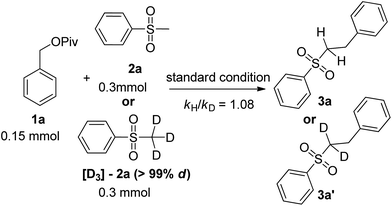
Nickel-catalyzed α-benzylation of sulfones with esters via C–O activation†
Jing Xiaoa,
Jia Yanga,
Tieqiao Chen*a and
Li-Biao Han*ab
aState Key Laboratory of Chemo/Biosensing and Chemo Metrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, China. E-mail: chentieqiao@hnu.edu.cn
bNational Institute of Advanced Industrial Science and Technology (AIST), Tsukuba, Ibaraki 305-8565, Japan. E-mail: libiao-han@aist.go.jp
First published on 20th April 2016
Abstract
The nickel-catalyzed α-benzylation of sulfones with readily available benzylic alcohol derivatives was achieved via C–O activation. The transformation was complete in 30 minutes using a simple Ni(COD)2 as a catalyst without any additional ligands. A variety of substituted sulfones including the building block for bioactive compound eletriptan were synthesized by using the strategy.
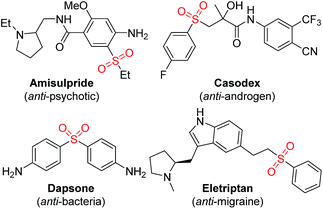
Sulfones are valuable common intermediates in organic synthesis.1 They are also key motifs in many biologically active compounds as exemplified by amisulpride, dapsone, casodex and eletriptan (Fig. 1).2 The α-alkylation of simple sulfones is one of the most useful methods to access these functional sulfones.2 This process traditionally uses nucleophilic substitution reactions (the couplings of sulfones with alkyl halides in the presence of a stoichiometric amount of base such as n-BuLi and LiN(SiMe3)2), in which other functionalities could be damaged.3
In recent years, C–O compounds such as benzyl alcohols and phenol derivatives have attracted much attention as the green and inexpensive coupling reagents replacing the halocompounds in metal-mediated coupling reactions.4,5 It would be appealing to achieve the α-benzylation of simple sulfones for the preparation of substituted sulfones by cross-coupling of hydrocarbons with C–O compounds via C–O activation. Herein, we report a Ni-catalyzed α-benzylation of simple sulfones with the readily available benzylic alcohol derivatives. This transformation is complete in 30 minutes with high selectivity for the mono-benzylation product. Moreover, unlike C–O functionalization where phosphine ligands or carbene ligands are usually required, this reaction can occur efficiently in the presence of Ni(COD)2 without any additional ligands. This transformation provides an alternative method for the α-benzylation of simple sulfones.
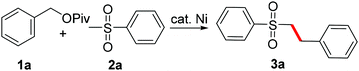
We chose benzyl pivalate 1a and methyl phenyl sulfone 2a as the model reaction and the obtained results were compiled in Table 1. Phosphine ligands or carbene ligands showed a dramatic effect in nickel-catalyzed C–O functionalization,4,5 however it was found that this transformation could take place in the presence of the simple Ni(COD)2 without any additional ligands (entries 1–6). Thus, in the presence of 10 mol% Ni(COD)2 and 1.5 equiv. t-BuONa, the cross-coupling between 1a and 2a took place smoothly in toluene at 100 °C to produce the corresponding product 3a in 52% yield (entry 6). When 2.0 equiv. t-BuONa and 2.0 equiv. 2a were loaded, 74% yield of 3a was generated (entry 8). Further increasing the amount of 2a to 2.5 equiv. did not improve the reaction efficiency (entry 9). The cross-coupling reaction proceeded fast and could be complete in 30 minutes (entries 10–12). t-BuONa was essential for this reaction, as none or only a trace amount of 3a was detected under similar reaction conditions when t-BuOK or t-BuOLi was used (entries 13 and 14). As for the solvent, the transformation also proceeded readily in dioxane, but poorly in DMF (entries 15 and 16). Whether elevating or lowering the reaction temperature would reduce the yield of the product (entries 17 and 18). The nickel catalyst was also essential. In the absence of Ni(COD)2, no trace amount of product could be detected (entry 19). Worth noting is that no dibenzylated by-product was detected during the reaction. The main side-product of this reaction is 3,3-dimethyl-1-(phenylsulfonyl)butan-2-one via acylation, which may also act as the ligand in the catalytic system.6
| Run | Ligand | Base (equiv.) | 2a (equiv.) | T (min) | Yieldb |
|---|---|---|---|---|---|
a Conditions: a mixture of 1a (0.2 mmol), 2a, Ni(COD)2 (0.02 mmol), a phosphine ligand (P/Ni = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and a base in toluene (0.5 mL) was stirred at 100 °C for the time indicated.b GC yield using tridecane as an internal standard.c In dioxane.d In DMF.e 80 °C.f 120 °C.g In the absence of nickel catalyst. 1) and a base in toluene (0.5 mL) was stirred at 100 °C for the time indicated.b GC yield using tridecane as an internal standard.c In dioxane.d In DMF.e 80 °C.f 120 °C.g In the absence of nickel catalyst. |
|||||
| 1 | PCy3 | t-BuONa (1.5) | 1.2 | 60 | 55% |
| 2 | dcype | t-BuONa (1.5) | 1.2 | 60 | 40% |
| 3 | dppp | t-BuONa (1.5) | 1.2 | 60 | 28% |
| 4 | binap | t-BuONa (1.5) | 1.2 | 60 | 17% |
| 5 | xantphos | t-BuONa (1.5) | 1.2 | 60 | 38% |
| 6 | None | t-BuONa (1.5) | 1.2 | 60 | 52% |
| 7 | None | t-BuONa (1.5) | 1.5 | 60 | 63% |
| 8 | None | t-BuONa (2.0) | 2.0 | 60 | 74% |
| 9 | None | t-BuONa (2.0) | 2.5 | 60 | 73% |
| 10 | None | t-BuONa (2.0) | 2.0 | 30 | 75% |
| 11 | None | t-BuONa (2.0) | 2.0 | 15 | 71% |
| 12 | None | t-BuONa (2.0) | 2.0 | 5 | 36% |
| 13 | None | t-BuOLi (2.0) | 2.0 | 30 | N.D. |
| 14 | None | t-BuOK (2.0) | 2.0 | 30 | Trace |
| 15c | None | t-BuONa (2.0) | 2.0 | 30 | 52% |
| 16d | None | t-BuONa (2.0) | 2.0 | 30 | N.D. |
| 17e | None | t-BuONa (2.0) | 2.0 | 30 | 59% |
| 18f | None | t-BuONa (2.0) | 2.0 | 30 | 53% |
| 19g | None | t-BuONa (2.0) | 2.0 | 30 | N.D. |
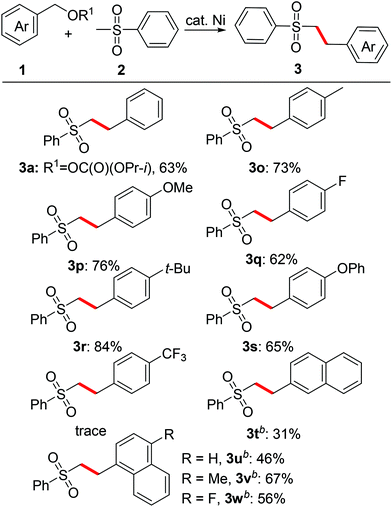
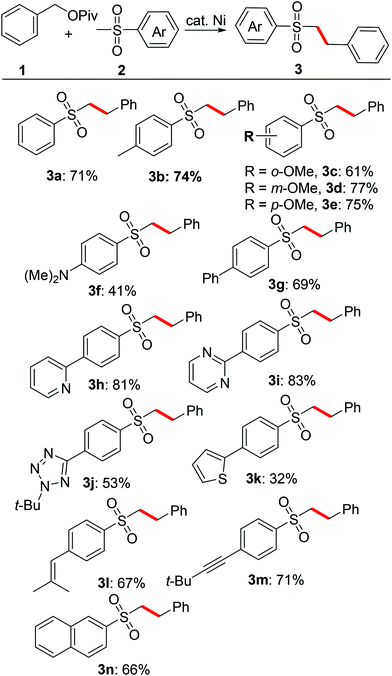
This transformation is applicable to other substrates. As shown in Table 2, a variety of sulfones coupled with benzylic esters produced the corresponding products in good yields under the present reaction conditions. Thus, substrates bearing functional groups such as methyl, methoxy (o, m, p-position), phenyl and amine on the benzene ring could all readily couple with benzyl pivalate, giving the corresponding substituted sulfones in moderate to good yields (3a–3g). Heterocyclic-containing sulfones such as pyridine, pyrimidine, tetrazole and thiophene also served well and the corresponding substituted sulfones were generated successfully (3h–3k). Alkenyl and alkynyl groups survived in the current catalytic system, facilitating further functionalization of the coupling products. 2-(Methylsulfonyl)naphthalene 2n was also converted to the corresponding sulfones in 66% yield under the standard reaction conditions.
| a Conditions: 1 (0.2 mmol), 2 (0.4 mmol), Ni(COD)2 (10 mol%), t-BuONa (0.4 mmol), toluene (0.5 mL), 100 °C, 0.5 h. Isolated yield. |
|---|
 |
The substrate scope of benzylic alcohol derivatives was further explored. As shown in Table 3, a carbonate coupled with 2a smoothly, giving the corresponding sulfone 3a in 63% yield. Benzylic alcohol pivalates containing methyl, methoxy, fluorine and t-Bu groups performed well in this catalytic system. Derivative with an easily hydrogenolytic group phenoxy was also proved to be a good coupling partner under the present reaction conditions and the corresponding coupling product 3s was produced in 65% yield.7 However, substrate bearing electron-withdrawing group CF3 gave only a trace amount of product. Naphthylmethyl pivalates all went through this transformation to give the corresponding products in moderate to good yields (3t–3w). No trace amount of product was detected when phenyl pivalate was used as substrate in the catalytic system.
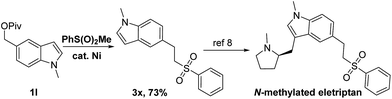
The building block for the bioactive molecule eletriptan was synthesized successfully by using the present cross-coupling strategy (Scheme 1). Pivalate 1l generated via methylation and esterification of the commercially available indole-5-methanol readily reacted with methyl phenylsulfone, producing the corresponding product 3x in 73% yield. Sulfone 3x could be converted to N-methylated eletriptan using literature methods.8 Thus, our method could be a new alternative method for the synthesis of eletriptan.9
It should be noted in the present catalytic system, 3a could be readily prepared in gram scales. As shown in Scheme 2, 3a was obtained in 65% isolated yield in 7 mmol scale under the standard reaction conditions.
In order to gain insight into the reaction mechanism, two separate kinetic isotope effect (KIE) experiments were carried out. A kinetic isotope effect (kH/kD = 1.08) was obtained, indicating that the C–H cleavage perhaps was not the rate-determining step in the reaction (Scheme 3).
Although the mechanism is not fully understood yet, on the basis of the above results and previous investigations,5,10–12 a plausible mechanism is proposed in Scheme 4. Ni(0) complex A firstly adds to benzylic alcohol derivatives via oxidative addition,10 generating complex B, followed by ligand exchange with sulfone by the aid of a base to yield C. Reductive elimination of C produces the corresponding coupling product 3 and regenerates Ni(0) complex A.
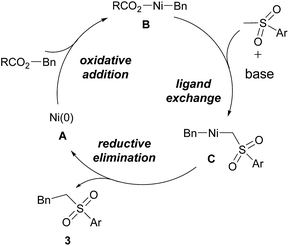 | ||
| Scheme 4 Proposed mechanism for the Ni-catalyzed cross-coupling between sulfones and benzylic alcohol derivatives. | ||
Conclusions
In summary, we have achieved the α-benzylation of sulfones using the readily available benzylic alcohol derivatives (ester, carbonate) as the alkylating reagent. The simple Ni(COD)2 without any additional ligands enables this transformation to complete in half an hour, providing an alternative practical method for the synthesis of various valuable sulfones including the building block of bioactive compound eletriptan.Acknowledgements
Partial financial supports from NSFC (21373080, 21573064, 21403062), HNNSF 2015JJ3039, the Fundamental Research Funds for the Central Universities (Hunan University) are gratefully acknowledged.Notes and references
- (a) A. El-Awa, M. N. Noshi, X. M. du Jourdin and P. L. Fuchs, Chem. Rev., 2009, 109, 2315 CrossRef CAS PubMed; (b) J.-E. Bäckvall, R. Chinchilla, C. Naájera and M. Yus, Chem. Rev., 1998, 98, 2291 CrossRef; (c) N. S. Simpkins, Tetrahedron, 1990, 46, 6951 CrossRef CAS; (d) P. D. Magnus, Tetrahedron, 1977, 33, 2019 CrossRef CAS; (e) C. Naájera and M. Yus, Tetrahedron, 1999, 55, 10547 CrossRef.
- (a) M. Maitre, C. Ratomponirina, S. Gobaille, Y. Hodé and V. Hechler, Eur. J. Pharmacol., 1994, 256, 2, 211 Search PubMed; (b) M. Artico, R. Silvestri, E. Pagnozzi, B. Bruno, E. Novellino, G. Greco, S. Massa, A. Ettorre, A. G. Loi, F. Scintu and P. L. Colla, J. Med. Chem., 2000, 43, 1886 CrossRef CAS PubMed; (c) R. L. Lopez de Compadre, R. A. Pearlstein, A. J. Hopfinger and J. K. Seyde, J. Med. Chem., 1987, 30, 900 CrossRef CAS PubMed; (d) E. Willems, P. D. Vries, J. P. C. Heiligers and P. R. Saxena, Naunyn-Schmiedeberg's Arch. Pharmacol., 1998, 358, 212 CrossRef CAS; (e) V. D. Harding, R. J. Macrae and R. J. Ogilvie, US 6110940 A, 2000.
- (a) G. W. Penton and C. K. J. Ingold, Chem. Soc., Abstr., 1929, 2388 CAS; (b) T. Murakami and K. Furusawa, Synthesis, 2002, 479 CrossRef CAS; (c) G. S. Misra and R. S. Asthana, J. Physiol., 1957, 4, 270 CAS; (d) S. H. Pine, G. Shen, J. Bautista, C. Sutton Jr, W. Yamada and L. Apodaca, J. Org. Chem., 1990, 55, 2234 CrossRef CAS; (e) C. A. G. Baker-Glenn, A. G. M. Barrett, A. A. Gray, P. A. Procopiou and M. Ruston, Tetrahedron Lett., 2005, 46, 7427 CrossRef CAS.
- For reviews on C–O activation, see: (a) M. Tobisu and N. Chatani, Acc. Chem. Res., 2015, 48, 1717 CrossRef CAS PubMed; (b) S. Z. Tasker, E. A. Standley and T. F. Jamison, Nature, 2014, 509, 299 CrossRef CAS PubMed; (c) J. Yamaguchi, K. Muto and K. Itami, Eur. J. Org. Chem., 2013, 2013, 19 CrossRef CAS; (d) D.-G. Yu, B.-J. Li and Z.-J. Shi, Acc. Chem. Res., 2010, 43, 1486 CrossRef CAS PubMed; (e) J. Cornella, C. Zarate and R. Martin, Chem. Soc. Rev., 2014, 43, 8081 RSC; (f) M. Tobisu and N. Chatani, Top. Organomet. Chem., 2013, 44, 35 CrossRef CAS; (g) B. M. Rosen, K. W. Quasdorf, D. A. Wilson, N. Zhang, A. M. Resmerita, N. K. Garg and V. Percec, Chem. Rev., 2011, 111, 1346 CrossRef CAS PubMed; (h) T. Chen and L.-B. Han, Angew. Chem., Int. Ed., 2015, 54, 8600 CrossRef CAS PubMed.
- For recent examples on Ni-catalyzed cross coupling of C–O compounds with hydrocarbons via C–O activation, see: (a) J. Cornella, E. P. Jackson and R. Martin, Angew. Chem., Int. Ed., 2015, 54, 4075 CrossRef CAS PubMed; (b) E. Koch, R. Takise, A. Studer, J. Yamaguchi and K. Itami, Chem. Commun., 2015, 51, 855 RSC; (c) T. Mukai, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2010, 12, 1360 CrossRef CAS PubMed; (d) J. Xiao, T. Chen and L.-B. Han, Org. Lett., 2015, 17, 812 CrossRef CAS PubMed; (e) K. Muto, J. Yamaguchi and K. Itami, J. Am. Chem. Soc., 2012, 134, 169 CrossRef CAS PubMed; (f) L. Meng, Y. Kamada, K. Muto, J. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2013, 52, 10048 CrossRef CAS PubMed; (g) J. Wang, D. M. Ferguson and D. Kalyani, Tetrahedron, 2013, 69, 5780 CrossRef CAS; (h) A. R. Ehle, Q. Zhou and M. P. Watson, Org. Lett., 2012, 14, 1202 CrossRef CAS PubMed; (i) M. R. Harris, M. O. Konev and E. R. Jarvo, J. Am. Chem. Soc., 2014, 136, 7825 CrossRef CAS PubMed; (j) R. Matsubara and T. F. Jamison, J. Am. Chem. Soc., 2010, 132, 6880 CrossRef CAS PubMed; (k) R. Takise, K. Muto, J. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2014, 53, 6791 CrossRef CAS PubMed.
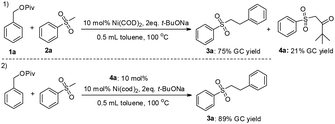
- Under the standard conditions, Under the standard conditions, the model reaction proceeded readily to generate the product 3a and side-product 4a in 75% and 21% GC yield, respectively (eqn (1)). After purification of 4a, we performed a control experiment (eqn (2)). We found the yield of product 3a was increased from 75% to 89% by addition of 10 mol% 4a. It is reasonable to deduce that 4a may act as the internal ligand.
. - (a) A. G. Sergeev and J. F. Hartwig, Science, 2011, 332, 439 CrossRef CAS PubMed; (b) A. G. Sergeev, J. D. Webb and J. F. Hartwig, J. Am. Chem. Soc., 2012, 134, 20226 CrossRef CAS PubMed.
- (a) X. Peng, M. Zhang, J. Zhao and X. Chen, CN. 103408535, 2013; (b) D. C. Cole, W. J. Lennox, J. R. Stock, J. W. Ellingboe, H. Mazandarani, D. L. Smith, G. Zhang, G. J. Tawa and L. E. Schechter, Bioorg. Med. Chem. Lett., 2005, 15, 4780 CrossRef CAS PubMed.
- (a) E. Negishi, Handbook of organopalladium chemistry for organic synthesis, John Wiley & Sons Inc, New York, 2002 Search PubMed; (b) M. R. Pullagurla, J. B. Rangisetty, N. Naidu, N. Maddela, R. Nagarapu and P. R. Polagani, WO 2010049952 A3, 2010; (c) M. R. Pullagurla, J. B. Rangisetty, N. Naidu, N. Maddela, R. Nagarapu and P. R. Polagani, US 20110207943, 2011.
- K. Muto, J. Yamaguchi, A. Lei and K. Itami, J. Am. Chem. Soc., 2013, 135, 16384 CrossRef CAS PubMed.
- (a) X. Hong, Y. Liang and K. N. Houk, J. Am. Chem. Soc., 2014, 136, 2017 CrossRef CAS PubMed; (b) H. Xu, K. Muto, J. Yamaguchi, C. Zhao, K. Itami and D. G. Musaev, J. Am. Chem. Soc., 2014, 136, 14834 CrossRef CAS PubMed; (c) Q. Lu, H. Yu and Y. Fu, J. Am. Chem. Soc., 2014, 136, 8252 CrossRef CAS PubMed.
- We also considered the radical mechanism. However, when the model reaction was performed by addition of 3.0 equiv. BHT or galvinoxyl, 3a was still produced in 62% and 65% yields, respectively. The results indicate that this transformation may not be a radical process.
Footnote |
| † Electronic supplementary information (ESI) available: General information, typical procedure, characterized data, copies of 1H and 13C NMR spectra. See DOI: 10.1039/c6ra07130a |
| This journal is © The Royal Society of Chemistry 2016 |