Glucose sensing and low-threshold field emission from MnCo2O4 nanosheets
Kusha Kumar Naika,
Ruchita T. Khareb,
Mahendra A. More*b,
Dattatray J. Late*c and
Chandra Sekhar Rout*a
aSchool of Basic Sciences, Indian Institute of Technology, Bhubaneswar 751013, Odisha, India. E-mail: csrout@iitbbs.ac.in
bCenter for Advanced Studies in Material Science and Condensed Matter Physics, Department of Physics P Pune University, Pune 411007, India. E-mail: mam@physics.unipune.ac.in
cPhysical & Materials Chemistry Division, CSIR-National Chemical Laboratory, Dr Homi Bhabha Road, Pune 411008, Maharashtra, India. E-mail: dj.late@ncl.res.in
First published on 16th March 2016
Abstract
Manganese cobalt oxide (MnCo2O4) nanosheets were grown on nickel (Ni) foam by a simple electrodeposition method. The as-synthesized nanosheets were characterized using X-ray diffraction and scanning electron microscopy. The Ni foam supports the growth of MnCo2O4 nanosheets without any aggregation, thereby increasing its catalytic and electronic properties. The electrochemical studies show that MnCo2O4 exhibits excellent electrocatalytic activity towards glucose sensing applications. The MnCo2O4 based glucose sensor shows a good sensitivity value of 8.2 μA μM−1 cm−2, with a response time of 19 s. In addition to this, field emission studies of as-synthesized MnCo2O4 reveal a low turn-on field value of 1.9 V μm−1 and good emission current stability, demonstrating MnCo2O4 nanosheets as a good field emitter material.
1. Introduction
Ternary transition metal oxides with spinel structure have attracted tremendous attention in various research fields due to their unique crystal structure and the presence of intrinsic vacant sites in the interstitial space of the crystal structure. Again, due to its great flexibility of the structure in hosting various metal ions and reciprocal substitutions between the two sub lattices promote its intrinsic material properties such as optical, electronic and catalytic properties.1,2 Furthermore, the spinel structured metal oxides are thermodynamically stable and naturally comprise of mixed-valance metal cations, so it exhibit synergetic enhancement factor in the electrochemical activities and are excellent performer in applications like lithium ion battery,3 oxygen reduction reaction,4 supercapacitor,5 photo catalytic water splitting6 and hydrogen peroxide sensor etc.7Among various electrode materials available for sensing applications, spinel ternary metal oxides have been considered as a promising, effective and scalable materials because of its excellent catalytic properties of transition metal ions with variable oxidation states, high electrical conductivity, low cost and environmental friendliness compared to single component metal oxides.8 Manganese cobalt oxide (MnCo2O4) is a spinel metal oxide with Mn2+ ions present in the tetrahedral sites, Co3+ ions in the octahedral positions and O2− ions tend to coordinate both tetrahedral and octahedral positions of a unit cell to form the face centre cubic (FCC) structure.9,10 Due to its fascinating regular arrangement, homogeneous distribution of ions in the respective lattice sites and the captivating arrangement of the unfilled and unpaired d-orbital electrons of both Mn and Co atoms in a unit cell of MnCo2O4, its electrocatalytic activities and hopping may be enhanced, which are more essential for the sensing applications.11,12 Further, beside the MnCo2O4 electrochemical properties, the synthesized material possesses vertical growth direction, fabulous morphology with interlinked nanostructure and contain large number of active edges. Thus, it is expected that the as-prepared MnCo2O4 material can act as a promising functional material.8,13
In particular, many approaches have already been explored on the development of non-enzymatic glucose sensors based on transition metal oxides.14,15 The development of highly sensitive glucose sensor is becoming important due to their applications in blood sugar detection, pharmaceutical analysis and food industry.16,17 However, still it has been a great challenge to fabricate a glucose sensor with high sensitivity, reliability with low cost and fast response time.16,18,19 Thus the development of electrochemical sensors based on novel materials with high electrocatalytic and bio-catalytic properties are one of the emerging research field.20,21
Field emission is a phenomenon where electrons tunnel from condensed matter phase to vacuum under the application of a strong electric field (∼107 V cm−1) and the emitted electrons are nearly mono-energetic. Thus field emitters can be used as an electron source in high resolution electron microscopes to fabricate flat panel displays, or to discharge spacecraft from induced charges.13 Transition metal oxides are fascinating functional materials having s-shell of the metallic positive ions are always fully filled by electrons; the d-shells of them may be not completely filled. This characteristic brings them unique properties which involve reactive electronic transition, good electrical characteristics and so on.22–25 To obtain high sensing activities and good field emission properties from the novel materials, we have attempted to create a multiple mixed-valence system based on a spinel structure with more than one metal cation, including cobalt and manganese. In this study, we demonstrate the amperometric non-enzymatic glucose sensing properties of electrodeposited MnCo2O4 thin film. The thin film of MnCo2O4 nanosheet arrays possess a sensitivity of 8.2 μA μM−1 cm−2 with a linear range value of 20–100 μM towards glucose. In addition to this, it also possesses a low turn on field value of 1.9 V μm−1 with good emission stability.
2. Experimental section
2.1 Material synthesis
Thin film of MnCo2O4 nanosheet arrays were deposited on Ni foam by chronoamperometry technique using a potentiostat/galvanostat (PG262A, Techno science instruments, Bangalore, India). At first, the glass cell and Ni foam were cleaned in ethanol–acetone mixture and then in de-ionized water (DI). In a beaker with 10 mL of DI water, 0.01 M of manganese chloride and 0.02 M of cobalt nitrate hexahydrate were added to make a clear solution. After the complete dissolution of salts, 0.02 M of potassium chloride (KCl) was added as an additional electrolyte to increase the conductivity of the solution. The three electrodes viz. Pt as a counter electrode, Ag/AgCl as a reference electrode and Ni foam as a working electrode were dipped into the solution. A potential of −1.1 V was applied for 180 s and temperature of the solution was maintained at 70 °C throughout the deposition process. At this temperature and high potential, Mn2+, Co3+ and OH− ions reacted with each other and nucleated to form MnCo2O4. Then by the driving force and diffusion process, the nucleated MnCo2O4 molecules were conglomerated on the interface of Ni foam to formed nanosheets. After that, Ni foam was washed several times in DI water to remove unreacted KCl and dried at room temperature. Further, the as-prepared sample was calcined at 500 °C for 6 hours in air. Finally, a thin film of black coloured MnCo2O4 nanosheets was obtained on the Ni foam.2.2 Material characterization
The crystalline nature of the as-synthesized MnCo2O4 was characterized by X-ray diffraction (XRD) (Bruker D8 advanced diffractometer using Cu-Kα radiation (λ = 1.54184 Å)). Morphology, composition and elemental mapping of the thin film sample were examined by FESEM (MERLIN Compact with GEMINI I electron column, Zeiss Pvt. Ltd, Germany) equipped with energy dispersive X-ray spectroscopy (EDAX). For SEM images, the sample was attached to a carbon tape then loaded to sample holder and gold sputtered to avoid the discharging of the sample. After gold sputtering, the sample was loaded and FESEM images were taken.2.3 Electrochemical measurement
To study the electrochemical sensing properties of as-synthesized MnCo2O4 nanosheets towards glucose, cyclic voltammetry (CV) and chronoamperometry (CA) experiments have been carried out in 0.1 M of NaOH solution. The CA experiments have been carried out at a constant applied voltage of 0.4 V under stirred condition.2.4 Field emission measurement
The field emission (FE) investigations of MnCo2O4 nanosheets on Ni foam were carried out in a planar diode configuration in a metal ultrahigh vacuum (UHV) chamber which was evacuated to a base pressure of ∼1 × 10−8 mbar. The vacuum chamber is equipped with a rotary back turbo molecular pump, sputter ion pump and titanium sublimation pump. In a typical arrangement, the MnCo2O4 sample was pasted on a copper stub with the help of a vacuum compatible silver paste and acts as cathode. A semi-transparent phosphor screen (diameter ∼ 50 mm) was held parallel to the cathode at a distance of 1 mm separation, which acted as an anode. The field emission current (I) vs. applied voltage (V) measurements have been carried out using Keithley 6514 electrometer and Spellman high voltage DC power supply. The stability of field emission current was investigated using a computer controlled data acquisition system with a sampling interval of 10 seconds. The field emission images were recorded using a digital camera (Canon SX150IS).3. Result and discussion
3.1 Characterizations of the MnCo2O4 nanosheets
Fig. 1(a) and (b) show the low and high magnification SEM images of the synthesized MnCo2O4 thin film. From the SEM images it is clear that the nanosheets are uniform, homogeneous, sparsely populated and well adhered to the Ni foam substrate. The nanosheets are interlinked with each other showing more stable and energetic structure which is more suitable for the biosensor and field emission applications. The thickness of the nanosheet is ∼20–40 nm and length is 1–2 μm. Fig. 1(c) shows the XRD patterns of the MnCo2O4 thin film and all the peaks are corresponding to (111), (220), (311), (400), (511), (440) and (531) planes of pure MnCo2O4 nanosheets (JCPDS-231237). No other characteristic peaks of any impurities are observed inferring that the as-prepared material possesses high purity and high crystallinity. The EDX spectrum (Fig. 1(d)), of the as-prepared thin film confirms the presence of Mn, Co and O. The elemental mapping data of MnCo2O4 shows uniform distribution of Mn, Co and O elements in the deposited material as shown in Fig. 2.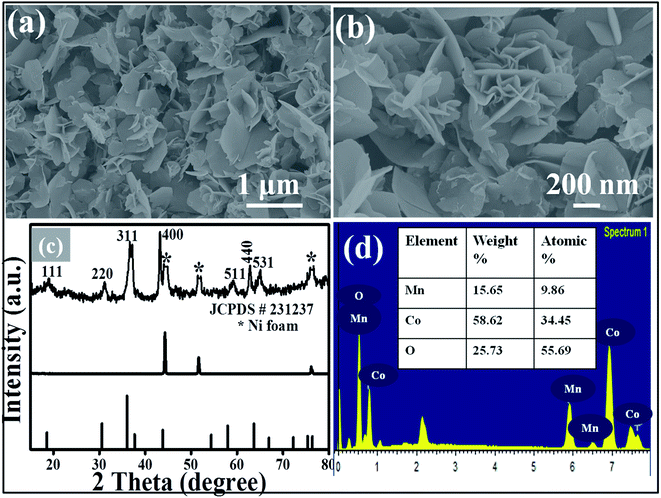 | ||
| Fig. 1 ((a) and (b)) Low and high magnification FESEM images of MnCo2O4 nanosheet arrays grown on Ni foam. (c) XRD pattern, (d) EDAX spectrograph with percentage of elements. | ||
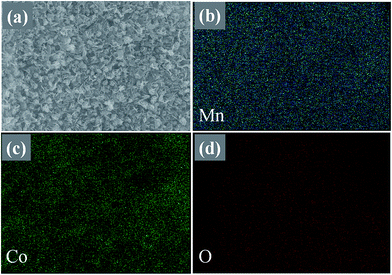 | ||
| Fig. 2 (a) SEM image of large area thin film of MnCo2O4 nanosheets, corresponding elemental mapping of the elements: (b) Mn, (c) Co and (d) O respectively. | ||
3.2 Biosensing application
To study the electrocatalytic activity of the MnCo2O4 nanosheets towards glucose molecules, CV experiments have been performed in 0.1 M of NaOH solution by applying potential range of −0.2 to 0.8 V (Fig. 3(a)). During the electrochemical cycling process, the metal ions present in the electrode surface oxidize and reduce which lead to the anodic and cathodic peaks as shown in Fig. 3(a). The anodic peak at 0.47 V and the cathodic peak at 0.26 V are assigned to MnCo2O4 electrode.5 When 100 μM of glucose molecule dropped into the solution, the semi acetal position of the hydrogen atoms of the glucose molecule detach from the mother glucose molecule and converts into gluconolactone scarifying two electrons into the solution and finally the detached glucose molecule adsorbs on the surface of the electrode. The electrochemical oxidation and reduction of Mn and Co ions species and glucose molecules occur simultaneously in the same applied potential range and the redox of adsorbent ions directly related to the redox of glucose molecules.The sensing mechanism of the glucose sensor can be understood as follows: when the MnCo2O4 electrode dipped into the solution, the OH− radical and water molecules present in the electrolytic solution attached with the electrode material and formed into manganese oxy-hydrated (MnOOH) and cobalt oxy-hydrated (CoOOH) molecules as in eqn (1).
| MnCo2O4 + OH− + H2O ↔ MnOOH + 2CoOOH + e− | (1) |
| Mn2+ + Co2+ ↔ Mn3+ + Co3+ + 2e− | (2) |
| Mn3+ + Co3+ + glucose → Mn2+ + Co2+ + gluconolactone | (3) |
| MnO + CoO + OH− − e− ↔ MnOOH + CoOOH | (4) |
The reduction of metal ions and oxidation of glucose molecules determine the response and detection of the glucose molecules as mentioned in eqn (3). The rate of reduction of metal ions and rate of oxidation of glucose molecules govern the sensing performance. Thus, the redox peak of metal ions determine the response and detection of glucose molecules.28 The amplitude of the oxidation peaks and anodic peaks current (Ip) increase respectively in each addition of glucose molecules due to more and more numbers of electrons liberate from the participated glucose molecules. The glucose detection is an anodic response and the anodic peaks of the MnCo2O4 nanosheets with each addition of glucose molecules are accounted for the amount of detection of the glucose molecules. Similar mechanisms involved for glucose sensing of other spinel metal oxides have been reported10,29,30 and we have followed the same mechanism here. Fig. 3(b) shows the change in the anodic peak current with respect to glucose concentration and it shows linear dependence.
The effect of scan rate on glucose oxidation has been investigated by performing cyclic voltammetry in 0.1 M NaOH containing 100 μM of glucose at different scan rates as in Fig. 3(c). From the data, it can be seen that the anodic and cathodic peak currents are found to be linearly proportional to the scan rates, indicating that the occurred electrochemical reaction is a diffusion-controlled process which is shown in Fig. 3(d).31,32
The CA experiment has been performed by applying the constant potential of 0.4 V and the respective data is shown in Fig. 4(a). When the glucose solution was added into the electrolyte solution, the current increased and achieved 95% of saturation at 19 s indicating the response of the fabricated sensor as shown in Fig. 4(d). The calibration graph is plotted by finding the stepwise current value against glucose concentration. The data shows that MnCo2O4 possess good linear range of detection in the range from 20–100 μM with the sensitivity of 8.2 μA μM−1 cm−2 as in Fig. 4(b). The limit of detection (LOD) of the fabricated glucose sensor is calculated by using the formula
 | (5) |
Interference study is the primary characteristics of the biosensors to know the effect of co-existing interfering species like dopamine (DA), uric acid (UA), lactic acid (LA), ascorbic acid (AA) and maltose (MA). Fig. 4(c) shows the interference data of MnCo2O4 glucose sensor. It is observed that the material possesses good anti-interference properties in the presence of other interfering species. In order to check the reproducibility of the fabricated sensor, five identical electrodes have been prepared and tested. All the sensors showed similar sensing performance with a relative standard deviation value of 5%. Table 1 shows the comparison of the performance of fabricated glucose sensor data with other reported spinel metal oxides based sensors and it is evident that MnCo2O4 nanosheets reported here possess good electrocatalytic activity towards glucose compared to other reported spinel materials.
3.3 Field emission study
In this work we have also investigated the field emission properties of MnCo2O4 nanosheets for the first time. The modified Fowler–Nordheim (F–N) equation for nanometric field emitters, in terms of the current density (J) and the applied electric field (E = V/d, where V is the voltage applied between the flat emitter cathode and the anode screen and d is the separation between cathode and anode) can be expressed as,
 | (6) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) J/E2 versus 1/E), using the following equation33,34
J/E2 versus 1/E), using the following equation33,34
 | (7) |
Fig. 5(b) shows the corresponding F–N plot. The F–N plot shows a linear behaviour with a tendency towards saturation in the high field region, indicating metallic nature of the emitter.
Furthermore, stability is an important parameter for the use of emitter in some applications. The emission current stability of MnCo2O4 nanosheets is monitored at two different preset values of emission current for nearly three hours. Fig. 5(c) shows the emission current versus time (I–t) plot for MnCo2O4 emitters at present value of 1 μA and 10 μA. The emitter exhibits good emission current stability with the emission current fluctuations within ±10% of average value. The spike type fluctuations observed in the emission current may results from the adsorption/desorption and ion bombardment of residual gas molecules.35–42 During adsorption/desorption events, the local work function of the MnCo2O4 emitter varies depending upon the nature of the adsorbent molecule (either electropositive/electronegative on the MnCo2O4 emitter surface). Also, ion bombardment of residual gas molecules due to the presence of high electrostatic field leads to mechanical damage creating and destroying the emission sites at the emitter's surface resulting in fluctuations.
4. Conclusion
In conclusion, MnCo2O4 shows excellent sensitivity towards glucose. The electrode showed the excellent sensitivity value of 8.2 μA μM−1 cm−2 with response time of 19 s. The turn-on field for MnCo2O4 at 10 μA cm−2 is found to be only 1.9 V μm−1 which is considerably lower than the reported oxide and sulphide based field emitters. Also the maximum emission current density of 563 μA cm−2 is achieved at an applied field of only 3.8 V μm−1. Thus a MnCo2O4 serves as a potential and ideal candidate for glucose sensing and field emission based applications.Acknowledgements
R. T. K. would like to thanks UGC-BSR for JRF fellowship. Dr C. S. Rout and Dr D. J. Late would like to thank DST (Government of India) for the Ramanujan fellowship. This work was supported by the DST-SERB Fast-track Young scientist (Grant No. SB/FTP/PS-065/2013), Ramanujan Fellowship research grants (Grant No. SR/S2/RJN-21/2012 and SR/S2/RJN-130/2012) and UGC-UKIERI thematic awards (Grant No. UGC-2013-14/005); NCL-MLP project grant 028626, DST-SERB Fast-track Young scientist project Grant No. SB/FT/CS-116/2013, Broad of Research in Nuclear Sciences (BRNS Grant No. 34/14/20/2015 (Government of India), partial support by INUP IITB project sponsored by DeitY, MCIT, Government of India.References
- T. R. Paudel, A. Zakutayev, S. Lany, M. d'Avezac and A. Zunger, Adv. Funct. Mater., 2011, 21, 4493–4501 CrossRef CAS
.
- F. Li, J. Liu, D. G. Evans and X. Duan, Chem. Mater., 2004, 16, 1597–1602 CrossRef CAS
.
- J. Li, S. Xiong, X. Li and Y. Qian, J. Mater. Chem., 2012, 22, 23254–23259 RSC
.
- E. Lee, J.-H. Jang and Y.-U. Kwon, J. Power Sources, 2015, 273, 735–741 CrossRef CAS
.
- Y. Xu, X. Wang, C. An, Y. Wang, L. Jiao and H. Yuan, J. Mater. Chem. A, 2014, 2, 16480–16488 CAS
.
- D. M. Robinson, Y. B. Go, M. Mui, G. Gardner, Z. Zhang, D. Mastrogiovanni, E. Garfunkel, J. Li, M. Greenblatt and G. C. Dismukes, J. Am. Chem. Soc., 2013, 135, 3494–3501 CrossRef CAS PubMed
.
- C.-C. Kuo, W.-J. Lan and C.-H. Chen, Nanoscale, 2014, 6, 334–341 RSC
.
- K. K. Naik, R. T. Khare, R. V. Gelamo, M. A. More, R. Thapa, D. J. Late and C. S. Rout, Mater. Res. Express, 2015, 2, 95011 CrossRef
.
- L. Schreyeck, A. Wlosik and H. Fuzellier, J. Mater. Chem., 2001, 11, 483–486 RSC
.
- Y. Zhang, L. Luo, Z. Zhang, Y. Ding, S. Liu, D. Deng, H. Zhao and Y. Chen, J. Mater. Chem. B, 2014, 2, 529–535 RSC
.
- Y. Zhang, S. Liu, Y. Li, D. Deng, X. Si, Y. Ding, H. He, L. Luo and Z. Wang, Biosens. Bioelectron., 2015, 66, 308–315 CrossRef CAS PubMed
.
- X. Ge, Y. Liu, F. W. T. Goh, T. S. A. Hor, Y. Zong, P. Xiao, Z. Zhang, S. H. Lim, B. Li, X. Wang and Z. Liu, ACS Appl. Mater. Interfaces, 2014, 6, 12684–12691 CAS
.
- K. K. Naik, R. Khare, D. Chakravarty, M. A. More, R. Thapa, D. J. Late and C. S. Rout, Appl. Phys. Lett., 2014, 105, 233101 CrossRef
.
- S.-W. Cheong, Nat. Mater., 2007, 6, 927–928 CrossRef CAS PubMed
.
- M. M. Rahman, A. J. S. Ahammad, J.-H. Jin, S. J. Ahn and J.-J. Lee, Sensors, 2010, 10, 4855 CrossRef CAS PubMed
.
- K. Tian, M. Prestgard and A. Tiwari, Mater. Sci. Eng., C, 2014, 41, 100–118 CrossRef CAS PubMed
.
- L.-C. Jiang and W.-D. Zhang, Biosens. Bioelectron., 2010, 25, 1402–1407 CrossRef CAS PubMed
.
- S. Park, H. Boo and T. D. Chung, Anal. Chim. Acta, 2006, 556, 46–57 CrossRef CAS PubMed
.
- Y. Zhang, Y. Wang, J. Jia and J. Wang, Sens. Actuators, B, 2012, 171–172, 580–587 CrossRef CAS
.
- C. Yuan, H. Bin Wu, Y. Xie and X. W. (David) Lou, Angew. Chem., Int. Ed., 2014, 53, 1488–1504 CrossRef CAS PubMed
.
- T. Guo, M.-S. Yao, Y.-H. Lin and C.-W. Nan, CrystEngComm, 2015, 17, 3551–3585 RSC
.
- M.-J. Lee, S. Han, S. H. Jeon, B. H. Park, B. S. Kang, S.-E. Ahn, K. H. Kim, C. B. Lee, C. J. Kim, I.-K. Yoo, D. H. Seo, X.-S. Li, J.-B. Park, J.-H. Lee and Y. Park, Nano Lett., 2009, 9, 1476–1481 CrossRef CAS PubMed
.
- M. Chhowalla, H. S. Shin, G. Eda, L.-J. Li, K. P. Loh and H. Zhang, Nat. Chem., 2013, 5, 263–275 CrossRef PubMed
.
- V. V. Sysoev, B. K. Button, K. Wepsiec, S. Dmitriev and A. Kolmakov, Nano Lett., 2006, 6, 1584–1588 CrossRef CAS PubMed
.
- C. N. R. Rao, Annu. Rev. Phys. Chem., 1989, 40, 291–326 CrossRef CAS
.
- S.-H. Liao, S.-Y. Lu, S.-J. Bao, Y.-N. Yu and M.-Q. Wang, Anal. Chim. Acta, 2016, 905, 72–78 CrossRef CAS PubMed
.
- Z. Shahnavaz, F. Lorestani, W. P. Meng and Y. Alias, J. Solid State Electrochem., 2015, 19, 1223–1233 CrossRef CAS
.
- K. K. Naik, S. Kumar and C. S. Rout, RSC Adv., 2015, 5, 74585–74591 RSC
.
- J. Yang, M. Cho and Y. Lee, Biosens. Bioelectron., 2016, 75, 15–22 CrossRef CAS PubMed
.
- Z. Yu, H. Li, X. Zhang, N. Liu, W. Tan, X. Zhang and L. Zhang, Biosens. Bioelectron., 2016, 75, 161–165 CrossRef CAS PubMed
.
- K. K. Naik and C. S. Rout, RSC Adv., 2015, 5, 79397–79404 RSC
.
- R. K. Srivastava, S. Srivastava, T. N. Narayanan, B. D. Mahlotra, R. Vajtai, P. M. Ajayan and A. Srivastava, ACS Nano, 2012, 6, 168–175 CrossRef CAS PubMed
.
- R. H. Fowler and L. Nordheim, Proc. R. Soc. London, Ser. A, 1928, 119, 173–181 CrossRef CAS
.
- G. N. Fursey, Field emission in vacuum microelectronics, Springer Science & Business Media, 2007 Search PubMed
.
- R. T. Khare, R. V. Gelamo, M. A. More, D. J. Late and C. S. Rout, Appl. Phys. Lett., 2015, 107, 123503 CrossRef
.
- M. B. Erande, S. R. Suryawanshi, M. A. More and D. J. Late, Eur. J. Inorg. Chem., 2015, 2015, 3102–3107 CrossRef CAS
.
- R. B. Sharma, D. J. Late, D. S. Joag, A. Govindaraj and C. N. R. Rao, Chem. Phys. Lett., 2006, 428, 102–108 CrossRef CAS
.
- D. J. Late, M. A. More, D. S. Joag, P. Misra, B. N. Singh and L. M. Kukreja, Appl. Phys. Lett., 2006, 89, 123510 CrossRef
.
- D. J. Late, B. Liu, H. S. S. R. Matte, V. P. Dravid and C. N. R. Rao, ACS Nano, 2012, 6, 5635–5641 CrossRef CAS PubMed
.
- D. J. Late, Y.-K. Huang, B. Liu, J. Acharya, S. N. Shirodkar, J. Luo, A. Yan, D. Charles, U. V. Waghmare, V. P. Dravid and C. N. R. Rao, ACS Nano, 2013, 7, 4879–4891 CrossRef CAS PubMed
.
- C. S. Rout, P. D. Joshi, R. V. Kashid, D. S. Joag, M. A. More, A. J. Simbeck, M. Washington, S. K. Nayak and D. J. Late, Sci. Rep., 2013, 3, 3282 Search PubMed
.
- R. V. Kashid, D. J. Late, S. S. Chou, Y.-K. Huang, M. De, D. S. Joag, M. A. More and V. P. Dravid, Small, 2013, 9, 2730–2734 CrossRef CAS PubMed
.
- S. Ratha, R. T. Khare, M. A. More, R. Thapa, D. J. Late and C. S. Rout, RSC Adv., 2015, 5, 5372–5378 RSC
.
| This journal is © The Royal Society of Chemistry 2016 |



