APTES assisted surface heparinization of polylactide porous membranes for improved hemocompatibility
Jinglong Liab,
Fu Liu*a,
Xuemin Yua,
Ziyang Wua,
Yunze Wanga,
Zhu Xionga and
Jidong Heb
aNingbo Institute of Materials Technology & Engineering, Chinese Academy of Sciences, Ningbo, 315201, P. R. China. E-mail: fu.liu@nimte.ac.cn; Tel: +86-574-86685256
bTsingtao University of Science & Technology, Qingdao, 266000, P. R. China
First published on 25th April 2016
Abstract
Surface heparinization is considered an efficient strategy to improve the hemocompatibility of polymeric membranes. We aim to realize the feasible surface heparinization of polylactide (PLA) membranes by means of a colourless 3-aminopropyltriethoxysilane (APTES) functionalized platform. APTES conjugated heparin (Hep–APTES) was first synthesized through the amidation reaction and then anchored onto PLA membrane via surface attachment, condensation, multilayer formation. The surface chemistry was confirmed by Fourier Transform Infrared-Attenuated Total Reflectance (ATR-FTIR) and X-ray Photoelectron Spectroscopy (XPS). The influences of heparinization time and micro-swelling agent (DMAc) on the heparin density, stability and sustained-release were investigated. The hydrophilicity, flux and morphology were also studied by contact angle, pure water flux and Scanning Electron Microscope (SEM). The hemocompatibility was evaluated by the activated partial thromboplastin time (APTT), prothrombin time (PT), the content of fibrinogen (FIB), platelet and Bovine Serum Albumin (BSA) adsorption respectively. All results demonstrated that APTES assisted surface heparinization provided a feasible and efficient strategy to improve the hemocompatibility of PLA membrane.
1. Introduction
Polymeric membranes have been intensively studied and widely applied for blood purification, such as polymethylmethacrylate (PMMA),1 cellulose acetate (CA),2,3 polyvinyl alcohol (PVA),4 polyurethane (PU),5 polyethersulfone (PES),6 polysulfone (PSf).7 Compared to these conventional petroleum-based materials, bio-based polylactide (PLA) provided an alternative choice due to the carbon-neutral and sustainable property, easy processability and controllable degradation in particular biomedical fields such as artificial blood vessel, periosteum cell culture scaffold and nerve channel guide channel.8–12 Despite the attracting virtues, the rough surface or hydrophobic characteristic of PLA porous membrane may cause unfavorable platelet adhesion, protein adsorption or even bacterial propagation, finally lead to the adverse reactions such as serious thrombosis, hemolysis, complement activation or inflammation.Versatile biocompatible modification approaches based on the conventional materials have been attempted in laboratory or enterprise.13–16 In particular, designs integrating heparin modified membranes have been attracting much attention to improve biocompatibility and biofunctionality, and versatile methods have been developed and summarized as follows: (1) surface coating, covalently bonded heparin-benzyl was synthesized and then coated onto the membrane surface for the prolonged extracorporeal lung assist.17 Besides, heparin can be directly coated onto the human serum albumin (AL) containing poly(hydroxyethyl methacrylate) hydrogel networks to effectively increase the blood compatibility and prevent thrombus formation.18 (2) Surface grafting, heparin can be directly grafted on the membrane surfaces through the amide reaction between –COO− and –NH2 using 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC) as catalyst.19 For example, Yang et al. grafted heparin onto the carboxyl-induced polyacrylonitrile (PAN) membrane or chitosan modified PAN membrane via EDC.20 A novel heparin-immobilized polyethersulfone (PES) was also synthesized by Hou et al. PES was initially sulfonated with chlorosulfonic acid and then 1,6-hexanediamine was grafted to the –SO3H groups of sulfonated PES, which subsequently reacted with heparin through a covalent bond by using EDC as catalyst.21 (3) Blending, the heparin-like surface can be obtained by blending sulfonated polyethersulfone (SPES) and poly(acrylonitrile-co-acrylic acid-co-vinyl pyrrolidone) (P(AN-AA-VP)), which provided sulfonic acid (–SO3H) and carboxylic acid groups (–COOH), respectively.22 (4) Layer-by-layer (LBL) assembly, Yi et al. designed novel 3D multifunctional nanolayers on biomedical membrane surfaces via LBL self-assembly of nanogels and heparin-like polymers. The Ag nanogels were assembled onto membrane surfaces by electrostatic interaction, which was further assembled with heparin-like polymers.23 Therefore, the designing principle of surface heparinization or surface heparinization-mimicking is highly depending on the materials and the appropriate physicochemical interactions to elicit the favorable biological responses or biocompatibility in blood purification, artificial organs and other clinical blood contacting medical fields.24 The above immobilized heparin on the membrane surface, unlike soluble heparin, also inhibits the initial contact activation coagulation enzymes through an antithrombin III (AT-III)34 mediated pathway, and thus shows better anticoagulant properties and refrains the possibilities of massive dialysis haemorrhage risk.25 Heparin and heparin-like surface can restrain factors Xa, XIa, IXa, and IIa (thrombin) to improve the biocompatibility without compromising thrombo-resistant properties.26
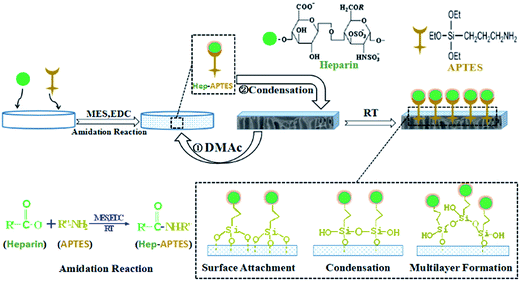
As summarized in our introduction, a versatile methods including surface coating, physical blending, layer-by-layer (LBL) assembly and surface grafting were widely to realize the heparinization for improving the hemocompatibility. Different from above all strategies, the firstly synthesized Hep–APTES conjugates will promote the amidation reaction between the –COOH group of heparin moieties and –NH2 group of APTES. Moreover, it can avoid the degradation of PLA porous membrane. In our previous study, heparin was grafted onto PLA membrane surface via dopamine as a linker, and consequently improved the hemocompatibility, suppressed the adhesion of platelet, extended plasma recalcification time, and also decreased hemolysis ratio of PLA porous membrane.27 Nevertheless, the slow dopamine deposition may cause deep color, low efficiency and contamination.28 APTES serves as superglue to anchor the target biomolecules on a solid surface and also acts as a spacer, allowing more steric freedom to the biomolecules during the immobilization step for higher specific activity.26 In general, APTES bonds to a substrate in three different ways. Surface attachment and condensation will react with its neighboring surface adsorption APTES. In the third scenario, the molecule of APTES is easy to transform into multilayer formation due to the formation of steric hindrance.26 Besides, the APTES spacer is colorless and the approach will not darken the original polymeric membrane.
In the present paper, we first synthesized APTES conjugated heparin (Hep–APTES) through the amidation reaction between –COOH groups of heparin moieties and –NH2 groups of APTES by using N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC·HCl) as catalyst and 4-morpholineethanesulfonic acid (MES) as buffer solution, and then Hep–APTES was immobilized onto PLA membrane surface via self-polycondensation including surface attachment, condensation and multilayer formation. Compared to the traditional heparin conjugated onto APTES modified substrate method, the firstly synthesized Hep–APTES conjugates will promote the amidation reaction between the –COOH group of heparin moieties and –NH2 group of APTES. Moreover, it can avoid the degradation of PLA porous membrane. The mechanism of heparin grafted on PLA membrane with APTES as a spacer was illustrated in Fig. 1. The surface chemistry, morphology, hydrophilicity/hydrophobicity, protein fouling resistance and hemocompatibility of the modified PLA membranes were also investigated in detail.
2. Experimental section
2.1 Materials
Polylactide (2003D, Natural works, US) was used to prepare PLA porous membrane. And polyoxyethylene (PEO) (CP, Mv 100 kD, Aladdin) was used as pore-forming agent. N,N-Dimethylacetamide (DMAc), 3-aminopropyltriethoxysilane (APTES), toluidine blue (TB), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC·HCl) and bovine serum albumin (BSA) with the purity of AR obtained from Aladdin, China were all directly used. 4-Morpholineethanesulfonic acid (MES), heparin sodium with the purity of AR was purchased from Sinopharm of China. The anticoagulant cattle whole blood was purchased from Beijing Pingrui Biotechnology Company, China. Ellagic acid was obtained from Sysmex Corporation, Japan. G-250 was purchased from Amresco, US.2.2 Preparation of PLA porous membrane
The PLA membranes were prepared via the classical non-solvent induced phase separation technique. The specific process is as follows: PLA (18 g) and PEO (6 g) were dissolved in DMAc (76 g) at 85 °C with stirring speed of 180 rpm for 12 h. The dissolved solution was kept for another 12 h at reduced pressure to release bubbles. The obtained solution was casted onto a clean glass plate with a casting knife's slit width of 200 μm and then immersed into a deionized water bath at 25 °C. The solidified PLA membrane was taken out from the water bath and thoroughly cleaned by frequently changed water to remove residual solvent. Finally PLA porous membranes were obtained for further modification and characterization.2.3 Surface heparinization of PLA porous membranes
APTES conjugated heparin (Hep–APTES) was first synthesized through the amidation reaction between –COOH groups of heparin moieties and –NH2 groups of APTES by using N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC·HCl) as catalyst and 4-morpholineethanesulfonic acid (MES) as buffer solution. The specific procedure is illustrated in Fig. 1 as follows: 1 g of heparin sodium powder was first dissolved in 50 mL MES buffer solution (0.05 mol L−1, 5 mL ethanol was added to regulate the hydrolysis of APTES), and then a certain 0.2811 g of coupling catalyst EDC·HCl was dropped in. Afterwards, 0.1622 g of APTES was added into the above solution to start the amidation reaction. Hep–APTES was therefore produced after sufficient stirring for 40 minutes at room temperature. Afterwards, a piece of PLA membrane (4 × 4 cm2) was immersed into the above prepared solution to initiate the surface heparinization reaction including surface attachment, condensation and multilayer formation of Hep–APTES for 4 h, 8 h, 12 h respectively. 0.5 mL of DMAc was also added to assist the immobilization of heparin on PLA porous membrane as a swelling agent. The membranes were washed thoroughly with physiological saline solution (0.9 wt%) and heparin immobilized PLA membranes (Hep–PLA membranes) were obtained finally for further characterization.2.4 Characterization of modified PLA membranes
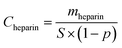 | (1) |
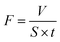 | (2) |
2.4.4.1 Activated partial thromboplastin time (APTT). 10 mL anticoagulant cattle whole blood was centrifuged at 3000 rpm for 15 min to prepare platelet-poor plasma (PPP). The membrane (0.4 × 0.4 cm2) was placed in a 48-well cell culture plate while dropping 0.1 mL PPP into the plate at 37 °C for 5 min, and then 0.1 mL ellagic acid (37 °C, water bath) was dropped into the above solution. After shaking incubation for 5 min, PLA membrane was taken out, and 0.1 mL CaCl2 (0.025 mol L−1, 37 °C) aqueous solution was dropped into the PPP (transferred to a reagent tube). The consuming time was recorded by semi-automated coagulation analyzer as the activated partial thromboplastin time APTT of the PLA membrane.
2.4.4.2. Prothrombin time (PT). The membrane (0.5 × 0.5 cm2) was placed in a 48-well cell culture plate while dropping 0.1 mL PPP into the plate at 37 °C for 10 min. Then the PT reagent (0.2 mL) was added to reaction cup and the coagulation time (prothrombin time) was determined by PUN-2048A coagulation analyzer.
2.4.4.3. Platelet adhesion. Anticoagulant cattle whole blood (10 mL) was centrifuged at 1000 rpm for 15 min to obtain platelet-rich plasma (PRP). 0.1 mL PRP was dropped into the 24-well cell culture plate at 37 °C and then the modified PLA membranes (1.0 × 1.0 cm2) was immersed into the plate for 1 h. After incubation, the unstable platelets adsorbed on the samples membrane surface were removed with physiological saline for three times. Then the modified PLA membranes were immersed in 2.5 wt% glutaraldehyde for a night in order to fix the adsorbed platelets. However, the membrane surface still contains much free water. Furthermore, a series of ethanol in water solution (25, 50, 75, 85, 95, and 100 vol% respectively) to dehydrate the sample in sequence as soon as possible. The morphologies and quantities of the platelets on the PLA membranes surface were observed and analyzed by scanning electronic microscopy (SEM).
2.4.4.4. Non-specific protein adsorption. The non-specific protein (BSA) adsorption on the modified PLA membrane was determined by the Bradford method. The specific operation method is as follows: (1) the standard solution with BSA concentration of 0.01, 0.02, 0.04, 0.06, 0.08, 0.1 mg mL−1 respectively and then adding a certain amount of G-250. (2) The standard solution was measured by UV-visible spectrophotometry with the absorption of 595 nm. (3) The membrane samples were immersed into BSA solution at 25 °C for 6 h. The amount of BSA adsorbed on the surface of the membranes was calculated by comparing the absorption peak with the standard curve.
2.4.4.5. The content of fibrinogen (FIB) transferring to fibrin. The constant value of plasma was diluted with buffer solution to prepare calibration curves (volume ratio of 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1
10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, 1
15, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20). The plasma was determined by the different dilution degree of 200 μL, and it was preheating at 37 °C for 3 min. Then the thrombin solution (100 μL) was added to reaction cup and the coagulation time was determined by PUN-2048A coagulation analyzer. The measured plasma diluted with buffer solution by volume ratio of 1
20). The plasma was determined by the different dilution degree of 200 μL, and it was preheating at 37 °C for 3 min. Then the thrombin solution (100 μL) was added to reaction cup and the coagulation time was determined by PUN-2048A coagulation analyzer. The measured plasma diluted with buffer solution by volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The membrane (1.0 × 1.0 cm2) was placed in a 48-well cell culture plate while dropping 200 μL test plasma (PPP) into the plate at 37 °C for 10 min. And then added the thrombin solution 100 μL into the reaction cup. The sample content of FIB can be read directly from the standard curve.
10. The membrane (1.0 × 1.0 cm2) was placed in a 48-well cell culture plate while dropping 200 μL test plasma (PPP) into the plate at 37 °C for 10 min. And then added the thrombin solution 100 μL into the reaction cup. The sample content of FIB can be read directly from the standard curve.
3. Results and discussion
3.1 Surface chemistry confirmation
XPS were used to analyze the chemistry of the surface heparinized PLA membranes. The binding energy and the element concentration was shown in Fig. 2(a). The original PLA membrane only showed the absorption peaks of O 1s and C 1s. In comparison, all modified PLA membranes showed decreased content of O 1s and C 1s, while the percentage of the oxygen elements was increased with increasing the reaction time from 4 h to 8 h and 12 h mainly due to the immobilization of Hep–APTES. More importantly, new adsorption peaks ascribed to nitrogen (N 1s), sulfur (S 2s and S 2p) and silicon (Si 2p) appeared distinctly for modified PLA membrane HEP04-M, HEP08-M and HEP12-M respectively. Furthermore, the increased contents of all new elements indicated the surface heparinization was enhanced with the reaction time. To be more specific, the newly appeared Si 2p indicated the presence of self-condensed APTES. The appearance of sulfur (S 2s and S 2p) implied the existence of –SO3− assigned to heparin. Interestingly, the appearance of N 1s cannot be identified to APTES or heparin clearly. Therefore, the heparin modified PLA membrane was further characterized by ATR-FTIR as show in Fig. 2(b).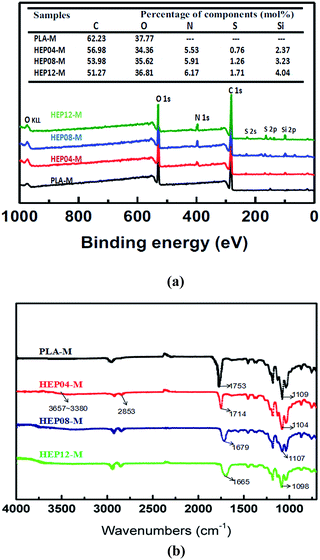 | ||
| Fig. 2 (a) XPS wide scans and elemental mole percentages, (b) ATR-FTIR spectra of PLA-M, HEP04-M, HEP08-M and HEP12-M. | ||
PLA membrane modified by Hep–APTES showed the strong absorption bands at 1109 cm−1 assigned to the ester group C–O bond stretching vibration. But the corresponding absorption peak intensity at 1104 cm−1, 1107 cm−1 and 1098 cm−1 becomes weak with the reaction time due to the occurrence of Si–O–Si absorption peaks. For heparin modified PLA membranes, a new broad absorption at 3657–3380 cm−1 was assigned to the N–H/O–H stretching vibrations of APTES or heparin. The peak at 2853 cm−1 was ascribed to C–H stretching vibrations of methylene from APTES. In contrast to the original PLA membranes displaying peak at 1753 cm−1 attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations, the corresponding absorption peak shifted gradually to 1714, 1679 and 1665 cm−1 respectively for the surface heparinized PLA membrane HEP04-M, HEP08-M and HEP12-M due to the influence of p–π conjugated phenomenon between the amide and carbonyl bonds. Thereof, it is inferred that the presence of amide bond obviously demonstrated the successful synthesis of APTES conjugated heparin (Hep–APTES) through the amidation reaction between –COOH groups of heparin moieties and –NH2 groups of APTES by using N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC·HCl) as catalyst and 4-morpholineethanesulfonic acid (MES) as buffer solution as depicted in Fig. 1. Both XPS and ATR-FTIR results manifested that the synthesized Hep–APTES was successfully anchored to PLA porous membrane via the complicated surface attachment, condensation and multilayer formation.
O stretching vibrations, the corresponding absorption peak shifted gradually to 1714, 1679 and 1665 cm−1 respectively for the surface heparinized PLA membrane HEP04-M, HEP08-M and HEP12-M due to the influence of p–π conjugated phenomenon between the amide and carbonyl bonds. Thereof, it is inferred that the presence of amide bond obviously demonstrated the successful synthesis of APTES conjugated heparin (Hep–APTES) through the amidation reaction between –COOH groups of heparin moieties and –NH2 groups of APTES by using N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC·HCl) as catalyst and 4-morpholineethanesulfonic acid (MES) as buffer solution as depicted in Fig. 1. Both XPS and ATR-FTIR results manifested that the synthesized Hep–APTES was successfully anchored to PLA porous membrane via the complicated surface attachment, condensation and multilayer formation.
3.2 Surface heparinization density, stability on PLA membrane
The content of heparin anchored onto PLA membrane surface was determined based on the fact that toluidine blue was closely binding to the polyanion substrate of heparin. Consequently, surface heparinization density on PLA membrane was able to be assayed through the TB colorimetric method as shown in Fig. 3.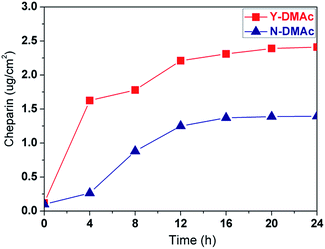 | ||
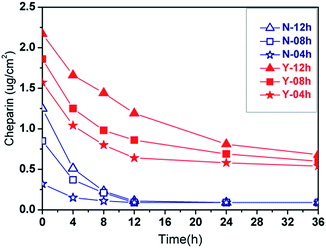
| Fig. 3 Surface heparinization density on PLA membrane over reaction time: where Y-DMAc indicating DMAc is involved in the reaction as a swelling agent, while N-DMAc indicating DMAc is not involved. | ||
As a whole, the surface heparinization density Cheparin on both Y-DMAc and N-DMAc PLA membrane was increased with the reaction time, indicating that more heparin was kinetically immobilized onto PLA membrane. Besides, in the first 12 h, Cheparin increased quickly, showing a higher reaction rate of APTES self-condensation. Subsequently, the Cheparin increased slowly due to the steric and electrostatic repulsion among Hep–APTES macromolecules. In particular, a small amount of DMAc in the reaction solution effectively enhanced the surface heparinization density as shown in Fig. 3. Y-DMAc displayed higher Cheparin than that of N-DMAc over 24 h. The maximum Cheparin of Y-DMAc and N-DMAc is 2.41 μg cm−2 and 1.42 μg cm−2 respectively. Diluted DMAc (∼1%) is regarded as a micro-swelling agent, which can swell the porous membrane to some extents and even dissolve the PEO. Thus more porous surface will be created to cause higher water permeability. Besides, Hep–APTES is more likely to enter the interior of the more porous membrane (as confirmed by the toluidine blue (TB) colorimetric method). A small amount of DMAc less than 1.0 vol% could induce the swelling of PLA membrane and the elution of PEO inevitably to promote more porous and rough surface, which is favorable to capture more Hep–APTES conjugates anchored to PLA membrane consequently. The porous and morphology can be identified by SEM pictures.
The stability of heparin anchored on PLA membrane was further investigated as shown in Fig. 4. The heparin modified PLA membranes were incubated in saline solution (0.9 wt%) for sustained-release performance measurement. In general, APTES bonds to a substrate in three different ways (surface attachment, condensation and multilayer formation, shown in Fig. 1). The polylactic acid membrane surface possessed no active hydroxyl, so there is no further covalent bonding interaction between the membrane and Hep–APTES conjugates except the above three interactions. The Hep–APTES of surface attachment is easy to be eluted. APTES related condensation reaction or multilayer formation on the surface will reduce the loss rate of heparin. It is demonstrated that Y-DMAc membrane contains more heparin on the surface than that of N-DMAc, in accordance with Fig. 3. Cheparin of Y-DMAc is still higher than 0.5 μg cm−2, while the heparin physically adsorbed or surface attached on N-DMAc are almost eluted by the saline when the incubation time is longer than 12 h. Hep–APTES can be immobilized more firmly onto Y-DMAc membrane attributing to the higher porosity. The porous surface provided more spaces and opportunities for the adsorption and subsequent condensation, multilayer formation and even hydrogen bonding interaction. The chemical sorption mainly contributed to the stable immobilization of Hep–APTES on PLA membrane. In the typical dialysis duration (∼4 h), both Y-DMAc and N-DMAc exhibited a rapid sustained-release, however, the Cheparin of N-12h and Y-12h is still 0.5 μg cm−2 and 1.7 μg cm−2 respectively in 4 h saline incubation. Therefore it is exhibited that the sustained-release of Hep–APTES conjugates can be well regulated through both physics adsorption and chemical sorption, besides, the swelling agent DMAc plays an important role of promoting the immobilization of Hep–APTES and stability as well.
3.3 Membrane hydrophilicity and flux
For the vast majority of biological materials, the hydrophilicity usually implied better compatibility. The original PLA membrane is relatively hydrophilic with the initial contact angel of 78°, and keeps slowly decaying with drop age despite of the intrinsic hydrophobic nature of PLA mainly due to the incorporation of compatible PEO as shown in Fig. 5. With surface heparinization, the modified PLA membrane including both Y-DMAc and N-DMAc membranes exhibited decreased contact angle and improved hydrophilicity. Besides, the contact angle of Y-DMAc PLA membrane (e.g. Y-12h) is lower than the counterpart of N-DMAc PLA membrane (e.g. N-12h). The contact angle of Y-12h decreased from 63°to 50°due to the immobilization of heparin on porous surface. The corresponding heparin density is around 2.25 μg cm−2, therefore, the enriched carbonyl, hydroxyl, and sulfonic acid groups of Hep–APTES spacers improved the hydrophilicity of PLA porous membrane significantly. | ||
| Fig. 5 Water contact angle variation with drop age for PLA and heparin modified PLA membranes with different reaction time. Hep-M: Y (DMAc involved) and N (DMAc not involved). | ||
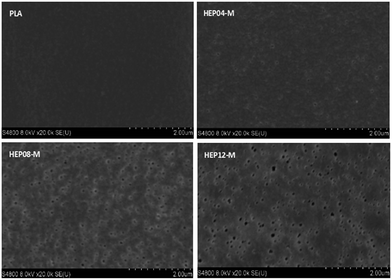
The flux of PLA membrane was comprehensively understood by measuring the pure water flux as shown in Fig. 6. The pure water flux of N-DMAc PLA membrane is around 350 L m−2 h−1, indicating that the surface heparinization did not influence the surface morphology despite the reaction time. In comparison, Y-DMAc PLA membrane modified by surface heparinization exhibited the improved flux. For example, the pure water flux of Y-DMAc membrane with reaction time 12 h can reach up to 767 L m−2 h−1, which is above two times higher than the counterpart. In contrast to the hydrophilicity, the porous surface played a more important role of providing more free water channels. With increasing the reaction time, more PEO was precipitated by the swelling agent DMAc and caused membrane pore size to larger. The resulted in more micropores on the surface as clearly depicted in Fig. 7.
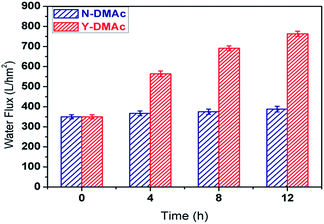 | ||
| Fig. 6 Pure water flux of N-DMAc and Y-DMAc PLA membrane modified by surface heparinization with different reaction time. | ||
3.4 Hemocompatibility
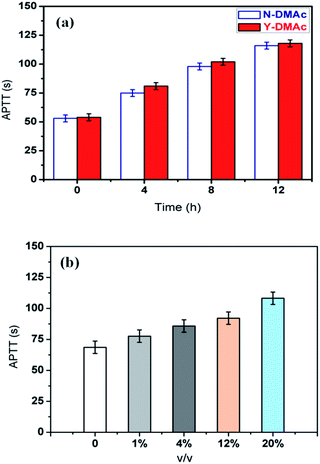
Hemocompatibility is the ability to inhibit the formation of thrombosis in the blood vessels on the surface of biomaterials. Hemolytic phenomenon, platelet adhesion, complement activation, temporary reduction of white blood cells are usually evaluated for the hemocompatibility. Owing to the direct contact between the membrane and blood in the process of dialysis, the applied are acquired to possess good blood compatibility besides membrane forming characteristics. And also, the coagulation factor level is crucial to evaluate the antithrombogenicity for heparin modified PLA membrane.However, measuring coagulation factor level is often of time-consuming and limited availability. Therefore, the activated partial thromboplastin time (APTT) assays are used as screening tests and surrogate markers of coagulation factor levels in trauma settings,32 and the results are exhibited in Fig. 8. It can be found that the APTT of the original PLA membrane was about 50 s. With the extension of reaction time, APTT values of both Y-DMAc and N-DMAc PLA membranes increased accordingly attributing to the enhanced immobilization of heparin, indicating the increased anticoagulation property as shown in Fig. 8(a). Surface heparinization density Cheparin of heparin modified PLA membrane was increased as previously shown in Fig. 3 by the physical adsorption and chemical sorption. Furthermore, heparin can act as a catalyst and restrain factors Xa, XIa, IXa, IIa,34 which was important for antithrombogenicity. However, the detailed observation of Y-DMAc and N-DMAc could be concluded that the APTT of Y-DMAc was slightly longer than that of N-DMAc, which is in accordance with the higher heparin density. Thereby, the APTT of Y-DMAc-12h can reach up to 118 s, which is two times higher than that of original one, demonstrating that the anticoagulation activity was thoroughly improved through the surface heparinization modification.
The effects of DMAc content as a swelling agent on APTT are also investigated in Fig. 8(b). It was found that APTT increased gradually with increasing DMAc fraction in the reaction mixture. The extended APTT (108.6 s) can be obtained when 20% of DMAc was applied. It has been verified that the addition of DMAc could induce the swelling of PLA and the surface pores forming as well. It can be inferred that higher concentration of DMAc will cause more porous surface, and more Hep–APTES conjugates will be absorbed on membrane to enhance the anticoagulation property as a result.
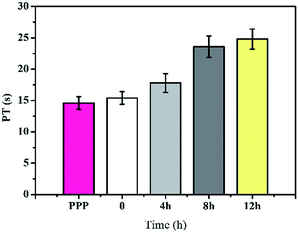
Generally, APTT was used to measure the inhibited efficacy of both the intrinsic and the common plasma coagulation pathways.33 However, prothrombin time (PT) is a sensitive and commonly used screening test for extrinsic coagulation system, which will inhibit efficacy of the common plasma coagulation pathways including factors I, II, V, X, XII. PT was commonly used to measure the time for the prothrombin into thrombin in the platelet-poor plasma (PPP). The longer clotting time indicates the slower conversion of prothrombin into thrombin, which will inhibit the production of thrombus as shown in Fig. 9. It can be found that the PT of the platelet-poor plasma (PPP) and original PLA membrane were about 14.6 and 15.4 s respectively. With the extension of reaction time, PT values of Y-DMAc membranes increased significantly (from 15.4 to 24.8 s). It is indicated that the anticoagulant activity increased with the heparin immobilization.
The heparin modified PLA membranes also inhibited the adhesion of platelets as imaged in Fig. 10. It can be seen that a large number of platelets were adhered on the surface of original PLA membranes due to the less hydrophilic nature and chemical structure of PLA. With increasing the immobilized heparin density (Fig. 3), the adhesive platelets are substantially reduced. When the reaction time is reach up to 8 h and 12 h, the platelets almost completely disappeared, indicating excellent hemocompatibility for HEP08-M and HEP12-M. It was thought that the improved hydrophilicity and electrostatic repulsion of heparin alleviate the adsorption and adhesion of the platelets. Meanwhile, the immobilized heparin can act on thrombin to inhibit the formation of fibrinogen.
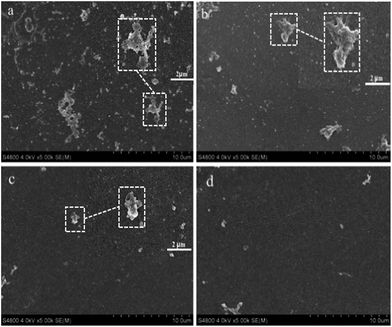 | ||
| Fig. 10 SEM images of heparin modified PLA membranes (DMAc is involved): (a) original PLA membrane, (b) HEP04-M, (c) HEP08-M and (d) HEP12-M. | ||
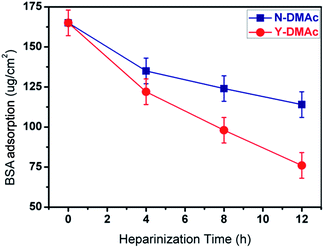
The adsorption of protein on the surface of the material was often used to simulate the mechanism of thrombus formation. The isoelectric point (PI) of BSA is about 4.9. When the pH value of the solution (pH = 7.4, simulated blood pH) is higher than BSA of the isoelectric point, BSA and heparin are negatively charged, electrostatic repulsion makes it difficult for BSA to adsorb to the surface of heparin modified membrane. The higher the density of heparin, the greater the electrostatic repulsion. Therefore, there was lower BSA adsorption on higher heparin density surface. BSA adsorption of PLA membranes with different heparinization time was investigated (Fig. 11). For original PLA membrane, the BSA adsorption amount was as high as 167.2 μg cm−2, implying serious protein adsorption and fouling as well. In case of heparin modified PLA membrane, the BSA adsorption amount decreased obviously (especially for Y-DMAc-12h, 73.1 μg cm−2), showing better biocompatibility and fouling resistance. All Y-DMAc membranes exhibited lower BSA adsorption than N-DMAc membranes mainly due to the higher heparin density and better hydrophilicity.
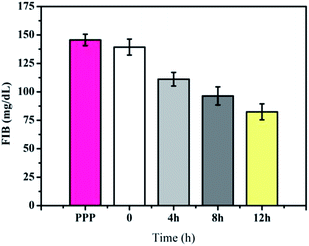
Heparin will block thromboplastin generation, resulting in fibrinogen cannot be hydrolyzed into a fibrin monomer. Therefore, FIB is used to measure the content of the fibrinogen transferring to fibrin as shown in Fig. 12 and the results were analyzed by PUN-2048A coagulation analyzer. The FIB of pure platelet-poor plasma (PPP) was about 145.6 mg dL−1, which was similar with pristine PLA membrane (139.3 mg dL−1). For the Hep–APTES membranes, the content of the FIB transferring to fibrin decreased obviously compared with the pristine PLA membrane. For example, the FIB of Hep–APTES membranes (grafting time 12 h) decreased to 82.4 mg dL−1. All APTT, PT, FIB, platelets and BSA adsorption results come to a conclusion that the PLA porous membrane modified by heparin showed significantly improved hemocompatibility.
4. Conclusion
In conclusion, surface heparinization of PLA membrane was successfully accomplished by using APTES as a spacer in a mild reaction condition. Hep–APTES conjugates were first synthesized the amidation reaction between –COOH and –NH2 groups and then anchored onto PLA membrane via the surface attachment, condensation, and multilayer formation interaction, which was verified by the XPS and ATR-FTIR results. Surface heparinization density Cheparin increased kinetically with the reaction time. The involvement of swelling agent DMAc promoted the immobilization of heparin and the stability as well. The highest Cheparin can reach up to 2.41 μg cm−2, and Cheparin of Y-12h PLA membrane is still 1.7 μg cm−2 after 4 h saline incubation. The flux was increased with reaction time due to the enhanced surface porosity and hydrophilicity. The APTT of Y-DMAc-12h can be extended to 118 s from 50 s of the original PLA membrane. And PT values of Y-DMAc membranes increased accordingly (from 15.4 to 24.8 s). In addition, the FIB for Hep–APTES membranes (grafting time was 12 h) decreased by 43.39% than that for the pure PLA membrane. All APTT, platelets and BSA adsorption results showed that surface heparinization significantly improved the hemocompatibility of PLA porous membrane.Acknowledgements
This work is supported by National Natural Science Foundation of China (51473177) and Youth Innovation Promotion Association of Chinese Academy of Sciences (2014258).Notes and references
- A. C. Yamashita and N. Tomisawa, Contrib. Nephrol., 2010, 166, 112–118 CrossRef CAS PubMed.
- M. Wang, J. Yuan, X. Huang, X. Cai, L. Li and J. Shen, Colloids Surf., B, 2013, 103, 52–58 CrossRef CAS PubMed.
- M. Rouabhia, J. Asselin, N. Tazi, Y. Messaddeq, D. Levinson and Z. Zhang, ACS Appl. Mater. Interfaces, 2014, 6, 1439–1446 CAS.
- M. Mahmoudi, A. Simchi, M. Imani, A. S. Milani and P. Stroeve, J. Phys. Chem. B, 2008, 112, 14470–14481 CrossRef CAS PubMed.
- Z. Wu, H. Chen, H. Huang, T. Zhao, X. Liu, D. Li and Q. Yu, Macromol. Biosci., 2009, 9, 1165–1168 CrossRef CAS PubMed.
- C. S. Zhao, T. Liu, Z. P. Lu, L. P. Cheng and J. Huang, Artif. Organs, 2001, 25, 60–63 CAS.
- C. S. Zhao, X. D. Liu, M. Nomizu and N. Nishi, Sep. Sci. Technol., 2004, 39, 3043–3055 CrossRef CAS.
- N. Mackiewicz, J. Nicolas, N. Handké, M. Noiray, J. Mougin, C. Daveu, H. R. Lakkireddy, D. Bazile and P. Couvreur, Chem. Mater., 2014, 26, 1834–1847 CrossRef CAS.
- C. M. Ding, Z. G. Qiao, W. B. Jiang, H. W. Li, J. H. Wei, G. D. Zhou and K. R. Dai, Biomaterials, 2013, 34, 6706–6716 CrossRef CAS PubMed.
- Y. Deng, J. K. Saucier-Sawyer, C. J. Hoimes, J. Zhang, Y. E. Seo, J. W. Andrejecsk and W. M. Saltzman, Biomaterials, 2014, 35, 6595–6602 CrossRef CAS PubMed.
- A. A. Appel, M. A. Anastasio, J. C. Larson and E. M. Brey, Biomaterials, 2013, 34, 6615–6630 CrossRef CAS PubMed.
- K. G. Battiston, J. W. Cheung, D. Jain and J. P. Santerre, Biomaterials, 2014, 35, 4465–4476 CrossRef CAS PubMed.
- B. Balakrishnan, D. S. Kumar, Y. Yoshida and A. Jayakrishnan, Biomaterials, 2005, 26, 3495–3502 CrossRef CAS PubMed.
- S. Sagnella and K. Mai-Ngam, Colloids Surf., B, 2005, 42, 147–155 CrossRef CAS PubMed.
- P. S. Liu, Q. Chen, X. Liu, B. Yuan, S. S. Wu, J. Shen and S. C. Lin, Biomacromolecules, 2009, 10, 2809–2816 CrossRef CAS PubMed.
- H. Fasl, J. Stana, D. Stropnik, S. Strnad, K. Stana-Kleinschek and V. Ribitsch, Biomacromolecules, 2010, 11, 377–381 CrossRef CAS PubMed.
- K. Ichinose, T. Okamoto, H. Tanimoto, A. Yoshitake, M. Tashiro, Y. Sakanashi, K. Kuwana, K. Tahara, M. Kamiya and H. Terasaki, Artif. Organs, 2004, 28, 993–1001 CrossRef CAS PubMed.
- G. Bayramoglu, M. Yilmaz, E. Batislam and M. Y. Arica, J. Appl. Polym. Sci., 2008, 109, 749–757 CrossRef CAS.
- X. S. Ren, L. Xu, J. X. Xu, P. Z. Zhu, L. Zuo and S. C. Wei, J. Biomater. Sci., Polym. Ed., 2013, 24, 1707–1720 CrossRef CAS PubMed.
- M. C. Yang and W. C. Lin, J. Polym. Res., 2002, 9, 201–206 CrossRef CAS.
- C. J. Hou, Q. Yuan, D. Q. Huo, S. J. Zheng and D. L. Zhan, J. Biomed. Mater. Res., Part A, 2008, 85, 847–852 CrossRef PubMed.
- M. Tang, J. M. Xue, K. L. Yan, T. Xiang, S. D. Sun and C. S. Zhao, J. Colloid Interface Sci., 2012, 386, 428–440 CrossRef CAS PubMed.
- Y. Xia, C. Cheng, R. Wang, H. Qin, Y. Zhang, L. Ma, H. Tan, Z. W. Gu and C. S. Zhao, Polym. Chem., 2014, 5, 5906–5919 RSC.
- C. Cheng, S. D. Sun and C. S. Zhao, J. Mater. Chem. B, 2014, 2, 7649–7672 RSC.
- C. P. R. Walker and D. Royston, Br. J. Anaesth., 2002, 88, 848–863 CrossRef CAS PubMed.
- S. K. Vashist, E. Lam, S. Hrapovic, K. B. Male and J. H. T. Luong, Chem. Rev., 2014, 114, 11083–11130 CrossRef CAS PubMed.
- A. L. Gao, F. Liu and L. X. Xue, J. Membr. Sci., 2014, 452, 390–399 CrossRef CAS.
- H. C. Yang, J. Q. Luo, Y. Lv, P. Shen and Z. K. Xu, J. Membr. Sci., 2015, 483, 42–59 CrossRef CAS.
- M. M. Wan, J. Y. Yang, Y. Qiu, Y. Zhou, C. X. Guan, Q. Hou, W. G. Lin and J. H. Zhu, ACS Appl. Mater. Interfaces, 2012, 4, 4113–4122 CAS.
- M. S. Ahola, E. S. Sailynoja, M. H. Raitavuo, M. M. Vaahtiob, J. I. Salonenb and A. U. O. Yli-Urpoa, Biomaterials, 2001, 22, 2163–2170 CrossRef CAS PubMed.
- L. H. Wang, Y. Cai, Y. H. Jing, B. K. Zhu, L. P. Zhu and Y. Y. Xu, J. Colloid Interface Sci., 2014, 422, 38–44 CrossRef CAS PubMed.
- S. Yuan, C. Ferrell and W. L. Chandler, Thromb. Res., 2007, 120, 29–37 CrossRef CAS PubMed.
- L. Ma, H. Qin, C. Cheng, Y. Xia, C. He, C. X. Nie, L. R. Wang and C. S. Zhao, J. Mater. Chem. B, 2014, 2, 363–375 RSC.
- J. M. Beaudet, A. Weyers, K. Solakyildirim, B. Yang, M. Takieddin, S. Mousa, F. Zhang and R. J. Linhardt, J. Pharm. Sci., 2011, 100, 3396–3404 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |