Hydrothermal conversion of layered hydroxide nanosheets into (Y0.95Eu0.05)PO4 and (Y0.96−xTb0.04Eux)PO4 (x = 0–0.10) nanocrystals for red and color-tailorable emission†
Zhihao Wanga,
Ji-Guang Li*ab,
Qi Zhua,
Xiaodong Lia and
Xudong Suna
aKey Laboratory for Anisotropy and Texture of Materials (Ministry of Education), School of Materials Science and Engineering, Northeastern University, Shenyang, Liaoning 110819, China
bAdvanced Materials Processing Unit, National Institute for Materials Science, Namiki 1-1, Tsukuba, Ibaraki 305-0044, Japan. E-mail: li.jiguang@nims.go.jp; Tel: +81-29-860-4394
First published on 22nd February 2016
Abstract
Tetragonal-structured (Y0.95Eu0.05)PO4 and (Y0.96−xTb0.04Eux)PO4 nanocrystals were sacrificially converted from layered rare-earth hydroxide (Ln2(OH)5NO3·nH2O, LRH) nanosheets via hydrothermal reaction and in the presence of ammonium dihydrogen phosphate (NH4H2PO4). Detailed characterizations of the materials were achieved by the combined techniques of FT-IR, ICP, XRD, FE-SEM, TEM, and optical spectroscopies, and the mechanisms of anion exchange and hydrothermal conversion were discussed. Phase structure and morphology of the product were found to heavily rely on the amount of PO43− relative to rare-earth ions. The effects of calcination were studied in detail with the (Y0.95Eu0.05)PO4 red phosphor nanocrystals. The (Y0.96−xTb0.04Eux)PO4 ternary phosphors present efficient Tb3+ → Eu3+ energy transfer through electric dipole–dipole interactions, with which the emission color can be facilely tuned from green to red via yellow by raising the Eu content. The efficiency of energy transfer was analyzed to be ∼40.7% at the optimal Eu3+ concentration of 8 at% (x = 0.08).
1. Introduction
The orthophosphate of YPO4 exhibits high thermal stability (melting point ∼2300 °C), extremely low water-solubility (solubility product ∼10−25 to 10−27),1,2 and facile substitution of the Y site with various lanthanide ions, and is thus widely employed as a host lattice for luminescence. Previous investigations manifested that the compound may crystallize as one of the following three phases depending on the extent of hydration: monoclinic YPO4·2H2O, hexagonal YPO4·0.8H2O and tetragonal YPO4,3–5 with the latter two being the most frequently encountered. Luminescent materials in nano-dimensions have been drawing keen research interest owing to their widespread applications in physics, chemistry, biology, optoelectronics, displays, and lighting.6–8 As for YPO4-based phosphors, C. X. Li et al. prepared nano/microcrystals with both hexagonal and tetragonal structures and well-defined uniform morphologies via hydrothermal reaction. Through a series of control experiments, they concluded that the initial solution pH, citrate chelate (Cit3−), and phosphate source (NaPO4, NH4H2PO4 or Na5P3O10) synergistically determine the phase structure and crystal shape of the final product. The luminescence behaviors of Tb3+, Eu3+, and Dy3+ in YPO4 were also studied, and multicolor emission in a single host lattice was achieved.9 Through a CTAB-assisted hydrothermal technique, P. Li et al. synthesized hexagonal structured (Y,Eu)PO4·0.8H2O of prismatic particle morphologies and discussed its thermal decomposition, morphology evolution, and luminescence.10 The growth pattern of nanocrystals is known to be governed by the intrinsic surface energy of the crystallographic face of the nuclei, the type and amount of adsorbed organic capping molecules, the time of crystal growth, and the molecular precursor,11 and morphology selection has always been a widely and intensively discussed topic.12–14 In this regard, the research group of Y. D. Li made extensive investigation into the crystallization habit of rare-earth orthophosphate and the factors influencing anisotropic growth under hydrothermal conditions.15,16Eu3+ and Tb3+ are well-known efficient activators for various host lattices to attain high quality red and green emissions, respectively. Meanwhile, energy transfer between two types of activators is being widely utilized in the phosphor field to tune the emission color, to produce a specific color that cannot be attained with one single type of activator, and to enhance the desired emission.17 The Eu3+/Tb3+ combination is undoubtedly one of the most frequently adopted activator pairs to achieve the aforementioned purposes, since the emission spectrum of Tb3+ presents significant overlap with the excitation spectrum of Eu3+, which allows efficient Tb3+ → Eu3+ resonant energy transfer.18–21 We thus synthesized in this work Eu3+ singly-doped and Eu3+/Tb3+ codoped YPO4 nanocrystals for red and color tunable emissions, via a hydrothermal nano-conversion technique where nanosheets (∼4 nm) of the Ln2(OH)5NO3·nH2O layered rare-earth hydroxide (LRH) were used as a new type of sacrificial precursor and ammonium dihydrogen phosphate (NH4H2PO4) as the phosphate source. The LRH compound is a recent type of layered inorganic materials, whose crystal structure is built up via alternative stacking of the [Ln2(OH)5(H2O)n]+ host layers and interlayer NO3− along the c-axis.21 The Ln3+ centers have the two types of coordination environments of [Ln(OH)7(H2O)] dodecahedron (8-fold coordination) and [Ln(OH)8(H2O)] monocapped square antiprism (9-fold coordination). Each LnO8 unit is linked to two other LnO8 and four LnO9 units via edge sharing to form a two-dimensional host layer parallel to the ab plane, with NO3− sandwiched between two adjacent layers as a free and exchangeable anion for charge balance.21 The classic synthesis of LRH via reflux and hydrothermal reactions generally yield micron-sized thick crystals,21–23 from which nanosheets are obtainable in poor yield and low efficiency via delamination.24–26 We very recently developed a “freezing-temperature crystallization” technique that can produce LRH nanosheets of only ∼4 nm thick in one step and large quantities.27 The significantly exposed hydroxide main layers by the thinness of the nanosheets would allow fast kinetics of phase conversion reactions. We systematically studied in this work the effects of PO43−/(Y,Ln)3+ (Ln = Eu, Tb/Eu) molar ratio, reaction time, and calcination on phase structure, morphology, and photoluminescent property of the (Y,Ln)PO4 nanocrystals. Color tunable luminescence was achieved by varying the Tb/Eu molar ratio and the efficiency and mechanism of Tb3+ → Eu3+ energy transfer were also analyzed in detail. We believe that the nanocrystal preparation can be transferred to technology for practical applications, since the synthesis of LRH is readily achievable via precipitation and the conversion of LRH into phosphate is fast under mild hydrothermal conditions.
2. Experimental section
2.1 Synthesis of LRH nanosheets and conversion into (Y,Ln)PO4 (Ln = Eu, Eu/Tb) nanophosphors
The starting lanthanide sources are Ln2O3 (Ln = Y, Tb, and Eu; 99.99% pure; Huizhou Ruier Rare-Chem. Hi Tech. Co. Ltd., Huizhou, China), and the nitrate solution of Ln was prepared by dissolving the oxide with a proper amount of hot nitric acid.In a typical synthesis, 150 ml of an aqueous solution containing 15 mmol of (Y0.95Eu0.05)3+ or (Y0.96−xTb0.04Eux)3+ was cooled to 2–5 °C under magnetic stirring, to which ammonium hydroxide solution (NH4OH, 1 mol L−1; analytical grade, Shenyang Chemical Reagent Factory, Shenyang, China) was dropwise added until pH ∼ 8.5 for direct precipitation of layered hydroxide nanosheets.27 The Eu content in the (Y0.96−xTb0.04Eux)3+ combination was varied in the range of x = 0–0.10 to reveal its effects on optical properties. After aging for 1 h, the precipitate was collected via centrifugation and was washed with distilled water three times to remove by-products, rinsed with absolute ethanol, followed by drying in an air oven at 50 °C for 24 h. For each run of anion exchange and hydrothermal reaction, 0.5 mmol of the LRH nanosheets was first dispersed in ∼70 ml of deionized water, to which a certain amount of ammonium dihydrogen phosphate (NH4H2PO4; 1 mol L−1, analytical grade, Shenyang Chemical Reagent Factory) was then dropwise added. The resultant suspension was constantly stirred for 30 min before being transferred into a Teflon-lined stainless steel autoclave of 100 ml capacity. The autoclave was tightly sealed and put into an electric oven preheated to 150 °C. After 24 h of reaction, the autoclave was left to cool naturally to room temperature and the hydrothermal product was collected via centrifugation. The wet precipitate, after being washed as aforementioned, was dried in the air at 70 °C for 24 h to yield a white powder for characterization and further processing. The effects of reaction parameters (Table 1) were studied with (Y0.95Eu0.05)PO4 for example.
| Sample ID | R = PO43−/(Y + Eu)3+ | Reaction time (h) |
|---|---|---|
| S1 | 1.5 | 0.5 (room temp.) |
| S2 | 1.5 | 3 |
| S3 | 1.5 | 6 |
| S4 | 1.5 | 9 |
| S5 | 1.5 | 15 |
| S6 | 1.5 | 24 |
| S7 | 1.1 | 24 |
| S8 | 1.2 | 24 |
| S9 | 2 | 24 |
2.2 Characterization techniques
Phase identification was performed via X-ray diffractometry (XRD, Model PW3040/60, Philips, Eindhoven, The Netherlands) operated at 40 kV/40 mA using nickel filtered Cu-Kα radiation (λ = 0.15406 nm) and a scanning speed of 4.0° 2θ per minute. Morphology and microstructure of the products were analyzed by field emission scanning electron microscopy (FE-SEM, Model JSM-7011F, JEOL, Tokyo, Japan) operated under 15 kV and transmission electron microscopy (TEM, FEM-3000F, JEOL) under 300 kV. Fourier transform infrared spectroscopy (FT-IR, Nicolet iS5, Thermal Fisher Scientific, USA) was performed by the standard KBr method. Element contents of the product was analyzed via inductively coupled plasma (ICP) spectroscopy (Model IRIS Advantage, Jarrell-Ash Japan, Kyoto, Japan) with a detection limit of 0.01 wt%. Photoluminescence and fluorescence decay of the YPO4:Ln3+ phosphors were analyzed at room temperature with an FP-8600 fluorospectrophotometer (Jasco, Tokyo).3. Results and discussion
3.1 Formation process of (Y0.95Eu0.05)PO4 nanocrystals
FT-IR analysis was performed on the three typical samples of S1, S4 and S6, and the results are presented in Fig. 1. The LRH precursor exhibits a sharp and strong absorption peak at 1385 cm−1, which is characteristic of uncoordinated nitrate anions widely observed for NO3−-LRHs.28 The absorption at ∼3448 cm−1 and the shallow shoulder near 1639 cm−1 prove the existence of hydration water and are assignable to the O–H stretching vibrations (ν1 and ν3) and the H–O–H bending mode (ν2), respectively.29 The band observed in the 3500–3700 cm−1 region (centered at 3587 cm−1) arises from stretching vibrations of hydroxyls (OH−).29,30 These findings conform well to the chemical formulae of NO3−-LRHs. For the anion-exchange product S1, it is clearly seen that the NO3− absorption at 1385 cm−1 and the hydroxyl vibration at ∼3587 cm−1 disappeared while new bands that correspond to the bending (ν4, at ∼531/638 cm−1) and stretching (ν3, at ∼1078 cm−1) vibrations of PO43− emerged in the IR spectrum.31 As no other species are clearly identifiable, it can thus be said that a hydrated phosphate has been formed even after very short (30 min) reaction at room temperature. Elemental analysis found 32.3 ± 0.1 wt% of Y, 2.92 ± 0.1 wt% of Eu and 12.3 ± 0.1 wt% of P for S1, leading to a Y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu
Eu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO4 molar ratio of 0.950
PO4 molar ratio of 0.950![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.050
0.050![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.030. The sample can thus be approximately expressed as (Y0.95Eu0.05)PO4·nH2O, where the n value was calculated from the Y content to be ∼4. This indicates that the phosphate anions released from NH4H2PO4 readily replace not only the interlayer NO3− but also the hydroxyls in the [Ln(OH)7H2O] and [Ln(OH)8H2O] polyhedrons that comprise the host layers of LRH owing to its significantly higher coordination capability.32 The IR spectra of samples S4 and S6 exhibit features similar to S1, but the weaker water absorptions at ∼3448 and 1639 cm−1 imply dehydration of the product under hydrothermal conditions. Dehydration would also lessen the intramolecular hydrogen bonding between H2O and PO43−, thus shifting the stretching mode of PO43− (around 1070 cm−1) towards lower energy (smaller wavenumber).33 TG analysis (Fig. S1†) found that all the three samples decompose via two major stages up to 500 °C, with the first one (up to ∼270 °C) largely due to the evaporation of surface absorbed water while the second one (∼270–500 °C) to the removal of hydration water. From the total weight losses of ∼23.01%, 14.06% and 9.6%, samples S1, S4 and S6 were calculated to have successively smaller n values of ∼3.11, 1.70 and 0.96, respectively.
1.030. The sample can thus be approximately expressed as (Y0.95Eu0.05)PO4·nH2O, where the n value was calculated from the Y content to be ∼4. This indicates that the phosphate anions released from NH4H2PO4 readily replace not only the interlayer NO3− but also the hydroxyls in the [Ln(OH)7H2O] and [Ln(OH)8H2O] polyhedrons that comprise the host layers of LRH owing to its significantly higher coordination capability.32 The IR spectra of samples S4 and S6 exhibit features similar to S1, but the weaker water absorptions at ∼3448 and 1639 cm−1 imply dehydration of the product under hydrothermal conditions. Dehydration would also lessen the intramolecular hydrogen bonding between H2O and PO43−, thus shifting the stretching mode of PO43− (around 1070 cm−1) towards lower energy (smaller wavenumber).33 TG analysis (Fig. S1†) found that all the three samples decompose via two major stages up to 500 °C, with the first one (up to ∼270 °C) largely due to the evaporation of surface absorbed water while the second one (∼270–500 °C) to the removal of hydration water. From the total weight losses of ∼23.01%, 14.06% and 9.6%, samples S1, S4 and S6 were calculated to have successively smaller n values of ∼3.11, 1.70 and 0.96, respectively.
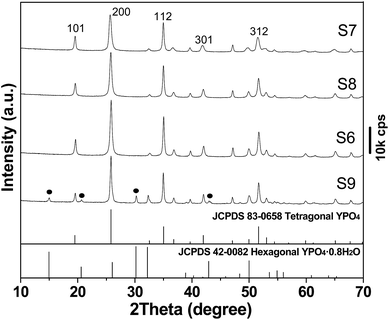
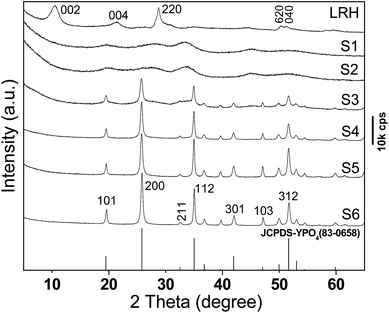
Fig. 2 shows XRD patterns of the pristine LRH and samples S1–S6. The LRH exhibits a series of 00l and non-00l diffractions that correspond to the layered rare-earth hydroxide of Ln2(OH)5NO3·nH2O.27 The characteristic LRH diffractions disappeared for S1, indicating collapse of the layered structure and disintegration of the hydroxide main layers, as also seen from the results of morphology analysis shown later. The seriously broadened diffraction peaks cannot be indexed to any crystalline form of rare-earth orthophosphate, possibly owing to its high water content. Though sample S2 is almost identical to S1, tetragonal (Y,Eu)PO4 (JCPDS No. 83-0658, space group: I41/amd) crystallizes in sample S3 via consumption of the unidentifiable phase. Pure tetragonal (Y,Eu)PO4 is formed for samples S4–S6, and gradually sharper XRD diffractions (better crystallinity) were observed along with increasing hydrothermal time.
 | ||
| Fig. 2 XRD patterns for the LRH, the room-temperature product S1, and the products obtained via hydrothermal reaction for 3 h (S2), 6 h (S3), 9 h (S4), 15 h (S5), and 24 h (S6). | ||
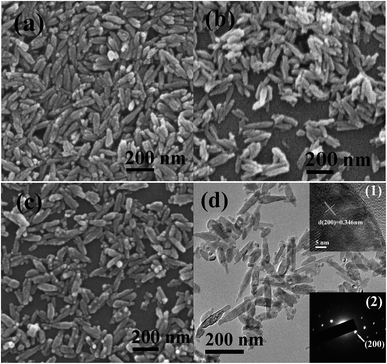
FE-SEM observation (Fig. 3a) revealed micron-sized and flower-like assemblies of LRH nanoflakes, while TEM analysis (Fig. 3b) found nanosheets of up to 3–6 nm in thickness. The very thin nature of the nanosheets is also perceivable from their flexibility. Selected area electron diffraction (SAED, the inset in Fig. 3b) yielded ring-like patterns that correspond to the (220) and (620) planes, further confirming that the hydroxide host layers of the LRH are well ordered as revealed by XRD. Sample S1 generally retains the flower-like skeletons of the LRH precursor (Fig. 3c), but the individual nanosheets were eroded and disintegrated by PO43− interaction at room temperature (Fig. 3d). HR-TEM (the inset 1 in Fig. 3d) revealed crystalline objects with interplanar spacing of ∼0.37 nm, and the very tiny crystallite size (∼5 nm) may explain the significantly broadened XRD peaks (Fig. 2, sample S1) and the rather vague diffraction rings (the inset 2 in Fig. 3d). The above observations may thus suggest that the hydrated product of (Y0.95Eu0.05)PO4·4H2O (S1) was formed via a dissolution-reprecipitation pathway. Though the 9 h product (S4, Fig. 3e) contains both dispersed and aggregated spindle-like particles, the 24 h product (S6, Fig. 3f) exclusively consists of monodispersed spindles. Longer reaction time favors Ostwald ripening and thus a more uniform particle morphology.
3.2 Phase and morphology evolution
Fig. 4 compares XRD patterns of the products synthesized with different PO43−/(Y + Eu)3+ molar ratio R, from which it is seen that those of up to R = 1.5 (S7, S8 and S6) are all well indexable with tetragonal YPO4 (JCPDS No. 83-0658, space group: I41/amd). The samples were calculated to have crystallite sizes of ∼32 nm for S7, 39 nm for S8 and 42 nm for S6 via broadening analysis of the (200) diffraction with the Scherrer equation. SEM observation found that increasing R from 1 to 1.5 led to gradually more elongated particles, and the aspect ratio was assayed to be ∼2.1 for S7 (Fig. 5a), ∼3.2 for S8 (Fig. 5b) and ∼3.6 for S6 (Fig. 3f). This is consistent with the report of Peng et al.,34,35 who suggested that a higher monomer concentration will encourage 1D crystal growth of phosphate. Further raising the R value to 2 (S9) led to partial crystallization of hexagonal phosphate (Fig. 4; JCPDS No. 42-0082, space group: P6222). Vanetsev et al. recently reported that hexagonal YPO4·0.8H2O tends to be lower in crystallinity than tetragonal YPO4 under the same synthetic conditions.36 The high PO43− concentration at R = 2 would fasten chemical reactions, preventing sufficient crystallization of the product and thus the formation of hexagonal phosphate.Low magnification TEM observation of sample S6 (Fig. 5c) found that the spindle-like particles have a length of about 200 nm and a width of about 35 nm. The aspect ratio calculated here (∼5.7) is larger than that (3.6) from SEM micrograph (Fig. 3f), since the SEM sample was sputtered with gold for electrical conductivity. Selected area electron diffraction (SAED, the inset in Fig. 5c) yielded sharp spots that correspond to the (200), (112) and (312) planes of tetragonal (Y0.95Eu0.05)PO4, indicating that the product is well crystallized. The excellent crystallinity is also evidenced by the well resolved lattice fringes of the individual nanocrystals (Fig. 5d), where the spacing of ∼0.259 nm corresponds well to the (112) plane of tetragonal YPO4 (d112 = 0.256 nm). Fourier transformation of the lattice fringe yielded well defined diffraction spots (the inset in Fig. 5d), indicating that each individual nanocrystal is of single crystalline.
3.3 The effects of calcination on structure and properties of (Y0.95Eu0.05)PO4
The effects of annealing on characteristics of the phosphate crystallites were investigated with sample S6 for example. It was found that, in the range of this study, calcination did not induce any change to the tetragonal phase structure (Fig. S2†) but led to gradually sharper XRD diffractions for the higher-temperature product owing to crystal perfection and growth. SEM observation revealed that the spindle-like morphologies were essentially retained at 800 (Fig. 6a) and 900 °C (Fig. 6b) but slightly fragmented at 1000 °C (Fig. 6c and d). HR-TEM analysis of the 1000 °C product (the inset (1) in Fig. 6d) clearly reveals the (200) plane with an inter-planar spacing of ∼0.346 nm, which is quite close to the 0.345 nm of YPO4 in the standard data file. SAED analysis (the inset (2) in Fig. 6d) yielded well defined spots, indicating not only high crystallinity but also single crystalline nature of each particle.The effects of calcination on photoluminescence of the (Y0.95Eu0.05)PO4 red phosphors are studied in Fig. 7 for sample S6. The PLE spectra (Fig. 7a) all exhibit excitation bands at ∼217 nm, which are assignable to the excitation of electrons from the 2p orbital of O2− in the PO43− group to the 4f orbital of Eu3+. The other excitations in the longer wavelength region are arising from the intra-4f6 electronic transitions of Eu3+. The phosphors exhibit sharp emissions upon UV excitation at 217 nm, which are associated with the transitions from the excited 5D0 state to the 7FJ (J = 0–4) ground states of Eu3+ as labeled in Fig. 7b.37,38 Intensities of the PLE and PL bands were greatly enhanced by calcination at 800 °C, mainly due to crystal perfection/growth and the elimination of fluorescence quenching water molecules, hydroxyls, and surface dangling bonds.39 Morphology of the nanocrystals does not appreciably affect emission peak position of the doped lanthanide ions since the luminescence only involves 4f electrons, which are well shielded by 5s25p6 electronic shells. Intensity of the emission, however, is clearly dependent on crystal morphology (size/shape). The asymmetry factor of luminescence (intensity ratio of 5D0 → 7F2 to 5D0 → 7F1 transitions) was calculated to be around 1.1, though a stronger 5D0 → 7F1 magnetic dipole transition was expected since the Eu3+ ions would replace the Y3+ in YPO4 to inherit a centrosymmetric D2d point symmetry.40,41 The abnormally higher intensities of the 5D0 → 7F2,4 emissions may mainly be due to the elongated crystallite morphology, which give rise to distortion of the Eu3+ local symmetry as well demonstrated for Sr3Ga2O5Cl2:Eu3+, Bi3+ phosphors.42
 | ||
| Fig. 7 PLE (a) and PL (b) spectra of the (Y0.95Eu0.05)PO4 phosphors (S6) calcined at various temperatures. | ||
Fluorescence decay kinetics of the (Y0.95Eu0.05)PO4 phosphors were studied for the 5D0 → 7FJ (J = 1, 2) emissions at 593 and 618 nm. The decay curves (Fig. S3 and S4†) can be fitted with the single-exponential function of I = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ) + B, where τ is the fluorescence lifetime, t the delay time, I the relative intensity of emission, and A and B are constants. The derived lifetime values are summarized in Table 2, where it is seen that the lifetime increased dramatically by calcination at 800 °C and then slightly decreases at higher annealing temperatures. The abrupt increase is primarily owing to the removal of luminescence quenching defects and surface species, while the subsequent decrease is mainly due to crystallite/particle growth.43 It is also observed that the 593 nm emission (5D0 → 7F1) has a longer lifetime than the 618 nm one (5D0 → 7F2) in each case, since the former arises from Eu3+ taking higher point symmetries and is parity-allowed while the latter from Eu3+ residing at less symmetric sites and is parity-forbidden. The Commission International de L'Eclairage (CIE) chromaticity coordinates were calculated to be about (0.62, 0.38) for the uncalcined powder and about (0.64, 0.36) for all the three calcined ones (Fig. S5†).
exp(−t/τ) + B, where τ is the fluorescence lifetime, t the delay time, I the relative intensity of emission, and A and B are constants. The derived lifetime values are summarized in Table 2, where it is seen that the lifetime increased dramatically by calcination at 800 °C and then slightly decreases at higher annealing temperatures. The abrupt increase is primarily owing to the removal of luminescence quenching defects and surface species, while the subsequent decrease is mainly due to crystallite/particle growth.43 It is also observed that the 593 nm emission (5D0 → 7F1) has a longer lifetime than the 618 nm one (5D0 → 7F2) in each case, since the former arises from Eu3+ taking higher point symmetries and is parity-allowed while the latter from Eu3+ residing at less symmetric sites and is parity-forbidden. The Commission International de L'Eclairage (CIE) chromaticity coordinates were calculated to be about (0.62, 0.38) for the uncalcined powder and about (0.64, 0.36) for all the three calcined ones (Fig. S5†).
| Conditions | Uncalcined | 800 °C | 900 °C | 1000 °C |
|---|---|---|---|---|
| 593 nm (ms) | 2.71 ± 0.04 | 4.74 ± 0.06 | 4.59 ± 0.03 | 4.46 ± 0.03 |
| 618 nm (ms) | 2.53 ± 0.04 | 4.45 ± 0.05 | 4.36 ± 0.04 | 4.31 ± 0.05 |
3.4 Characterization and optical properties of doubly doped (Y0.96−xTb0.04Eux)PO4
Since only a slight increment in emission intensity was observed for the (Y0.95Eu0.05)PO4 phosphor upon raising calcination from 900 to 1000 °C, the doubly doped (Y0.96−xTb0.04Eux)PO4 phosphors were thus synthesized with the hydrothermal conditions of sample S6, followed by calcination in flowing H2 (200 ml min−1) at 900 °C for 2 h. XRD analysis (Fig. S6†) shows that the products (x = 0–0.10) all exhibit diffractions corresponding to tetragonal YPO4. The lattice constants a and c, calculated from the (200) and (101) diffractions, become steadily larger with increasing Eu3+ addition (Table S1†), confirming the formation of solid solution.We firstly studied the excitation and emission behaviors of Tb3+ in the Eu3+-free sample of (Y0.96Tb0.04)PO4 (Fig. S7†). The excitation spectrum recorded by monitoring the intra-4f8 5D4 → 7F5 transition at 546 nm (Fig. S7a†) consists of two bands in the short UV region for the spin-allowed (low-spin, LS; at ∼222 nm) and spin-forbidden (high-spin, HS; at ∼266 nm) inter-configurational 4f8 → 4f75d1 transitions, respectively.18,44 The other bands in the longer UV region of 275–400 nm represent intra-4f8 transitions of Tb3+ (Fig. S7a,† the inset). The whole excitation spectrum is dominated by the LS transition at 222 nm. The PL spectrum (Fig. S7b†) obtained under 222 nm excitation consists of sharp lines ranging from 470 to 650 nm that are associated with transitions from the excited 5D4 state to the 7FJ (J = 6–2) ground states, with the green emission at 546 nm (5D4 → 7F5) being the most prominent.
The excitation spectra of (Y0.96−xTb0.04Eux)PO4 are shown in Fig. S8a and b† for the red emission of Eu3+ at 618 nm and the green emission of Tb3+ at 546 nm, respectively. Monitoring the Eu3+ emission at 618 nm produced an overlapped excitation band in the ∼200–250 nm region due to superimposition of the LS transition of Tb3+ (∼222 nm) and the charge transfer (CT) excitation of Eu3+ (∼215 nm). Intensity of the 215 nm excitation improves monotonically with increasing Eu3+ content up to x = 0.08 and then decreases, indicating concentration quenching at x > 0.08. It is clearly seen from Fig. S8b† that the excitation spectrum of Tb3+ consists of both 4f8 → 4f75d1 and 4f–4f transitions, with the LS at ∼222 nm being the strongest in each case. The LS intensity remarkably and steadily decreases towards a higher Eu3+ content and finally becomes negligible at x = 0.10, suggesting the occurrence of efficient Tb3+ → Eu3+ energy transfer.
Since the LS band of Tb3+ and the CT band of Eu3+ significantly overlaps, we excited the (Y0.96−xTb0.04Eux)PO4 phosphors at 266 nm (the HS transition of Tb3+) to better analyze the mechanism and efficiency of Tb3+ → Eu3+ energy transfer. The obtained PL spectra are shown in Fig. 8, from which it is seen that the characteristic emissions of Tb3+ and Eu3+ emerged simultaneously for the codoped samples, but the Tb3+ emission was drastically weakened at a higher Eu3+ content (larger x, Fig. 8 and 9). This further confirms an efficient energy transfer (ET) from Tb3+ to Eu3+.20 The observed 620 nm luminescence of samples x = 0.02–0.10 is superimposed from the 618 nm emission of Eu3+ (5D0 → 7F2) and the 622 nm emission of Tb3+ (5D4 → 7F3), and the results of deconvolution are shown in Fig. 8 as inset. Luminescence properties of the phosphors are further analyzed in Fig. 9, where it is seen that the 618 nm (5D0 → 7F2) and 697 nm (5D0 → 7F4) emissions of Eu3+ similarly improve up to x = 0.08 (CTb+Eu = 12 at%) and then decrease, following the tendency observed from the excitation spectra (Fig. S8a†). The optimal total activator concentration of 12 at% found in this work is almost identical to the value determined for the (Y,Eu)PO4 binary phosphor.45,46 The red to green intensity ratios of I618/I546 and I697/I546 steadily increase towards a higher Eu3+ content, implying that the emission color can be tailored. The two intensity ratios are expected to increase further at x > 0.10 since the emission intensity of Tb3+ is approaching zero.
 | ||
| Fig. 8 Photoluminescence (PL) spectra of the (Y0.96−xTb0.04Eux)PO4 phosphors calcined at 900 °C. The inset shows deconvolution of the ∼620 nm emission by Gaussian fitting for samples x = 0.02–0.10. | ||
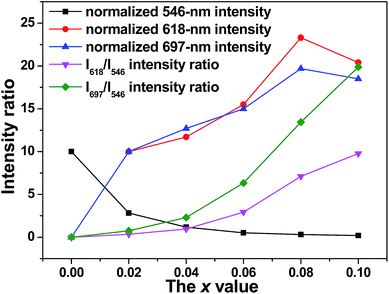 | ||
| Fig. 9 Relative emission intensities of Tb3+ (546 nm) and Eu3+ (618 and 697 nm) and the I618/I546 and I697/I546 intensity ratios, as a function of the Eu3+ content (the x value). | ||
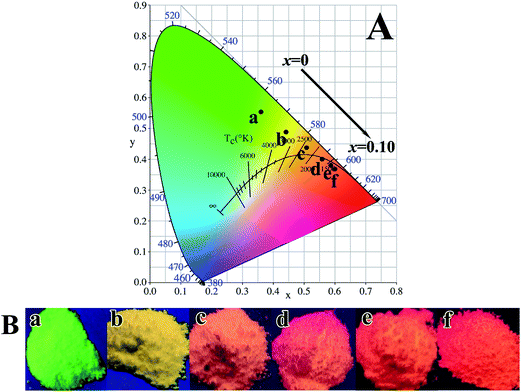
The process of Tb3+ → Eu3+ energy transfer is well studied and documented in the literature,17,20,21,45,47,48 and is schematically shown in Fig. S9.† Exciting the (Y0.96−xTb0.04Eux)PO4 phosphor at 266 nm raises electrons from the 4f8 ground state to the 4f75 d1 energy level of Tb3+, followed by relaxation to the 5D3 and then 5D4 state to yield the observed 5D4 → 7FJ (J = 3–6) emissions. Since the 5D4 state of Tb3+ lies above the 5D0,1 states of Eu3+ in the energy diagram for the excited states of rare-earth ions47,49 and the 5D4 → 7FJ Tb3+ emissions and the 7F0 → 5D0,1 Eu3+ excitations present significant spectral overlap, the resonant energy transfer from Tb3+ to Eu3+ may excite Eu3+ electrons to the 5D0,1 energy levels, and back-jumping of the excited electrons from the lowest lying 5D0 state to the 7FJ ground states (J = 1–4) then produces the observed Eu3+ red emissions.47 Emissions of the (Y0.96−xTb0.04Eux)PO4 phosphors under 266 nm excitation were calculated to have CIE chromaticity coordinates of around (0.36, 0.55) for x = 0, (0.44, 0.49) for x = 0.02, (0.51, 0.44) for x = 0.04, (0.56, 0.40) for x = 0.06, (0.59, 0.38) for x = 0.08 and (0.60, 0.37) for x = 0.10 (Fig. 10A). The emission color can thus be finely tuned from green to red via yellow with increasing x from 0 to 0.10, as also seen from the vivid multicolor luminescence of the phosphors under 254 nm irradiation from a hand-held UV lamp (Fig. 10B).
The mode of energy transfer (ET) is governed by the average separation distance (R) between the Tb3+ donor and Eu3+ acceptor, and ET may occur via either exchange interaction or electric multipole interaction. Exchange interaction generally requires an overlap of the donor and acceptor orbitals and an R value of less than 0.3–0.4 nm, or, otherwise, electric multipole interaction may dominate.50 The R value can be estimated from the following equation proposed by Blasse and Bril.51,52
 | (1) |
The energy transfer mechanism for exchange/multipolar interactions has been discussed by several authors,54–56 and can be analyzed by:
 | (2) |
 | (3) |
 | ||
| Fig. 11 Dependence of ln(IS0/IS) on C (a) and (IS0/IS) on C6/3 (b), C8/3 (c), and C10/3 (d) for the Tb3+ emission. | ||
Fluorescence decay kinetics of the 546 nm Tb3+ and 618 nm Eu3+ emissions were analyzed against the Eu3+ content (0 ≤ x ≤ 0.10) via single exponential fitting (Fig. S10 and S11†), and the derived lifetimes are summarized in Table 3. Apparently, the lifetime of Tb3+ dramatically decreased from ∼4.72 ms for x = 0 to 3.62 ms for x = 0.02, followed by slow yet steady shortening. The sudden decrease is mainly owing to Tb3+ → Eu3+ energy transfer and is frequently observed for donor/acceptor co-doped systems.57,58 The lifetime of Eu3+ emission continuously decreases with increasing x, which can be explained as follows. Raising the Eu3+ content shortens the separation distance among luminescent centers, which increases the probability of not only non-radiative Tb3+ → Eu3+ energy transfer but also radiative (resonant) energy transfer among them followed by non-radiative transfer to surface sites. This may also account for the continuous lifetime shortening of Tb3+ emission for x = 0.02–0.10.
| x | 0 | 0.02 | 0.04 | 0.06 | 0.08 | 0.10 |
| τR(Tb3+)/ms | 4.72 ± 0.02 | 3.62 ± 0.02 | 3.24 ± 0.03 | 3.11 ± 0.03 | 2.80 ± 0.06 | 2.70 ± 0.08 |
| τR(Eu3+)/ms | — | 4.21 ± 0.04 | 3.94 ± 0.05 | 3.71 ± 0.03 | 3.49 ± 0.02 | 3.18 ± 0.02 |
In the absence of concentration quenching, the efficiency (ηET) of Tb3+ → Eu3+ energy transfer can be calculated from the fluorescence lifetime with the equation
| ηET = 1 − τ/τ0 | (4) |
4. Conclusion
Red emitting (Y0.95Eu0.05)PO4 and multi-color emitting (Y0.96−xTb0.04Eux)PO4 nanophosphors have been successfully synthesized via sacrificial conversion of layered rare-earth hydroxide (LRH) nanosheets in the presence of ammonium dihydrogen phosphate (NH4H2PO4), followed by hydrothermal treatment and calcination. Under hydrothermal reaction at 150 °C and PO43−/RE3+ (RE: rare-earth) molar ratios of up to 1.5, tetragonal structured orthophosphate was suggested to crystallize via dehydration of a REPO4·4H2O intermediate formed at room temperature via dissolution of LRH. Longer hydrothermal time up to 24 h was found beneficial to the formation of monodispersed phosphate spindles. Partial crystallization of hexagonal orthophosphate was observed at the higher PO43−/RE3+ molar ratio of 2.0. Calcination up to 1000 °C did not change phase purity of the product but leads to greatly improved luminescence and altered fluorescence lifetime. With efficient Tb3+ → Eu3+ energy transfer, the emission color of (Y0.96−xTb0.04Eux)PO4 can be finely tuned from green to red by raising the content of Eu3+. The efficiency of energy transfer was assayed to be ∼40.7% at the optimal Eu3+ content of 8 at%, and the transfer was attributed to electric dipole–dipole interactions. Successively shortened fluorescence lifetime was observed for both the 546 nm green emission of Tb3+ and the 618 nm red emission of Eu3+ along with increasing Eu3+ content.Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (Grants No. 51172038, U1302272, and 51302032), the Fund of the State Key Laboratory of Advanced Technologies for Comprehensive Utilization of Platinum Metals (SKL-SPM-201505), the Special Fund for Fundamental Research in Central Universities (Grant No. N140204002), and Grant-in-Aid for Scientific Research (JSPS KAKENHI, No. 26420686).References
- F. H. Firsching and S. N. Brune, J. Chem. Eng. Data, 1991, 36, 93 CrossRef CAS.
- A. Rouanel, J. J. Serra, K. Allaf and V. P. Orlovskii, Inorg. Mater., 1981, 17, 76 Search PubMed.
- R. Kijkowska, E. Cholewka and B. Duszak, J. Mater. Sci., 2003, 38, 223 CrossRef CAS.
- S. Lucas, E. Champion, D. Bregiroux, D. Bernache-Assollant and F. Audubert, J. Solid State Chem., 2004, 177, 1302 CrossRef CAS.
- H. J. Song, L. Q. Zhou, L. Li, T. Wang, F. Hong and X. R. Luo, Mater. Sci. Eng., B, 2013, 178, 1012 CrossRef CAS.
- G. Hebbink, J. Stouwdam, D. Reinhoudt and E. Beggel, Adv. Mater., 2002, 14, 1147 CrossRef CAS.
- P. Schuetz and F. Caruso, Chem. Mater., 2002, 14, 4509 CrossRef CAS.
- M. Q. Tan, Z. W. Ye, G. L. Wang and J. L. Yuan, Chem. Mater., 2004, 16, 2494 CrossRef CAS.
- C. X. Li, Z. Y. Hou, C. M. Zhang, P. P. Yang, G. G. Li, Z. H. Xu, Y. Fan and J. Lin, Chem. Mater., 2009, 21, 4598 CrossRef CAS.
- P. Li, Y. Liu, Y. X. Guo, X. L. Shi, G. Q. Zhu and H. Q. Zuo, Ceram. Int., 2015, 41, 6621 Search PubMed.
- S. M. Lee, S. N. Cho and J. Cheon, Adv. Mater., 2003, 15, 441 CrossRef CAS.
- X. G. Peng, Adv. Mater., 2003, 15, 459 CrossRef CAS.
- Z. A. Peng and X. G. Peng, J. Am. Chem. Soc., 2001, 123, 1389 CrossRef CAS.
- Y. N. Xia, P. D. Yang, Y. G. Sun, Y. Y. Wu, B. Mayers, B. Gates, Y. D. Yin, F. Kim and H. Q. Yan, Adv. Mater., 2003, 15, 353 CrossRef CAS.
- R. X. Yan, X. M. Sun, X. Wang, Q. Peng and Y. D. Li, Chem.–Eur. J., 2005, 11, 2183 CrossRef CAS PubMed.
- Z. Y. Huo, C. Chen, D. R. Chu, H. H. Li and Y. D. Li, Chem.–Eur. J., 2007, 13, 7708 CrossRef CAS PubMed.
- J.-G. Li and Y. Sakka, Sci. Technol. Adv. Mater., 2015, 16, 014902 CrossRef PubMed.
- D. L. Geng, M. M. Shang, D. M. Yang, Y. Zhang, Z. Y. Cheng and J. Lin, J. Mater. Chem., 2012, 22, 23789 RSC.
- Q. Zhu, M. Xiong, J.-G. Li, W. G. Liu, Z. H. Wang, X. D. Li and X. D. Sun, RSC Adv., 2015, 5, 36122 RSC.
- X. L. Wu, J.-G. Li, J. K. Li, Q. Zhu, X. D. Li, X. D. Sun and Y. Sakka, Sci. Technol. Adv. Mater., 2013, 14, 015006 CrossRef PubMed.
- B. Lu, J.-G. Li, X. D. Sun and Y. Sakka, J. Am. Ceram. Soc., 2015, 98, 2480 CrossRef CAS.
- F. X. Geng, R. Z. Ma and T. Sasaki, Acc. Chem. Res., 2010, 43, 1177 CrossRef CAS PubMed.
- Q. Zhu, J.-G. Li, C. Y. Zhi, X. D. Li, X. D. Sun, Y. Sakka, D. Golberg and Y. Bando, Chem. Mater., 2010, 22, 4204 CrossRef CAS.
- L. F. Hu, R. Z. Ma, T. C. Ozawa and T. Sasaki, Chem.–Asian J., 2010, 5, 248 CrossRef CAS PubMed.
- K.-H. Lee, B. I. Lee, J. H. You and S.-H. Byeon, Chem. Commun., 2010, 46, 1461 RSC.
- Q. Zhu, J.-G. Li, X. D. Li, X. D. Sun, Y. Qi, M. Y. Zhu and Y. Sakka, Sci. Technol. Adv. Mater., 2014, 15, 014203 CrossRef PubMed.
- X. L. Wu, J.-G. Li, Q. Zhu, W. G. Liu, J. Li, X. D. Li, X. D. Sun and Y. Sakka, J. Mater. Chem. C, 2015, 3, 3428 RSC.
- F. X. Geng, Y. Matsushita, R. Z. Ma, H. Xin, M. Tanaka, N. Iyi and T. Sasaki, Inorg. Chem., 2009, 48, 6724 CrossRef CAS PubMed.
- J. A. Gadsden, Infrared Spectra of Minerals and Related Inorganic Compounds, Butterworth, Newton, 1975 Search PubMed.
- Z. L. Xiu, Z. S. Yang, M. K. Lu, S. W. Liu, H. P. Zhang and G. J. Zhou, Opt. Mater., 2006, 29, 431 CrossRef CAS.
- N. K. Sahu, R. S. Ningthoujam and D. Bahadur, J. Appl. Phys., 2012, 112, 014306 CrossRef.
- Z. H. Wang, J.-G. Li, Q. Zhu, X. D. Li and X. D. Sun, Dalton Trans., 2016 10.1039/c5dt01983d.
- S. A. Brandán, S. B. Díaz, R. C. Picot, E. A. Disalvo and A. B. Altabef, Spectrochim. Acta, Part A, 2007, 66, 1152 CrossRef PubMed.
- Z. A. Peng and X. G. Peng, J. Am. Chem. Soc., 2001, 123, 1389 CrossRef CAS.
- Z. A. Peng and X. G. Peng, J. Am. Chem. Soc., 2002, 124, 3343 CrossRef CAS PubMed.
- A. S. Vanetsev, E. V. Samsonova, O. M. Gaitko, K. Keevend, A. V. Popov, U. Mäeorg, H. Mändar, I. Sildos and Y. V. Orlovskii, J. Alloys Compd., 2015, 639, 415 CrossRef CAS.
- J.-G. Li, Q. Zhu, X. D. Li, X. D. Sun and Y. Sakka, Acta Mater., 2011, 59, 3688 CrossRef CAS.
- Q. Zhu, J.-G. Li, X. D. Li and X. D. Sun, Acta Mater., 2009, 57, 5975 CrossRef CAS.
- Q. Zhu, J.-G. Li, X. D. Li and X. D. Sun, Curr. Nanosci., 2010, 6, 496 CrossRef CAS.
- G. Blasse and B. C. Grabmaier, Lumin Mater, Springer Verlag, Berlin, Germany, 1994, p. 65 Search PubMed.
- P. C. de Sousa Filho and O. A. Serra, J. Phys. Chem. C, 2011, 115, 636 CrossRef CAS.
- Z. P. Ci, R. N. Guana, K. H. Niea, L. M. Liua, L. L. Hana, J. C. Zhanga and Y. H. Wang, Mater. Res. Bull., 2015, 70, 822 CrossRef CAS.
- Q. Zhu, J.-G. Li, R. Z. Ma, T. Sasaki, X. J. Yang, X. D. Li, X. D. Sun and Y. Sakka, J. Solid State Chem., 2012, 192, 229 CrossRef CAS.
- F. X. Geng, Y. Matsushita, R. Z. Ma, H. Xin, M. Tanaka, F. Izumi, N. Iyi and T. Sasaki, J. Am. Chem. Soc., 2008, 130, 16344 CrossRef CAS PubMed.
- W. H. Di, X. X. Zhao, S. Z. Lu, X. J. Wang and H. F. Zhao, J. Solid State Chem., 2007, 180, 2478 CrossRef CAS.
- J. J. Xiao, Y. Y. Gao, J. Zhang, Y. X. Liu and Q. B. Yang, J. Rare Earths, 2012, 30, 515 CrossRef CAS.
- G. H. Dieke and H. M. Crosswhite, Appl. Opt., 1963, 2, 675 CrossRef CAS.
- R. Q. Li, Y. Liu, N. N. Zhang, L. L. Lin, L. Liu, Y. M. Liang and S. C. Gan, J. Mater. Chem. C, 2015, 3, 3928 RSC.
- R. T. Wegh, A. Meijerink, R. J. Lamminmaki and H. Jorma, J. Lumin., 2000, 87–89, 1002 CrossRef CAS.
- D. L. Dexter and J. H. Schulman, Chem. Phys., 1954, 22, 1063 CAS.
- G. Blasse and A. Bril, J. Chem. Phys., 1969, 51, 3252 CrossRef CAS.
- G. Blasse, J. Solid State Chem., 1986, 62, 207 CrossRef CAS.
- G. K. Liu, V. V. Zhorin, S. T. Li and J. V. Beitz, J. Chem. Phys., 2000, 112, 373 CrossRef CAS.
- C. H. Huang, W. R. Liu and T. M. Chen, J. Phys. Chem. C, 2010, 114, 18698 CrossRef CAS.
- C. K. Chang and T. M. Chen, Appl. Phys. Lett., 2007, 90, 161901 CrossRef.
- W. J. Yang, L. Luo, T. M. Chen and N. S. Wang, Chem. Mater., 2005, 17, 3883 CrossRef CAS.
- W. Di, X. Wang, P. Zhu and B. Chen, J. Solid State Chem., 2007, 180, 467 CrossRef CAS.
- S. Mukherjee, V. Sudarsan, R. K. Vatsa, S. V. Godbole, R. M. Kadam, U. M. Bhatta and A. K. Tyagi, Nanotechnology, 2008, 19, 325704 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra00434b |
| This journal is © The Royal Society of Chemistry 2016 |