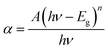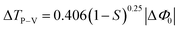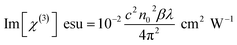Self-assembled supramolecular structure of 4-dimethylaminopyridinium p-hydroxy benzoate pentahydrate: synthesis, growth, optical and biological properties†
P. Sathya,
S. Pugazhendhi and
R. Gopalakrishnan*
Crystal Research Laboratory, Department of Physics, Anna University, Chennai-600025, India. E-mail: krgkrishnan@yahoo.com; krgkrishnan@annauniv.edu; Fax: +91 44 2235 8700; Tel: +91 44 2235 8710/8707
First published on 20th April 2016
Abstract
A single crystal of 4-dimethylaminopyridinium p-hydroxybenzoate pentahydrate (DMAPHB) was obtained via a slow evaporation solution growth technique. Single crystal X-ray diffraction studies of the as-grown crystal show that the crystal belongs to a monoclinic system with P21/c space group. The asymmetric part of the title compound contains three DMAPHB molecules and five water molecules. Interplay between a vast number of intermolecular interactions such as strong O–H⋯O, N–H⋯O and weak C–H⋯O hydrogen bonds are discussed. Spectroscopic studies such as FT-IR, FT-Raman, NMR and UV-Vis were carried out to reveal the molecular properties and optical behaviour. The mechanical strength of DMAPHB was examined using Vickers microhardness test. The thermal stability and melting point of the crystal were studied by TG-DTA analysis. The third order non-linear optical behaviour of the title compound was studied using the Z-scan technique. Antibacterial biological evaluation of the material was carried out using a resazurin reduction assay method against Escherichia coli, Staphylococcus aureus and Bacillus cereus.
Introduction
The synthesis and characterisation of new organic complexes have attracted many researchers due to their chemical, mechanical, thermal and photochemical stability rather than other inorganic materials.1 In particular, ionic organic crystals exhibit a wide range of pharmacological properties such as antibacterial, antiviral, anti-inflammatory and cytotoxicity towards cancer cell lines, including human-hepatoprotective activities, and have been extensively used in non-linear dynamics.2 Moreover, third order non-linear optical organic materials have gained a lot of consideration because of their significant applications in optical switching, optical data processing, optical limiting, signal processing and ultra-fast optical communications.3,4 Non-centrosymmetric organic crystals with good stability leads to third order susceptibility (χ(3)), molecular hyperpolarizability, higher non-linear absorption co-efficient and fast response time for non-linear optical properties.5 The nature of exhibiting both optical and biological properties of the title compound is highlighted due to its bifunctionality in commercial industry.The origin of optical non-linearities in organic materials is mainly due to the presence of delocalized π-conjugated donor–π–acceptor systems, which augments their asymmetric polarizability.6 The fundamental understanding of the structural and bonding features of novel materials is mandatory to improve the efficiency of the material. In the field of crystal engineering, non-covalent interactions, such as strong and weak hydrogen bonds, π⋯π interactions, halogen–halogen contacts and van der Waals forces, play a vital role because of their directionality, strength and flexibility.7 The occurrence of hydrogen bonds in molecular packing have long been recognized as the key factor for self-assembly or the molecular recognition process when extensive structures are formed from crystal building blocks or supramolecular synthons.8 The crystal structures of the functional solid-state materials from neutral or ionic blocks are primarily dominated by strong O/N–H⋯O/N and weak C/N–H⋯π hydrogen bonds or delocalized π-conjugated donor–π–acceptor systems, which hold vital strength and reproducibility, leading to a distinct advantage in the creation of molecular solids with desirable properties.9,10
Pyridinium and its derivatives are of great interest in the scientific community because of their peculiar properties due to their structural chemistry and biological activities. Numerous pyridines of commercial interest have applications in market areas where bioactivity is significant, such as in medicinal drugs and agricultural products.11 Methyl substituted pyridine compounds are broadly used in co-ordination and organometallic chemistry, catalysis and other physical and chemical investigations and are also involved in hydrogen-bonded interactions. Hydroxybenzoic acid and its derivatives exhibit antimicrobial activity against Gram positive and Gram negative microbes.12,13 Cationic–anionic organic non-centrosymmetric crystals have been investigated and reported.14–16
In this study, we synthesized a new organic complex of 4-dimethylaminopyridinium p-hydroxy benzoate pentahydrate (DMAPHB) grown as a bulk crystal. Its crystal structure in the context of a crystal engineering approach was investigated and it was found that the title compound crystallises in a non-centrosymmetric nature with a supramolecular structure owing to its bifunctionality. Spectral studies such as FTIR, FT-Raman, NMR and UV-Vis analysis have been carried out to confirm the nature of its molecular and optical behaviour. The thermal and mechanical stabilities were also examined. Third order non-linear optical behaviour of the title compound was examined by Z-scan techniques. The synthesized complexes were also screened for antibacterial and anticancer activities.
Materials and methods
Synthesis and crystal growth
DMAPHB salt was synthesised by dissolving an equimolar ratio of 4-dimethyl aminopyridine and p-hydroxybenzoic acid in methanol at room temperature. The solubility was estimated at room temperature in water, ethanol, methanol and a mixture of water and ethanol. Among the four solvents, methanol was found to be the better solvent for the bulk growth of the title compound. The saturated solution was allowed to agitate at constant speed for two days in a magnetic stirrer to achieve a homogeneous mixture. The solution was filtered into a large surface area glass vessel and allowed to evaporate in a constant temperature bath. The block-like single crystal was successfully grown after recrystallizing the materials twice over a period of 10 days. The as-grown DMAPHB crystals are shown in Fig. 1.Characterisation studies
The unit cell parameters and crystal structure of DMAPHB were determined from single crystal X-ray diffraction analysis. The Fourier transform infrared (FT-IR) spectra were obtained in the range of 400–4000 cm−1 using KBr pellets on a Bruker Alpha spectrometer. FT-Raman spectra were obtained between 4000 and 50 cm−1 on a Bruker RFS 100/S FT-Raman instrument using 1064 nm excitation from a Nd:YAG laser. The 1H and 13C spectra were acquired on a 500 MHz AVANCE III spectrometer at room temperature (500 MHz for 1H NMR and 125 MHz for 13C NMR) in deuterated methanol (CD3OD) with tetramethylsilane (TMS) as an internal standard and the chemical shifts reported in ppm. Optical absorption spectroscopy analysis was carried out using a CARY 5E UV-Vis spectrophotometer in the range of 200–800 nm. The hardness test was performed on a MATSUZAWA microhardness tester fitted with a Vickers diamond pyramidal indenter attached to an incident light microscope. The thermal stability and phase transition of the crystal were studied using thermogravimetric and differential thermal analysis using a NETZSCH STA 409 °C analyser. The third order non-linear optical parameters such as non-linear refractive index (n2), non-linear absorption coefficient (β) and susceptibility (χ(3)) were estimated using Z-scan studies. The dissolution rate and homogeneity of the resazurin solution were achieved using vortex mixer apparatus to perform biological examinations.Results and discussion
Single crystal X-ray diffraction analysis
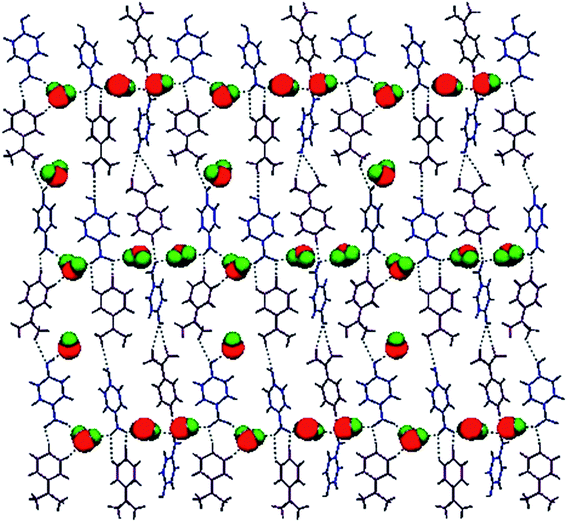
The as-grown crystal of DMAPHB was subjected to single crystal X-ray diffraction analysis using a Bruker AXS Kappa APEX II diffractometer equipped with graphite monochromated Mo Kα radiation (λ = 0.71073 Å) at room temperature with a crystal dimension of 0.30 × 0.25 × 0.20 mm3. Data collection, indexing and data reduction were extracted from APEX-2 Saint software.17 The crystal structure was solved by direct methods using the SHELXS-97 and refined by SHELXL-97 computer programs.18 The refinement was carried out using the full matrix least square method on F2 and all the non-hydrogen atoms were refined with anisotropic thermal parameters. Furthermore, the position of hydrogen atoms was identified from the difference electron density map. Finally, the anisotropic refinement coverage to R values of R1 = 0.0509 and wR2 = 0.1394 was performed. A total number of 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 979 reflections were recorded in the 2θ range of 1.14–25.00° of which 7892 reflections were considered as unique reflections with I > 2σ(I). The hydrogen atom positions were acknowledged from the difference electron density map. The thermal ellipsoid plot was drawn using the ORTEP programme19 at a 30% probability level for DMAPHB as depicted in Fig. 2. The anionic chain is shown in Fig. 3. The molecular packing diagram (Fig. 4) was generated using Mercury 2.4 software.20 The crystallographic data and refinement are illustrated in Table 1. The possible hydrogen bonds are listed in Table 2. The crystal structure of the title compound was deposited to the Cambridge Crystallographic Data Centre (CCDC No. 1061663†).
979 reflections were recorded in the 2θ range of 1.14–25.00° of which 7892 reflections were considered as unique reflections with I > 2σ(I). The hydrogen atom positions were acknowledged from the difference electron density map. The thermal ellipsoid plot was drawn using the ORTEP programme19 at a 30% probability level for DMAPHB as depicted in Fig. 2. The anionic chain is shown in Fig. 3. The molecular packing diagram (Fig. 4) was generated using Mercury 2.4 software.20 The crystallographic data and refinement are illustrated in Table 1. The possible hydrogen bonds are listed in Table 2. The crystal structure of the title compound was deposited to the Cambridge Crystallographic Data Centre (CCDC No. 1061663†).
| Mole | SHELXL |
| Empirical formula | C42H58N6O14 |
| Formula weight | 757.94 |
| Temperature | 296(2) K |
| Wavelength | 0.71073 Å |
| Crystal system, space group | Monoclinic, P21/c |
| Unit cell dimensions | a = 17.87(19) Å, α = 90° |
| b = 7.38(9) Å, β = 93.64(5)° | |
| c = 34.00(3) Å, γ = 90° | |
| Volume | 4480.9(8) Å3 |
| Z, calculated density | 4, 1.257 Mg m−3 |
| Absorption coefficient | 0.080 mm−1 |
| F(000) | 1636 |
| Crystal size | 0.30 × 0.25 × 0.20 mm3 |
| Theta range for data collection | 1.14 to 25.00° |
| Limiting indices | −21 ≤ h ≤ 21, −8 ≤ k ≤ 8, −40 ≤ l ≤ 40 |
| Reflections collected/unique | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 979/7892 [R(int) = 0.0681] 979/7892 [R(int) = 0.0681] |
| Completeness to theta = 25.00 | 100.0% |
| Max. and min. transmission | 0.9842 and 0.9764 |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 7892/15/612 |
| Goodness-of-fit on F^2 | 0.996 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0471, wR2 = 0.1268 |
| R indices (all data) | R1 = 0.0990, wR2 = 0.1806 |
| Extinction coefficient | 0.0047(6) |
| Largest difference peak and hole | 0.209 and −0.188 e A−3 |
| D–H⋯A | d(D–H) | d(H⋯A) | d(D⋯A) | 〈(DHA) |
|---|---|---|---|---|
| O(1A)–H(1)⋯O(4W) | 0.82 | 1.80 | 2.592(3) | 163.1 |
| O(2C)–H(2)⋯O(1W)#1 | 0.82 | 1.89 | 2.705(3) | 172.7 |
| N(1C)–H(1C)⋯O(1C) | 1.01(4) | 1.63(4) | 2.632(3) | 169(3) |
| N(1B)–H(1B)⋯O(2B) | 1.06(4) | 1.59(4) | 2.645(3) | 174(3) |
| N(1A)–H(1A)⋯O(3A) | 0.92(3) | 1.84(3) | 2.744(3) | 168(3) |
| O(4W)–H(7W)⋯O(3B) | 0.825(10) | 1.857(12) | 2.661(3) | 164(3) |
| O(1W)–H(1W)⋯O(2A) | 0.824(10) | 1.871(11) | 2.694(3) | 177(3) |
| O(5W)–H(10W)⋯O(1W)# | 0.825(10) | 1.965(12) | 2.784(3) | 171(3) |
| O(1W)–H(2W)⋯O(2W) | 0.827(10) | 1.878(11) | 2.703(4) | 175(3) |
| O(4W)–H(8W)⋯O(3C) | 0.823(10) | 1.878(12) | 2.686(3) | 167(3) |
| O(5W)–H(9W)⋯O(3W)#2 | 0.822(10) | 1.918(14) | 2.728(4) | 168(3) |
| O(2W)–H(4W)⋯O(1C)#3 | 0.828(10) | 1.927(13) | 2.746(4) | 170(4) |
| O(3W)–H(6W)⋯O(3A)#4 | 0.817(10) | 2.070(16) | 2.851(4) | 160(4) |
| O(2W)–H(3W)⋯O(3A)#5 | 0.820(10) | 2.003(17) | 2.772(4) | 156(4) |
| O(3W)–H(5W)⋯O(2B) | 0.822(10) | 1.886(11) | 2.706(3) | 175(5) |
Structural description
The combination of a symmetrical crystal scaffold and the occurrence of reliable hydrogen bonding sites is an effectual way to obtain open framework structures.21 In the present investigation, DMAPHB crystallizes in a monoclinic crystal system with P21/c space group. The compound structure was composed of three p-dimethylaminopyridinium cations, three p-hydroxy benzoate anions and five water molecules in the asymmetric unit. The hydrogen atom of the carboxylic group of (COOH) in p-hydroxy benzoic acid moiety gets dissociated and transferred to the ring nitrogen atom of the p-dimethylaminopyridine moiety and reveals the salt formation between the two molecules. The water molecules present in the compound play an important role in the assembly of the solid-state structure and the multiple hydrogen bonding interactions around the water molecules are particularly interesting. The oxygen atom of the water molecule acts as both a donor and acceptor. The basic framework and molecular packing between the ions in DMAPHB are primarily decided by O–H⋯O, N–H⋯O and weak C–H⋯O hydrogen bonds. The three p-hydroxybenzoate anions and five water molecules exhibit twelve O–H⋯O hydrogen bonds. The OH groups of the three anions are involved in O–H⋯O hydrogen bonds with neighbouring water molecules. The carboxylate (COO−) groups of the anions display three O–H⋯O hydrogen bonds with the water molecules and three N–H⋯O with the ring nitrogen atom of the cations.The benzoate anion and water molecule form two O–H⋯O hydrogen bonds with the carboxylate (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O) and O–H groups, which leads to the formation of a 1D infinite chain along the ‘a’ axis. Moreover, the infinite chain was connected with the p-dimethylaminopyridinium cations through N–H⋯O hydrogen bonds to generate a two dimensional self-assembled molecular network through supramolecular R88 (34) and R54 (20) motifs. The 2D molecular network linked by O–H⋯O hydrogen bonds with water molecules to form a three dimensional network construct a zig-zag sheet, as shown in Fig. 5. In addition, the weak C–H⋯O interactions observed between the methyl group of C atom with the O–H group of the anion stabilize the supramolecular architectures.
C–O) and O–H groups, which leads to the formation of a 1D infinite chain along the ‘a’ axis. Moreover, the infinite chain was connected with the p-dimethylaminopyridinium cations through N–H⋯O hydrogen bonds to generate a two dimensional self-assembled molecular network through supramolecular R88 (34) and R54 (20) motifs. The 2D molecular network linked by O–H⋯O hydrogen bonds with water molecules to form a three dimensional network construct a zig-zag sheet, as shown in Fig. 5. In addition, the weak C–H⋯O interactions observed between the methyl group of C atom with the O–H group of the anion stabilize the supramolecular architectures.
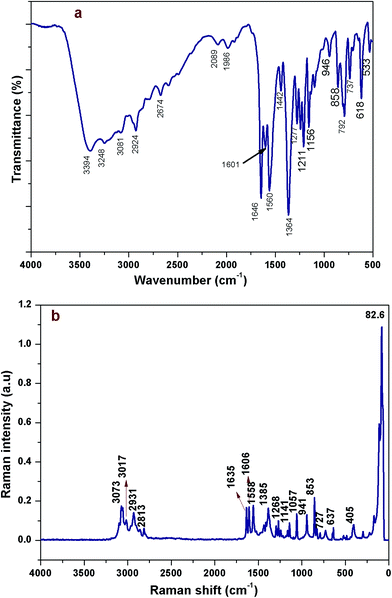
FTIR and FT-Raman spectroscopy analyses
The functional analysis of the DMAPHB crystal was identified by obtaining the FT-IR and FT-Raman spectra, which are shown in Fig. 6a and b, respectively.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) O vibrations. Both carbon and oxygen atoms have the same vibration in the same frequency. Normally carbonyl group vibrations occur in the region from 1800 to 1700 cm−1. In the present investigation, the C
O vibrations. Both carbon and oxygen atoms have the same vibration in the same frequency. Normally carbonyl group vibrations occur in the region from 1800 to 1700 cm−1. In the present investigation, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching mode was observed at 1646 cm−1 in the FT-IR spectrum.
O stretching mode was observed at 1646 cm−1 in the FT-IR spectrum.NMR spectroscopy analysis
NMR spectroscopy plays an incredible role for confirming the structure of the title compound and provides (i) a relationship between the numbers of signals in the spectrum and the number of different types of hydrogen atoms in the molecules and (ii) splitting of the primary signal (doublet, triplet and quartet). The 1H and 13C NMR spectra are shown in Fig. 7a and b, respectively.In the 1H NMR spectrum of DMAPHB, a sharp signal appearing at δ = 3.170 ppm was assigned to the methyl proton in the 4-dimethylaminopyridinium moiety. The N–H and COOH protons do not show a signal because of fast deuterium exchange taking place at these two groups, with D2O used as the solvent. The aromatic proton peaks are usually observed between δ = 6.85 ppm and 8.10 ppm. The aromatic O–H proton shows an intense singlet signal at 5.2 ppm.
In the 13C NMR spectra, the carbon signal at δ = 47.63 ppm has been assigned to the methyl carbon atom in the 4-dimethylaminopyridinium moiety. The aromatic carbon atoms are shown in range between δ = 106.6 ppm and 140.70 ppm. The resonance peak at δ = 173.08 ppm indicated the keto (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) carbon. A peak at δ = 160.10 ppm was attributed to the C–OH carbon atom in the p-hydroxybenzoate moiety.
O) carbon. A peak at δ = 160.10 ppm was attributed to the C–OH carbon atom in the p-hydroxybenzoate moiety.
UV-visible spectroscopy studies
The optical absorption and cut-off wavelength of the crystalline material is one of the important physical parameters used to highlight the optical properties.22 The UV-Vis absorption spectrum of the DMAPHB crystal was obtained in the wavelength range of 200–800 nm, as shown in Fig. 8a and b. In the spectrum, the UV cut-off wavelength of the compound was found at 345 nm, which is due to π → π* electronic transitions. Moreover, the lower cut-off wavelength and wide emission observed between 345 and 800 nm without any absorption shows that the title crystal can be used for optical applications.The optical absorption coefficient (α) was calculated using the following relationship,
where A is a constant, Eg is the optical band gap, h is Planck's constant and ν is the frequency of the incident photons. The band gap has been calculated by plotting (αhν)2 vs. hν and extrapolating the linear part near the onset of the absorption edge to the energy axis. The optical band gap energy value of the title compound was 3.6 eV.
Vickers microhardness test
The mechanical strength of the as-grown crystal was studied using a Vickers microhardness tester fitted with a diamond pyramidal indenter. The indentations were made on the sample with various applied loads in the range of 5–50 g. The indentation time was kept constant as 5 s for each variable load. The Vickers microhardness HV was computed using the relationship, HV = 1.8544P/d2 kg mm−2, where P is the applied load in kg, d is the indentation diagonal average length in mm and 1.8544 is a constant of the geometrical factor for the diamond pyramid indenter. A plot between the HV and applied load P is shown in Fig. 9a. From the plot, it is observed that the hardness value increases with increasing load. When it reaches 50 g, cracks developed around the indentation mark owing to the release of internal stresses and a decrease in the microhardness.23 Furthermore, the plot clearly explains the reverse indentation size effect (RISE) of DMAPHB.The work hardening coefficient (n) of the material was calculated using the relation, P = kdn, where ‘k’ is the arbitrary constant of a given material and ‘n’ is the work hardening coefficient. Onitsch24 and Hanneman25 pointed out that ‘n’ lies between 1 and 1.6 for moderately hard materials and more than 1.6 for soft category materials, where n is the Meyer's index. According to Meyer's law, the relationship between load and the size of indentation can be correlated. The plot of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P vs. log
P vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d (Fig. 9b), which gives the work hardening coefficient (n) that was found to be 2.81 followed the least-squares fitting method.
d (Fig. 9b), which gives the work hardening coefficient (n) that was found to be 2.81 followed the least-squares fitting method.
Thermal studies
Thermogravimetric (TG) and differential thermal analyses (DTA) were carried out to study the thermal behaviour of the title compound. The TGA/DTA curve of DMAPHB was recorded using a NETZSCH STA 409 °C analyser under a nitrogen atmosphere at a heating rate of 5 °C min−1 in the temperature range of 50–600 °C. The resultant thermal curve for DMAPHB is depicted in Fig. 10, which shows that the crystal is thermally stable up to 192 °C. There is a weight loss in the temperature range of 192–250 °C due to the liberation of volatile substances. The DTA curve shows a sharp exothermic peak at 192.08 °C, which is assigned as the melting point of DMAPHB. Moreover, the sharpness of the exothermic peak shows the good degree of crystallinity and purity of the material. The endothermic curve observed at 225.44 °C corresponds to the occurrence of decomposition.The melting point of the compound was additionally examined using a melting point apparatus (VEEGO MODEL: VMP-PM). Powdered DMAPHB was loaded in a capillary tube and placed in the apparatus for study. Upon observation through the lens, the material starts melting at 192 °C, which indicates the melting temperature of the material. This is in accordance with the TG/DTA measurements. Hence, it can be concluded that the compound has good thermal stability up to 192 °C.
Z-scan studies
From the single crystal X-ray diffraction studies, it is found that the as-grown crystal belongs to the centrosymmetric space group P21/c. It is well known centrosymmetric crystals possess inversion symmetry; thus, the second order susceptibility χ(2) becomes zero. Hence, the crystal prominently obeys third order non-linearity, which can be confirmed by the Z-scan technique developed by Sheik Bahae et al.26 It is a simple and highly sensitive single beam technique used for measuring the Kerr non-linearity of the optical material, i.e., Z-scan is a self-focusing measurement technique that involves a laser beam focusing through a thin sample and the transmitted light collected through a far-field aperture.27 Here, the sample acts like an intensity dependent lens, where the effective focal length changes when the beam intensity changes. This effect reflects the intensity distribution at the aperture in the far-field. The amount of energy transmitted through the aperture depends on the sample location along the z-axis and the sign of the refractive index (n2).28 Hence, the measurement method is called the Z-scan technique.In the present study, a continuous-wave diode-pumped Nd:YAG laser with a wavelength of 532 nm was focused through a lens with a focal length of 3.5 cm. The sample was moved along the axial direction of the laser beam (+z to −z) and the transmitted beam from the sample was collected using a digital power photodetector.
In an open aperture Z-scan technique, the entire transmitted beam through the sample was collected using the detector and the non-linear absorption coefficient (β) was estimated using the following relationship,28
To determine the sign and magnitude of the non-linear refractive index (n2) of the title compound, the closed aperture Z-scan technique was performed. Since the closed aperture transmittance was affected by the non-linear refraction and absorption of the compound.29 The sample is moved towards the focus of the lens (z = 0), when the beam irradiance increases leading to pre-focal peak and the sample moved away from the focus, where the beam irradiance decreases rapidly leading to a post-focal valley. This indicates the negative non-linearity of the sample. The closed aperture Z-scan trace of DMAPHB is given in Fig. 11a. To obtain the non-linear refractive index (n2), the on-axis non-linear phase shift ΔΦ0 was determined by fitting the closed aperture trace (Fig. 11a) using the following approximate equation,
ΔTP–V measures the difference between the normalised peak and valley transmittance (i.e. TP − TV). The linear transmittance and non-linear refractive index (n2) of the material was calculated using the following relation,
where K = 2π/λ and λ is the laser wavelength.
The real (Re[χ(3)]) and imaginary (Im[χ(3)]) parts of the third-order non-linear susceptibility (χ(3)) can be calculated from the non-linear refractive index n2 and non-linear absorption coefficient β using the following equations,30
where ε0 is the vacuum permittivity (8.8518 × 10−12 F m−1) and c is the velocity of light in a vacuum. The absolute value of the third-order non-linear optical susceptibility χ(3) is
| |χ(3)| = [(Re(χ(3)))2 + (Im(χ(3)))2]1/2 |
The calculated third-order non-linear parameters such as refractive index (n2), non-linear absorption coefficient (β), non-linear susceptibility (χ(3)) and other non-linear factors are given in Table 3. Fig. 11a shows a peak–valley characteristic, which is clearly attributed to the negative non-linearity of the samples. The non-linear refractive index n2 of the sample was in the order of 10−8 and the variance between the peak and valley (ΔTP–V) was 0.3. Hence, the sample exhibits self-defocusing nature at 532 nm. Moreover, the open aperture Z-scan graph in Fig. 11b reveals that the sample displays saturable absorption and the intensity of transmittance beam increases as the intensity of the incident beam decreases. Hence, based on the overall studies, the material under investigation may represent an improved non-linear response in optical limiting purposes.
| n2 (cm2 W−1) | β (cm W−1) | Re[χ(3)] esu | Im[χ(3)] esu | χ(3) esu |
|---|---|---|---|---|
| 1.267 × 10−8 | −0.651 × 10−3 | 3.215 × 10−7 | −6.988 × 10−8 | 1.016 × 10−13 |
Biological studies
Antibacterial activities
Organic acids such as benzoic, sorbic, lactic and propionic acids are used in food preservation and in pharmaceutical industries. Hydroxybenzoic acid has strong antiseptic and germicidal properties. The enhancement of antiseptic properties and destructive effect of microbes are due to the presence of the –COOH group in hydroxybenzoate. The microbial growth can be controlled by the carboxylated phenol group and is externally used as an antiseptic and employed in lotions and ointments.The anti-bacterial behaviour and minimum inhibitory concentration (MIC) of the title compound was studied using a resazurin reduction assay method31 against the standard bacteria: Staphylococcus aureus, Escherichia coli and Bacillus cereus. The resazurin dye solution was prepared via dissolving a 270 mg resazurin tablet in 40 mL of sterile distilled water. 96 well plates were prepared under aseptic conditions and 200 μL of the DMAPHB compound (1 mg mL−1) in 5% (v/v) dimethyl sulfoxide (DMSO) was pipetted into the sterile 96 wells plates. 100 μL of nutrient broth was added to the wells for the bacterial cells. Serial dilutions were performed using a micropipette with sterile pipette tips such that each well contains 100 μL of the test material in serially descending concentrations. 10 μL of resazurin dye solution and 10 μL of bacterial suspension (5 × 106 cells per mL) were added to each well to achieve a concentration of 5 × 105 cells per mL. The commercially available antibiotic streptomycin (against bacteria) was used as a positive control in the assay plate and placed in an incubator at 37 °C for 24 h.
The change in color of the dye from blue or purple to pink indicates that the cells are viable. The enzyme oxido-reductase present in the bacterial cells converts resazurin to resorufin, which is pink in color. When the color of the dye remains blue, it indicates that there is no activity of viable cells. The synthesised compound was added to kill the bacterial cells during incubation, which was determined from the blue or purple color of dye in the respective wells. The pink color formation in the wells even after treating with the test compounds or commercial drug indicates the presence of viable cells. Thus, the least dilution in which the color remains blue was taken as the MIC value of the respective compound. DMAPHB showed activity against the bacterial pathogens.
The MIC value of DMAPHB revealed that the effective inhibition of the growth of the Gram positive bacteria Escherichia coli was more or less similar to that found for the antibiotic streptomycin. It was concluded that DMAPHB has good antibacterial activity. Further development of this compound may have better results in its biological applications. Table 4 shows the antibacterial performance of DMAPHB with the standard drug as the control experiment and Fig. 12 shows the minimum inhibitory concentration (MIC) of the compound. The MIC values of DMAPHB are 6.25 μg mL−1, 1.56 μg mL−1 and 6.25 μg mL−1 against Staphylococcus aureus, Escherichia coli and Bacillus cereus, respectively.
| MIC μg mL−1 | |||
|---|---|---|---|
| Test material | Bacterial pathogens | ||
| Staphylococcus aureus | Escherichia coli | Bacillus cereus | |
| DMAPHB compound | 6.25 | 3.12 | 6.25 |
| Streptomycin | 1.56 | 1.56 | 1.56 |
Anti-cancer activities
Fig. 13 shows the cytotoxicity of the hepatoprotective activities of DMAPHB tested against HepG2 cell lines using the MTT (3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay method. The sensitive and consistent colorimetric MTT assay method was used to measure viability, proliferation and activation of the cells.32 Epidemiological studies give evidence of an inverse dose-dependent relationship between coffee consumption and the risk of human hepatocarcinoma independent of its etiology.33 The half maximal inhibitory concentration (IC50) is employed to measure the cytotoxicity of the compounds against the cancer cell lines. 5 mg mL−1 of MTT dye stock solution was prepared in sterilized phosphate buffered saline. The lysis solution was prepared (0.6 mL acetic acid + 99.4 mL dimethyl sulphoxide) and used as the control for the further experiments. HepG2 cells were incubated with suitable concentrations of DMAPHB (1 to 10 μg) for 48 h. After this period, MTT dye (100 μL per mL of stock solution) was added to each well and the culture plates incubated for 3 h in a CO2 incubation chamber. The cultured cells were transferred to the premarked 96 well plates and then the optical densities (OD) of the lysate were measured using a wavelength 595 nm ELISA reader. The percentage OD values (OD for metal specimen/OD for control group × 100%) were calculated. A higher value of the OD represents that the cell is more viable. DMAPHB reveals good anticancer properties against the hepatocellular carcinoma HepG2 cell line at higher concentrations, whereas at low concentrations, 91% of the cancer cells are viable. The 4% cancer cells are viable in 10 μg concentration of title compound. The IC50 value of the compound was found to be 4.90 μg mL−1 against HepG2 cells as obtained from these results. The pyridine ring present in DMAPHB may play an important role in the cytotoxic behaviour and exhibits good anticancer activity. More studies on this compound for its biological functions are underway. The results are summarised in Table 5.| Cell viability in % | Concentration in μg |
|---|---|
| 91 | 1 |
| 87 | 2 |
| 79 | 3 |
| 64 | 4 |
| 51 | 5 |
| 35 | 6 |
| 20 | 7 |
| 10 | 8 |
| 5 | 9 |
| 4 | 10 |
Conclusions
A new organic single crystal of DMAPHB was grown via a slow evaporation solution growth method at room temperature. The structural investigation of the compound was studied using single crystal XRD studies, which revealed the molecular structure and the formation of hydrogen bonds in the crystal. The molecular confirmation of the title compound was identified using FTIR and FT-Raman and NMR spectroscopy. The UV-Vis spectroscopy studies show the crystal cut-off wavelength at 345 nm. The Vickers microhardness test reveals that the as-grown crystal is classified in the soft material category and other mechanical parameters were evaluated. The TG/DTA curve shows that the melting point and material is thermally stable up to 192 °C. The single beam Z-scan experimental results confirmed the non-linear absorption coefficient and negative value of the third-order non-linear refractive index, which are required for optical limiting applications. From the present investigation, we conclude that DMAPHB is a new candidate for NLO device applications. The MIC values of DMAPHB against a variety of bacterial strains were evaluated and performed using a resazurin reduction assay method. DMAPHB was best at inhibiting the growth of the Gram negative bacteria Escherichia coli due to the heterocyclic pyridine and carboxylated phenol molecules present in the title compound and hence, it can be used in pharmaceutical industry.Acknowledgements
One of the authors P. Sathya thanks N. Karthikeyan, Research Scholar, Department of Physics, Anna University, Chennai, for fruitful scientific discussions.References
- G. R. Desiraju, Angew. Chem., Int. Ed., 2007, 46, 8342–8356 CrossRef CAS PubMed.
- R. L. Sutherland, Handbook of Nonlinear Optics, Marcel Dekker, Inc., 1996 Search PubMed.
- C. C. Evans, M. Bagieu-Beucher, R. Masse and J.-F. Nicoud, Nonlinearity Enhancement by Solid-State Proton Transfer: A New Strategy for the Design of Nonlinear Optical Materials, Chem. Mater., 1998, 10, 847–854 CrossRef CAS.
- J. A. Delaire and K. Nakatani, Linear and nonlinear optical properties of photochromic molecules and materials, Chem. Rev., 2000, 100, 1817–1845 CrossRef CAS PubMed.
- B. B. Koleva, T. Kolev, R. W. Seidel, T. T. sanev, H. Mayer-Figge, M. Spiteller and W. S. Sheldrick, Spectroscopic and structural elucidation of 4-dimethylaminopyridine and its hydrogensquarate, Spectrochim. Acta, Part A, 2008, 71, 695–702 CrossRef PubMed.
- J. Zyss, R. Masse, M. Bagieu-Beucher and J. Levy, Adv. Mater., 1993, 5(20), 120–124 CrossRef CAS.
- R. A. Abramovitch, The Chemistry of Heterocyclic Compounds, Pyridine and Its Derivatives, John Wiley & Sons, an Interscience publication, 2008, vol. 14 Search PubMed.
- J. J. D. De Jong, L. N. Lucas, R. M. Kellogg, J. H. van Esch and B. L. Feringa, Reversible Optical Transcription of Supramolecular Chirality into Molecular Chirality, Science, 2004, 304, 278–281 CrossRef CAS PubMed.
- C. Alosious Gonsago, H. Merina Albert, J. Karthikeyan, P. Sagayaraj and A. Joseph Arul Pragasam, Mater. Res. Bull., 2012, 47, 1648–1652 CrossRef.
- B. Chattopadhyay, U. Das, M. Mukherjee and A. K. Mukherjee, CrystEngComm, 2013, 15, 1077–1085 RSC.
- E. F. V. Scriven and R. Murugan, Pyridine and Pyridine derivatives, Kirk-Othmer Encyclopedia of Chemical Technology, 2005 Search PubMed.
- D.-K. Bucar, R. F. Henry, X. Lou, R. W. Duerst, L. R. Mac Gillivray and G. G. Z. Zhang, Cocrystals of Caffeine and Hydroxybenzoic Acids Composed of Multiple Supramolecular Heterosynthons: Screening via Solution-Mediated Phase Transformation and Structural Characterization, Cryst. Growth Des., 2009, 9, 1932–1943 CAS.
- J. Bourke, C. F. Brereton, S. V. Gordon, Ed C. Lavelle and E. M. Scanlan, The synthesis and biological evaluation of mycobacterial p-hydroxybenzoic acid derivatives (p-HBADs), Org. Biomol. Chem., 2014, 12, 1114 CAS.
- I. P. Bincy and R. Gopalakrishnan, Studies on synthesis, growth and characterization of a novel third order nonlinear optical 4-dimethylaminopyridinium p-toluenesulfonate single crystal, Opt. Mater., 2014, 37, 267–276 CrossRef CAS.
- B. B Koleva, T. Kolev, R. W. Seidel, T. T. sanev, H. Mayer-Figge, M. Spiteller and W. S. Sheldrick, Spectroscopic and structural elucidation of 4-dimethylaminopyridine and its hydrogensquarate, Spectrochim. Acta, Part A, 2008, 71, 695–702 CrossRef PubMed.
- A. Arunkumar and P. Ramasamy, Synthesis, crystal structure, spectral and thermal properties of 4-dimethylaminopyridinium salicylate monohydrate, Appl. Phys. A: Mater. Sci. Process., 2013, 111, 1165–1173 CrossRef CAS.
- Bruker, APEX2, SAINT and SADABS, Bruker AXS Inc., Madison, Wisconsin, USA, 2004 Search PubMed.
- G. M. Sheldrick, Short history of SHELX, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- L. J. Farrugia, ORTEP-3 for Windows - a version of ORTEP-III with a Graphical User Interface (GUI), J. Appl. Crystallogr., 1997, 30, 565 CrossRef CAS.
- C. F. Macrae, I. J. Bruno, J. A. Chisholm, P. R. Edgington, P. McCabe, E. Pidcock, L. Rodriguez-Monge, R. Taylor, J. Vande Streek and P. A. Wood, Mercury CSD 2.0 – new features for the visualization and investigation of crystal structures, J. Appl. Crystallogr., 2008, 41, 466–470 CrossRef CAS.
- S. Aitipamula, G. R. Desiraju, M. Jasko lski, A. Nangia and R. Thaimattamb, Multiple molecules in the crystallographic asymmetric unit. Self host–guest and doubly interpenetrated hydrogen bond networks in a pair of keto-bisphenols, CrystEngComm, 2003, 5(78), 447–450 RSC.
- J. Kulakowska and S. Kucharski, New chromophoric monomers with nonlinear optical properties, Eur. Polym. J., 2000, 36, 1805–1815 CrossRef CAS.
- K. Sangwal, M. Hordyjewicz and B. Surowska, Microindentation hardness OF SrLaAlO4 and SrLaGaO4 single crystals, J. Optoelectron. Adv. Mater., 2002, 4, 875–882 CAS.
- E. M. Onitsch, The present status of testing the hardness of materials, Mikroskopie, 1947, 2, 131 Search PubMed.
- M. Hanneman, Metall. Manch., 1941, 23, 135 Search PubMed.
- M. Sheik-Bahae, A. A. Said and E. W. Van Stryland, High-sensitivity, single-beam n2 measurements, Opt. Lett., 1989, 14, 955 CrossRef CAS PubMed.
- M. Sheik-Bahae, A. A. Said, T. Wei, D. J. Hagan and E. W. Van Stryland, Sensitive measurement of optical nonlinearities using a single beam, IEEE J. Quantum Electron., 1990, 26, 760 CrossRef CAS.
- M. Sheik-Bahaei and E. W. Van Stryland, in Z-Scan Measurements of Optical Nonlinearities, Characterization Techniques and Tabulations for Organic Nonlinear Materials, ed. M. G. Kuzyk and C. W. Dirk, Marcel Dekker, Inc., 1998, pp. 655–692 Search PubMed.
- K. Senthil, S. Kalainathan, A. Ruban Kumara and P. G. Aravindan, Investigation of synthesis, crystal structure and third-order NLO properties of a new stilbazolium derivative crystal: a promising material for nonlinear optical devices, RSC Adv., 2014, 4, 56112 RSC.
- T. Cassano, R. Tommasi, M. Ferrara, F. Babudri, G. M. Farinola and F. Naso, Substituent-dependence of the optical nonlinearities in poly(2,5-dialkoxy-p-phenylenevinylene) polymers investigated by the Z-scan technique, Chem. Phys., 2001, 272, 111–118 CrossRef CAS.
- S. D. Sarker, L. Nahar and Y. Kumarasamy, Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of photochemical, Methods, 2007, 42, 321–324 CrossRef CAS PubMed.
- T. Mosmann, Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity tests, J. Immunol. Methods, 1983, 65, 55–63 CrossRef CAS PubMed.
- L. Dalbem Rocha, M. Costa Monteiro and A. Junger Teodoro, Anticancer Properties of Hydroxycinnamic Acids, J. Cancer Res. Clin. Oncol., 2012, 1, 2 Search PubMed.
Footnote |
| † CCDC 1061663. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra00283h |
| This journal is © The Royal Society of Chemistry 2016 |