg. Med. Chem. Lett., 2013, 23, 5498–5502 CrossRef CAS PubMed.
- A. Stößel, M. Schlenk, S. Hinz, P. Küppers, J. Heer, M. Gütschow and C. E. Müller, J. Med. Chem., 2013, 56, 4580–4596 CrossRef PubMed.
- N. T. Tzvetkov, S. Hinz, P. Küppers, M. Gastreich and C. E. Müller, J. Med. Chem., 2014, 57, 6679–6703 CrossRef CAS PubMed.
- A. Burns and S. Iliffe, BMJ, 2009, 338, b158 CrossRef PubMed.
- M. Garcia-Alloza, F. Gil-Bea, M. Diez-Ariza, C.-H. Chen, P. T. Francis, B. Lasheras and M. Ramirez, Neuropsychologia, 2005, 43, 442–449 CrossRef CAS PubMed.
- J. L. Cummings, W. Ross, J. Absher, J. Gornbein and L. Hadjiaghai, Alzheimer Dis. Assoc. Disord., 1995, 9, 87–93 CrossRef CAS PubMed.
- Y. Christen, Am. J. Clin. Nutr., 2000, 71, 621–629 Search PubMed.
- W. R. Markesbery, Free Radical Biol. Med., 1997, 23, 134–147 CrossRef CAS PubMed.
- J. Sterling, Y. Herzig, T. Goren, N. Finkelstein, D. Lerner, W. Goldenberg, I. Miskolczi, S. Molnar, F. Rantal and T. Tamas, J. Med. Chem., 2002, 45, 5260–5279 CrossRef CAS PubMed.
- T. Thomas, Neurobiol. Aging, 2000, 21, 343–348 CrossRef CAS PubMed.
- D. R. Liston, J. A. Nielsen, A. Villalobos, D. Chapin, S. B. Jones, S. T. Hubbard, I. A. Shalaby, A. Ramirez, D. Nason and W. F. White, Eur. J. Pharmacol., 2004, 486, 9–17 CrossRef CAS PubMed.
- A. Samadi, M. Chioua, I. Bolea, C. de los Rios, I. Iriepa, I. Moraleda, A. Bastida, G. Esteban, M. Unzeta and E. Galvez, Eur. J. Med. Chem., 2011, 46, 4665–4668 CrossRef CAS PubMed.
- I. Bolea, J. Juarez-Jimenez, C. de los Rıos, M. Chioua, R. Pouplana, F. J. Luque, M. Unzeta, J. Marco-Contelles and A. Samadi, J. Med. Chem., 2011, 54, 8251–8270 CrossRef CAS PubMed.
- A. Samadi, C. de los Ríos, I. Bolea, M. Chioua, I. Iriepa, I. Moraleda, M. Bartolini, V. Andrisano, E. Gálvez and C. Valderas, Eur. J. Med. Chem., 2012, 52, 251–262 CrossRef CAS PubMed.
- O. M. Bautista-Aguilera, G. Esteban, I. Bolea, K. Nikolic, D. Agbaba, I. Moraleda, I. Iriepa, A. Samadi, E. Soriano, M. Unzeta and J. Marco-Contelles, Eur. J. Med. Chem., 2014, 75, 82–95 CrossRef CAS PubMed.
- H. Zheng, M. B. Youdim and M. Fridkin, ACS Chem. Biol., 2010, 5, 603–610 CrossRef CAS PubMed.
- M.-Y. Wu, G. Esteban, S. Brogi, M. Shionoya, L. Wang, G. Campiani, M. Unzeta, T. Inokuchi, S. Butini and J. Marco-Contelles, Eur. J. Med. Chem., 2015 DOI:10.1016/j.ejmech.2015.10.001.
- L. Wang, G. Esteban, M. Ojima, O. M. Bautista-Aguilera, T. Inokuchi, I. Moraleda, I. Iriepa, A. Samadi, M. B. Youdim and A. Romero, Eur. J. Med. Chem., 2014, 80, 543–561 CrossRef CAS PubMed.
- O. M. Bautista-Aguilera, A. Samadi, M. Chioua, K. Nikolic, S. Filipic, D. Agbaba, E. Soriano, L. de Andres, M. I. Rodríguez-Franco and S. Alcaro, J. Med. Chem., 2014, 57, 10455–10463 CrossRef CAS PubMed.
- C. Lu, Q. Zhou, J. Yan, Z. Du, L. Huang and X. Li, Eur. J. Med. Chem., 2013, 62, 745–753 CrossRef CAS PubMed.
- Y. Sun, J. Chen, X. Chen, L. Huang and X. Li, Bioorg. Med. Chem., 2013, 21, 7406–7417 CrossRef CAS PubMed.
- S.-S. Xie, X. Wang, N. Jiang, W. Yu, K. D. Wang, J.-S. Lan, Z.-R. Li and L.-Y. Kong, Eur. J. Med. Chem., 2015, 95, 153–165 CrossRef CAS PubMed.
- M. Huang, S.-S. Xie, N. Jiang, J.-S. Lan, L.-Y. Kong and X.-B. Wang, Bioorg. Med. Chem. Lett., 2015, 25, 508–513 CrossRef CAS PubMed.
- R. Farina, L. Pisani, M. Catto, O. Nicolotti, D. Gadaleta, N. Denora, R. Soto-Otero, E. Méndez-Álvarez, C. S. Passos and G. Muncipinto, J. Med. Chem., 2015, 58, 5561–5578 CrossRef CAS PubMed.
- M. K. Hawkins and A. A. Baumeister, El papel de la “serendipity” en la ontogenia de la moderna psicofarmacología, in Historia de la Psicofarmacología. La consolidación de la psicofarmacología como disciplina científica: aspectos éticolegales y perspectivas de futuro, ed. F. López-Muñoz and C. Alamo, Editorial Médica Panamericana, Madrid, 2007, pp. 1525–1538 Search PubMed.
- G. E. Crane, Psychiatr. Res. Rep., 1957, 8, 142–152 CAS.
- R. M. Pinder and J. H. Wieringa, Med. Res. Rev., 1993, 13, 259–325 CrossRef CAS PubMed.
- D. Leysen and R. M. Pinder, Annu. Rep. Med. Chem., 1994, 29, 1–12 Search PubMed.
- J. C. Soares and S. Gershon, Drugs, 1996, 52, 477–482 CrossRef CAS PubMed.
- S. Salmans, Depression: Questions You Have – Answers You Need. People's Medical Society, 1997, ISBN 978-1-882606-14-6 Search PubMed.
- M. Karuppasamy, M. Mahapatra, S. Yabanoglu, G. Ucar, B. N. Sinha, A. Basu, N. Mishra, A. Sharon, U. Kulandaivelu and V. Jayaprakash, Bioorg. Med. Chem., 2010, 18, 1875–1881 CrossRef CAS PubMed.
- F. Chimenti, S. Carradori, D. Secci, A. Bolasco, B. Bizzarri, P. Chimenti, A. Granese, M. Yanez and F. Orallo, Eur. J. Med. Chem., 2010, 45, 800–804 CrossRef CAS PubMed.
- K. Senturk, O. U. Tan, S. Y. Ciftci, G. Ucar and E. Palaska, Arch. Pharm., 2012, 345, 695–702 CrossRef PubMed.
- G. Jo, S. H. Sung, Y. Lee, B.-G. Kim, J. Yoon, H. O. Lee, S. Y. Ji, D. Koh, J.-H. Ahn and Y. Lim, Bull. Korean Chem. Soc., 2012, 33, 3841–3844 CrossRef CAS.
- J. Reniers, S. Robert, R. Frederick, B. Masereel, S. Vincent and J. Wouters, Bioorg. Med. Chem., 2011, 19, 134–144 CrossRef CAS PubMed.
- M. Prashanth, H. D. Revanasiddappa, K. Lokanatha Rai and B. Veeresh, Bioorg. Med. Chem. Lett., 2012, 22, 7065–7070 CrossRef CAS PubMed.
- Y. Bandaruk, R. Mukai, T. Kawamura, H. Nemoto and J. Terao, J. Agric. Food Chem., 2012, 60, 10270–10277 CrossRef CAS PubMed.
- O. Demirkiran, Phytochem. Lett., 2012, 5, 700–704 CrossRef CAS.
- V. H. Masand, D. T. Mahajan, A. M. Manikrao, N. Mahajan, P. N. Khatale, R. D. Jawarkar and T. B. Hadda, J. Comput. Methods Mol. Des., 2011, 1, 24–28 CAS.
- O. M. Abdelhafez, K. M. Amin, H. I. Ali, M. M. Abdalla and R. Z. Batran, J. Med. Chem., 2012, 55, 10424–10436 CrossRef CAS PubMed.
- O. M. Abdelhafez, K. M. Amin, H. I. Ali, M. M. Abdalla and R. Z. Batran, Neurochem. Int., 2013, 62, 198–209 CrossRef CAS PubMed.
- C. Mattsson, P. Svensson and C. Sonesson, Eur. J. Med. Chem., 2014, 73, 177–186 CrossRef CAS PubMed.
- S. N. Khattab, S. Y. Hassan, A. A. Bekhit, A. M. El Massry, V. Langer and A. Amer, Eur. J. Med. Chem., 2010, 45, 4479–4489 CrossRef CAS PubMed.
- L. Shi, Y. Yang, Z.-L. Li, Z.-W. Zhu, C.-H. Liu and H.-L. Zhu, Bioorg. Med. Chem., 2010, 18, 1659–1664 CrossRef CAS PubMed.
- S. Valente, S. Tomassi, G. Tempera, S. Saccoccio, E. Agostinelli and A. Mai, J. Med. Chem., 2011, 54, 8228–8232 CrossRef CAS PubMed.
- F. Pettersson, P. Svensson, S. Waters, N. Waters and C. Sonesson, J. Med. Chem., 2012, 55, 3242–3249 CrossRef CAS PubMed.
- K. Lewellyn, D. Bialonska, N. D. Chaurasiya, B. L. Tekwani and J. K. Zjawiony, Bioorg. Med. Chem. Lett., 2012, 22, 4926–4929 CrossRef CAS PubMed.
- J. G. Villarinho, R. Fachinetto, F. d. V. Pinheiro, G. d. S. Sant'Anna, P. Machado, P. A. Dombrowski, C. d. Cunha, D. d. A. Cabrini, M. A. P. Martins and H. G. Bonacorso, Prog. Neuro-Psychopharmacol. Biol. Psychiatry, 2012, 39, 31–39 CrossRef CAS PubMed.
- B. Mathew, A. Jerad Suresh and S. Anbazhagan, J. Saudi Chem. Soc., 2012, 1–8 Search PubMed.
- E. Bonaiuto, A. Milelli, G. Cozza, V. Tumiatti, C. Marchetti, E. Agostinelli, C. Fimognari, P. Hrelia, A. Minarini and M. L. Di Paolo, Eur. J. Med. Chem., 2013, 70, 88–101 CrossRef CAS PubMed.
- L. Pisani, M. Barletta, R. Soto-Otero, O. Nicolotti, E. Mendez-Alvarez, M. Catto, A. Introcaso, A. Stefanachi, S. Cellamare and C. Altomare, J. Med. Chem., 2013, 56, 2651–2664 CrossRef CAS PubMed.
- M. K. Srivastav, M. Shamshuddin and S. Shantakumar, Am. J. Chem., 2013, 3, 14–22 Search PubMed.
- J. Kumar, G. Chawla, H. Gupta, M. Akhtar, O. P. Tanwar and M. Bhowmik, Afr. J. Pharm. Pharmacol., 2013, 7, 1523–1530 CrossRef.
| This journal is © The Royal Society of Chemistry 2016 |