The effect of rol genes on phytoecdysteroid biosynthesis in Ajuga bracteosa differs between transgenic plants and hairy roots†
Abstract
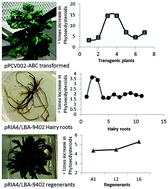
Phytoecdysteroids are secondary metabolites biosynthesized by plants as a defense strategy against phytophagous insects. These are also used in certain medical preparations to reduce depression and prevent infections. We studied the effect of rol genes on phytoecdysteroid biosynthesis in Ajuga bracteosa by transformation through Agrobacterium tumefaciens strain GV3101 harboring pPCV002-ABC. Transformation was confirmed by PCR. Among seven independently generated transgenic lines, a significant increase of phytoecdysteroids was observed in lines 3 and 4 (6728 and 6759 μg g−1 total phytoecdysteroids, respectively) that was 14.5-fold higher than the control plants. Both these lines showed relatively high expression of the rolC gene verified by semi-quantitative RT-PCR and densitometric analysis. We also obtained transgenic hairy root lines of A. bracteosa by transformation with A. rhizogenes strains LBA-9402, A4 and ARqua1. Semi-quantitative RT-PCR and densitometric molecular imaging of 9 transgenic root lines obtained from LBA 9402 revealed a high rolC expression. Transgenic hairy root lines also exhibited phytoecdysteroid production, particularly lines A4-2 and 9402-01 that showed a yield of 4449 and 4123 μg g−1 dry weight, respectively. Complete transgenic plants developed from hairy roots had more phytoecdysteroid content than the parent hairy root lines, suggesting the presence of a possible sink (leaves). Considering the ecdysteroid negative feedback inhibition, we hypothesize that the unavailability of a suitable sink prevents further biosynthesis of phytoecdysteroids in hairy roots. Moreover, sengosterone was not detected in untransformed plants, pPCV002-ABC-generated transgenic plants or untransformed roots, but its presence in some high ecdysteroid-producing hairy root lines suggests its de novo biosynthesis in roots.

 Please wait while we load your content...
Please wait while we load your content...