Enhanced total phenolic and isoflavone aglycone content, antioxidant activity and DNA damage protection of soybeans processed by solid state fermentation with Rhizopus oligosporus RT-3
Abstract
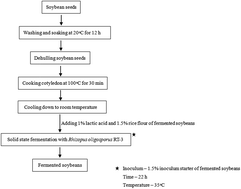
In this study, Rhizopus oligosporus RT-3, which was first isolated in our group, was used for solid state fermentation of soybeans (R. oligosporus-fermented soybeans, RFS) in a short time (22 h). The effects of fermentation on total phenolic content (TPC), isoflavone composition, antioxidant activity and DNA damage protection of soybeans were investigated. Besides, the effects of different polarity solvents in extracting antioxidant compounds were also tested. The results showed that fermentation significantly enhanced TPC and isoflavone aglycone content, antioxidant activity and DNA damage protection, but decreased the content of isoflavone glucosides. The enhanced antioxidant activity of RFS could be ascribed to the markedly higher levels of TPC and isoflavone aglycones achieved during fermentation. The study also indicated that extraction solvents had significant effects on TPC and isoflavone glucoside and aglycone content, and antioxidant activity. The water extract of RFS showed the highest TPC, ABTS˙+ and hydroxyl radical scavenging activity and chelating ability, whereas the 80% ethanol extract of RFS exhibited the strongest DPPH radical scavenging activity, reducing power and antioxidant capacity determined by a silver nanoparticle-based method. TPC and isoflavone aglycone content were highly correlated with antioxidant activity. In addition, bioaccessibility studies also demonstrated that RFS exhibited higher TPC and isoflavone aglycone bioaccessibility, as well as stronger antioxidant activity compared to non-fermented soybeans. Thus, this study demonstrated that RFS with enhanced TPC and isoflavone aglycone content, antioxidant activity and DNA damage protection which could be considered a good source of natural antioxidants in the prevention of oxidative damage-induced diseases, and it can serve as a nutraceutical and functional food/ingredient in health promotion and disease risk reduction.

 Please wait while we load your content...
Please wait while we load your content...