APTES assisted surface heparinization of polylactide porous membranes for improved hemocompatibility
Abstract
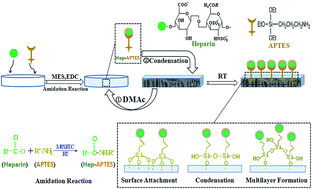
Surface heparinization is considered an efficient strategy to improve the hemocompatibility of polymeric membranes. We aim to realize the feasible surface heparinization of polylactide (PLA) membranes by means of a colourless 3-aminopropyltriethoxysilane (APTES) functionalized platform. APTES conjugated heparin (Hep–APTES) was first synthesized through the amidation reaction and then anchored onto PLA membrane via surface attachment, condensation, multilayer formation. The surface chemistry was confirmed by Fourier Transform Infrared-Attenuated Total Reflectance (ATR-FTIR) and X-ray Photoelectron Spectroscopy (XPS). The influences of heparinization time and micro-swelling agent (DMAc) on the heparin density, stability and sustained-release were investigated. The hydrophilicity, flux and morphology were also studied by contact angle, pure water flux and Scanning Electron Microscope (SEM). The hemocompatibility was evaluated by the activated partial thromboplastin time (APTT), prothrombin time (PT), the content of fibrinogen (FIB), platelet and Bovine Serum Albumin (BSA) adsorption respectively. All results demonstrated that APTES assisted surface heparinization provided a feasible and efficient strategy to improve the hemocompatibility of PLA membrane.

 Please wait while we load your content...
Please wait while we load your content...