Phenolic metabolites from mangrove-associated Penicillium pinophilum fungus with lipid-lowering effects†
Chongming Wua,
Yang Zhaob,
Ran Chena,
Dong Liub,
Mingyue Liua,
Peter Prokschc,
Peng Guo*a and
Wenhan Lin*b
aPharmacology and Toxicology Research Center, Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing 100193, P. R. China. E-mail: pguo@implad.ac.cn; Fax: +86-10-57833235; Tel: +86-10-57833235
bState Key Laboratory of Natural and Biomimetic Drugs, Peking University, Beijing 100191, P. R. China. E-mail: whlin@bjmu.edu.cn; Fax: +86-10-82802724; Tel: +86-10-82806188
cInstitute für Pharmazeutische Biologie und Biotechnologie, Heinrich-Heine-Universität Düsseldorf, Universitätsstr. 1, Geb.26.23, 40225 Düsseldorf, Germany
First published on 17th February 2016
Abstract
Chemical examination of the mangrove-associated fungus Penicillium pinophilum (H608) resulted in the isolation of 16 phenolic metabolites, including a new metabolite, namely 5′-hydroxypenicillide (1). The structure of the new compound was determined by extensive spectroscopic analyses, in association with the Mosher method for configurational assignment. All compounds were tested for inhibitory effects against oleic acid (OA)-elicited lipid accumulation in HepG2 cells, while eight compounds (4, 7–8, and 11–15) exhibited inhibition toward lipid accumulation at a dose of 10 μM with no cytotoxic effect. Further investigation revealed six compounds (4, and 11–15) that significantly suppressed intracellular total cholesterol (TC) and triglycerides (TGs). A real-time quantitative PCR indicated that compounds 4, 11, and 13–15 dramatically decreased the expression of fatty acid synthase (FAS), acetyl-CoA carboxylase (ACC) and 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) in association with up-regulation of carnitinepalmitoyl transferase-1 (CPT-1). In addition, seven compounds (4, 8, 11, and 13–16) significantly reduced oxidized low-density lipoprotein stimulated lipid accumulation in RAW264.7 cells. Mechanistic study revealed that compounds 14–16 remarkably decreased CD36 and SR-1 transcription, while compounds 4 and 15 dramatically up-regulated PPARγ, LXRα and ABCG1 to promote cholesterol efflux. This work provided a group of new chemical entities as promising leads for the development of hypolipidemic and anti-atherosclerotic agents.
1. Introduction
Hyperlipidemia is known as abnormally elevated levels of lipids (high cholesterol and/or triglyceride levels) and/or lipoproteins in the blood, which raise the risk of cardiovascular (atherosclerosis and coronary heart diseases) and heart diseases.1–5 In addition, hyperlipidemia also aggravates other pathological conditions such as hypothyroidism and chronic kidney dysfunction.6 On the other hand, atherosclerosis,7 a progressive disease characterized by the formation and accumulation of lipid plaques in the arteries and by inflammatory responses, results in insufficient blood supply to organs and tissues, that induces death of cardiovascular disease.8,9 Low-density lipoprotein (LDL) plays a key role for the accumulation of extracellular and intracellular lipids in the arterial intima to develop atherogenesis.10,11 Foam cell formation is a main determinant of atherosclerotic lesions, in which macrophages express scavenger receptors on their plasma membranes and uptake oxidized LDL.12 Foam cells also secrete various inflammatory cytokines to accelerate the development of atherosclerosis. For instance, scavenger receptors CD36, SR-A1 and SR-A2 bind to and uptake excess oxLDL into macrophages,13 leading to the accumulation of excess cholesterol, which is toxic to cells. ATP-binding cassette (ABC) transporters (ABCA1 and ABCG1) induce the reverse cholesterol transport (RCT) pathway by mediating the translocation of cholesterol across cellular bilayer membranes.14–16 ABCA1 promotes the efflux of cholesterol to lipid-poor apolipoproteins such as apolipoprotein A1 (apoA1), while ABCG1 mediates cholesterol efflux to high-density lipoprotein (HDL).17–19 The expression of ABCA1 and ABCG1 is regulated by proliferator-activated receptor gamma (PPARγ)-dependent and liver X receptor alpha (LXRα)-dependent pathways, respectively.20,21 The marketed lipid-lowering agents with beneficial therapeutic effects are mainly classified into statins and fibrates,22 while rosiglitazone is used for the treatment of atherogenesis through the stimulation of cholesterol efflux by up-regulating ABCA1 to prevent foam cell formation. Among the statin derivatives, compactin (mevastatin) is the first fungal metabolite with a PKS-based scaffold to be isolated from the fungi Penicillium citrinum and P. brevicompactum, and it performed as a specific inhibitor of HMG-CoA reductase being highly effective in lowering plasma cholesterol levels in animals and men.23 The fungal product lovastatin, (mevinoline) structurally related to compactin as isolated from the fungus A. terreus, was the first marketed statin drug.24 Microbial transformation of compactin results in the lactone ring opening to form pravastatin, which shows a reduction in side effects compared with lovastatin and simvastatin.25 Thus, natural products are an excellent strategy for developing future effective and safe hypolipidemic and anti-atherosclerotic drugs.26 With the aim of discovering new bioactive natural products with lipid-lowering effects from marine-derived microorganisms, a cell model-based bioassay was performed. The results demonstrated that a mangrove soil-derived fungus Penicillium pinophilum can reduce lipid accumulation. Chromatographic separation of an active lipid-lowering ethyl acetate (EtOAc) fraction from the fungus led to isolation of 16 phenolic compounds (Fig. 1). In this paper, we report the inhibitory effects of the phenolic analogues on the lipid-lowering effects and oxLDL-induced foam cell formation, in addition to the potential mechanisms in RAW264.7 macrophages.2. Experimental
2.1. General procedures
Optical rotations were measured by an Autopol III automatic polarimeter (Rudolph Research Co., Ltd.). IR spectra were measured on a Thermo Nicolet Nexus 470 FT-IR spectrometer. The 1H and 13C NMR spectra were recorded on a Bruker Avance-400FT NMR spectrometer using TMS as an internal standard. HRESIMS spectra were obtained on a Bruker APEX IV 70 eV FT-MS spectrometer and on a Thermo DFS spectrometer using a matrix of 3-nitrobenzyl alcohol. EIMS (70 eV) were recorded on a Finnigan MAT 95 mass spectrometer. Column chromatography was carried out using silica gel (160–200, 200–300 mesh), and HF254 silica gel for TLC was obtained from Qingdao Marine Chemistry Co. Ltd. Sephadex LH-20 (18–110 μm) from Pharmacia. HPLC was performed on an Alltech 426 pump employing an UV detector, and the prevail C18 column (5 μm) was used for semi-preparative HPLC separation. A chiral-phase column (Phenomenex Lux, cellulose-2, 250 × 10 mm, 5 μm) was used for chiral analysis. 25-[N-[(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)-methyl] amino]-27-norcholesterol (25-NBD cholesterol), MTT, digitonin, simvastatin, rosiglitazone, Oil Red O and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). An intracellular cholesterol assay kit was purchased from Jian Cheng Biotechnology Company (Nanjing, China). Human oxLDL, ApoA1 and HDL were obtained from Yiyuan Biotechnologies (Guangzhou, China). A total RNA extraction reagent (RNAiso Plus), a Prime Script RT reagent kit, and a SYBR-Green PCR kit were purchased from Transgene Biotech, Inc. (Beijing, China). A luciferase assay kit was purchased from Promega Inc. (Beijing, China).2.2. Fungal strain and identification
The fungus Penicillium pinophilum (H608) was isolated from the mangrove sediment, which was collected from the Xiamen coastline, in May 2012. The strain was identified by comparing the morphological character and 18S rDNA (ITS) sequence with those of standard records. The morphological examination was performed by scrutinizing the fungal culture, the mechanism of spore production, and the characteristics of the spores. For inducing sporulation, the fungal strains were separately inoculated onto potato dextrose agar. All experiments and observations were repeated at least twice leading to the identification of the strain H608 as Penicillium pinophilum. The strain H608 was deposited at the State Key Laboratory of Natural and Biomimetic Drugs, Peking University, China, with the GenBank (NCBI) accession number KP901304.2.3. Fermentation
The fermentation was carried out in 30 Fernbach flasks (500 mL), each containing 100 g of rice. Distilled H2O (100 mL) was added to each flask, and the contents was soaked overnight before autoclaving at 15 psi for 30 min. Spore inoculum was prepared by suspending the seed culture in sterile, distilled H2O to give a final spore/cell suspension of 1 × 106 per mL. After cooling to room temperature, each flask was inoculated with 5.0 mL of the spore inoculum and incubated at 25 °C for 40 days.2.4. Extraction and isolation
The fermented material was extracted successively with EtOAc (3 × 500 mL). The EtOAc extract was evaporated to dryness under vacuum to afford a crude residue (11.7 g), which was then subjected to silica gel (200–300 mesh) vacuum column chromatography, eluting with PE/EtOAc (from 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 0
1 to 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, gradient) to obtain six fractions (F1 to F6). The 1H NMR spectra informed that the fraction F4 contains the components featured by phenolic compounds. Thus, fraction F4 (3.0 g) was chromatographed over C18 gel (ODS, MeOH/H2O = 3
1, gradient) to obtain six fractions (F1 to F6). The 1H NMR spectra informed that the fraction F4 contains the components featured by phenolic compounds. Thus, fraction F4 (3.0 g) was chromatographed over C18 gel (ODS, MeOH/H2O = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain four subfractions (SF4a–SF4d). SF4c (1.43 g) was purified on a silica gel column using hexane/acetone = 5
1) to obtain four subfractions (SF4a–SF4d). SF4c (1.43 g) was purified on a silica gel column using hexane/acetone = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 as an elutant to obtain 1 (18.0 mg), 2 (24.5 mg), 3 (7.6 mg), 4 (18.2 mg), 5 (8.7 mg), and 6 (24.8 mg). SF4b (310 mg) was subjected to RP-HPLC with a mobile phase of MeCN/H2O = 2
2 as an elutant to obtain 1 (18.0 mg), 2 (24.5 mg), 3 (7.6 mg), 4 (18.2 mg), 5 (8.7 mg), and 6 (24.8 mg). SF4b (310 mg) was subjected to RP-HPLC with a mobile phase of MeCN/H2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (2 mL min−1) to yield 16 (17.6 mg), 15 (7.8 mg), and 14 (23.5 mg). SF4d (110 mg) was subjected to RP-HPLC with a mobile phase of MeCN/H2O = 3
1 (2 mL min−1) to yield 16 (17.6 mg), 15 (7.8 mg), and 14 (23.5 mg). SF4d (110 mg) was subjected to RP-HPLC with a mobile phase of MeCN/H2O = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (2 mL min−1) to yield 7 (27.1 mg), 8 (17.2 mg), 9 (13.5 mg), 10 (11.7 mg), 11 (8.7 mg), 12 (6.7 mg), and 13 (18.3 mg).
1 (2 mL min−1) to yield 7 (27.1 mg), 8 (17.2 mg), 9 (13.5 mg), 10 (11.7 mg), 11 (8.7 mg), 12 (6.7 mg), and 13 (18.3 mg).
5′-Hydroxypenicillide (1): white solid. [α]25D −27.5 (c 0.05, MeOH); UV (MeOH) λmax (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 217 (1.74), 280 (1.12) nm; IR (KBr) νmax 3388, 2958, 1732, 1598, 1468, 1357, 1294, 1206 cm−1; for 1H and 13C NMR data, see Table 1; HRESIMS m/z 389.1594 [M + H]+ (calcd for C21H25O7, 389.1600).
ε) 217 (1.74), 280 (1.12) nm; IR (KBr) νmax 3388, 2958, 1732, 1598, 1468, 1357, 1294, 1206 cm−1; for 1H and 13C NMR data, see Table 1; HRESIMS m/z 389.1594 [M + H]+ (calcd for C21H25O7, 389.1600).
| No. | 1 | |
|---|---|---|
| δH (ppm, J in Hz) | δC (ppm) | |
| a Measured in DMSO-d6 at 400 MHz for 1H and 100 MHz for 13C. | ||
| 1 | 6.96, d (8.4) | 118.0 CH |
| 2 | 7.65, d (8.4) | 131.6 CH |
| 3 | 134.4 C | |
| 4 | 153.7 C | |
| 4a | 119.5 C | |
| 5 | 167.8 C | |
| 7 | 5.08, s | 69.1 CH2 |
| 7a | 127.5 C | |
| 8 | 6.34, d (1.6) | 120.3 CH |
| 9 | 138.7 C | |
| 10 | 6.77, d (1.6) | 118.7 CH |
| 11 | 148.9 C | |
| 11a | 142.2 C | |
| 1a | 151.4 C | |
| 13 | 2.16, s | 20.8 C |
| 1′ | 4.93, dd (3.0, 8.6) | 64.3 CH |
| 2′ | 1.21, m; 1.60, m | 43.2 CH2 |
| 3′ | 1.78, m | 33.8 CH |
| 4′ | 0.90, d (6.7) | 22.1 CH3 |
| 5′ | 4.36, d (6.0) | 69.4 CH2 |
| OMe | 3.80, s | 62.4 CH3 |
| 11-OH | 9.68, s | |
2.5. MPA esterification and 9-AMA esterification of 1
Compound 1 (4 mg, 0.01 mmol) was dissolved in dimethyl carbonate (4 mL), then DBU (0.6 mmol) was added. The solution was kept at 90 °C under magnetic stirring and monitored by TLC. After disappearance of 1, the solvent was evaporated under reduced pressure. The residue was solubilized with ethyl acetate (10 mL) and treated with a solution of 1 N HCl (5 mL). The final products were extracted with ethyl acetate (3 × 10 mL), and the reunited organic extracts were washed with a saturated solution of NaCl and dried over Na2SO4. After filtration and evaporation of the solvent, the methylated compound was purified by chromatography on a column using silica gel (160–200 mesh) eluting with CH2Cl2/CH3OH (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to yield 11-methylated 1 (3.8 mg).
2) to yield 11-methylated 1 (3.8 mg).
Both (R)- and (S)-MPA esters of 11-methylated 1 were obtained by the treatment of 11-methylated 1 (0.9 mg, respectively) with (R)- and (S)-MPA (3.1 mg), and dicyclohexylcarbodiimide (3.9 mg) in dry CDCl3 (0.6 mL) catalyzed with dimethylaminopyridine (2.32 mg) and stirred at RT overnight. The MPA esters, 1a (1.4 mg) and 1b (1.6 mg), were purified by semi-preparative HPLC using MeCN (100%) as a mobile phase.
Esters 1c and 1d were prepared separately by treatment of 11-methylated 1 (1 mg) with the corresponding (R)- and (S)-9-AMA acids (1.1 equiv.) in the presence of EDC (1.1 equiv.) and DMAP in dry CH2Cl2, under a N2 atmosphere. The reaction was stirred at room temperature for 12 h. The organic layer was washed sequentially with H2O, HCl (1 M), H2O, NaHCO3 (sat), and H2O, then dried (Na2SO4) and concentrated under reduced pressure to obtain the corresponding ester. Final purification was achieved by column chromatography on silica gel 160–200 mesh eluting with hexane–EtOAc (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to yield 1c (1.2 mg) and 1d (1.5 mg).
1) to yield 1c (1.2 mg) and 1d (1.5 mg).
2.6. HepG2 cell culture
HepG2 cells, which originated from the American Type Culture Collection (ATCC) (Manassas, VA, USA) and obtained from the Peking Union Medical College, were maintained in DMEM containing 10% fetal bovine serum (FBS) at 37 °C and 5% CO2. Before treatment, cells were kept in serum-free DMEM for 12 h then incubated with the indicated concentration of SN12 or with simvastatin (10 μM) in DMEM containing oleic acid (100 nM) for 24 h. The blank group was incubated with serum-free DMEM alone. Oil red O staining was performed as previously reported and the intracellular contents of the total lipid, total cholesterol and triglyceride were determined by kits according to the manufacturer’s instructions.2.7. Oleic acid (OA)-elicited lipid accumulation
HepG2 liver cells were maintained in DMEM supplemented with penicillin/streptomycin (100 μg mL−1) and 10% fetal bovine serum. The cells with 70–80% confluence were incubated in DMEM/oleic acid (100 μM) for 12 h and then were treated with the compounds (each, 10 μM) and the positive control simvastatin was in DMEM/100 μM oleic acid with DMEM/100 μM oleic acid as a blank for an additional 6 h. Subsequently, the cells were subjected to Oil Red O staining or TC and TG determination as described previously.42 Each experiment (n = 8 for Oil Red O staining or n = 3 for TC and TG determination) was repeated in triplicate.2.8. oxLDL-induced foam cell formation
RAW264.7 cells were maintained in DMEM supplemented with penicillin/streptomycin (100 μg mL−1) and 10% fetal bovine serum. The cells with 70–80% confluence were incubated with DMEM + oxLDL (50 mg mL−1) and individual compound (each, 10 μM) or the positive control rosiglitazone (10 μM) for 12 h. Subsequently, the cells were subjected to Oil Red O staining, photography and TC determination.2.9. Cholesterol uptake assay
Cholesterol uptake assays were performed using 25-NBD cholesterol in RAW264.7 macrophages. The cells were plated in 96-well clear-bottom black plates at 4 × 104 cells per well. Six hours later, the medium was removed, and the cells were labeled with 25-NBD cholesterol (5 μg mL−1) in aliquots of serum-free DMEM individually containing 10 μM of each of the experimental compounds or an equal volume of DMSO for the indicated time. Then, the cells were washed twice with phosphate buffered saline (PBS), and the amounts of cholesterol in the cells were measured using a Tecan Infinite M1000Pro Microplate Reader (TECAN Group Ltd., Shanghai, China; excitation 485 nm, emission 535 nm). Each uptake assay was performed in duplicate in three experiments.2.10. Cholesterol efflux assay
RAW264.7 cells were equilibrated with NBD-cholesterol (1 μg mL−1) for 12 h. The NBD-cholesterol labeled cells were washed with PBS and incubated in serum-free DMEM containing 50 μg mL−1 HDL and 10 μM of each experimental compound individually for 6 h. Fluorescence-labeled cholesterol released from the cells into the medium was measured with a Tecan Infinite M1000Pro Microplate Reader (TECAN Group Ltd., Shanghai, China). Cholesterol efflux was expressed as a percentage of fluorescence in the medium relative to the total amount of fluorescence detected in the cells and the medium. Each experiment was performed in triplicate with 3 replicates each time.2.11. Quantitative real-time PCR
Total RNA extraction, cDNA synthesis, and quantitative PCR assays were performed as described previously.43 Samples were cycled 40 times using a Fast ABI-7500 sequence detector (Applied Biosystems). ABI-7500 cycle conditions were conducted for 5 min at 95 °C, and were followed by 40 cycles of 15 s at 95 °C, 30 s at 60 °C, and 30 s at 72 °C. The cycle threshold (CT) was calculated under default settings for real-time sequence detection software (Applied Biosystems). At least three independent biological replicates were performed to check the reproducibility of the data. The gene-specific primers used for quantitative PCR are listed in Table 2.| Name | Forward (5′-3′) | Reverse (5′-3′) | |
|---|---|---|---|
| For HepG2 | FAS | CGGTACGCGACGGCTGCCTG | GCTGCTCCACGAACTCAAACACCG |
| ACC | TGATGTCAATCTCCCCGCAGC | TTGCTTCTTCTCTGTTTTCTCCCC | |
| HMGR | GGACCCCTTTGCTTAGATGAAA | CCACCAAGACCTATTGCTCTG | |
| CPT-1 | CGTCTTTTGGGATCCACGATT | TGTGCTGGATGGTGTCTGTCTC | |
| β-Actin | CCTGGCACCCAGCACAAT | GCCGATCCACACACGGAGTACT | |
| For RAW264.7 | PPARγ | GCAGCTACTGCATGTGATCAAGA | GTCAGCGGGTGGGACTTTC |
| LXRα | AGGAGTGTCGACTTCGCAAA | CTCTTCTTGCCGCTTCAGTTT | |
| ABCG1 | CAAGACCCTTTTGAAAGGGATCTC | GCCAGAATATTCATGAGTGTGGAC | |
| CD36 | CAAGCTCCTTGGCATGGTAGA | TGGATTTGCAAGCACAATATGAA | |
| SR-1 | TTAAAGGTGATCGGGGACAAA | CAACCAGTCGAACTGTCTTAAG | |
| β-Actin | ACACTGTGCCCATCTACGAG | CAGCACTGTGTTGGCATAGAG |
2.12. Western blot
HepG2 cells were lysed in lysis buffer containing 10% glycerol, 1% Triton X-100, 135 mM NaCl, 20 mM Tris (pH 8.0), 2.7 mM KCl, 1 mM MgCl2, and protease and phosphatase inhibitors (0.5 mM PMSF, 2 μM pepstatin, and 2 μM okadaic acid). Aliquots of samples were subjected to SDS-PAGE followed by transfer to polyvinylidenedifluoride (PVDF) membranes. Immunoblotting was performed using respective antibodies (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000). Following incubation with horseradish peroxidase-conjugated secondary antibody, proteins were detected with ECL plus kits.
1000). Following incubation with horseradish peroxidase-conjugated secondary antibody, proteins were detected with ECL plus kits.
2.13. Measurement of PPARγ promoter activity
A transactivation reporter assay in 293T cells was performed. Briefly, cells were transiently transfected with a PPARγ expression vector and a DR-1 luciferase reporter vector. At 6 h after transfection, the transfection mixture was replaced with fresh medium containing the appropriate agonist. Luciferase assays were performed after 24 h using a luciferase assay kit according to the manufacturer’s instructions.2.14. Cell viability assay
Cell viability was examined using an MTT assay. RAW264.7 macrophages in 96-well culture plates were treated with compounds with 50 μM digitonin as a cytotoxic control. The cells were incubated for 12 h, and MTT reagent (5 mg mL−1) was added to each well. After 2 h, the medium was removed and cells were lysed in 200 μL of DMSO. The absorbance at 565 nm was measured using a microplate reader (TECAN Group Ltd., Shanghai, China).2.15. Statistical analyses
The data are presented as the mean ± SEM. Differences were assessed by one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test. A probability level (p) of 0.05 was considered significant. SPSS 17.0 for Windows (SPSS, Chicago, IL, USA) was used for statistical analysis.3. Results and discussion
3.1. Structural elucidation
Compound 1 had a molecular formula of C21H24O7, as determined by HRESIMS (m/z 389.1594 [M + H]+, calcd 389.1600) and NMR data, requiring ten degrees of unsaturation. The 1H NMR spectrum exhibited resonances for three methyl groups, four aromatic protons belonging to meta- and ortho-spin systems, and a number of alkyl protons. The 13C NMR spectrum provided a total of 21 carbon resonances (Table 1), including 12 aromatic carbons for two phenyl rings and a carbonyl carbon. Analyses of 2D NMR (COSY, HMQC and HMBC) data established the basic skeleton of 1 to be a diphenyl ether lactone, closely related to the structure of penicillide.27 The distinction was only attributed to the substitution at the isopentyl side chain, in which a hydroxyl group at C-5′ (δC 69.4) was assigned by the HMBC correlations from H3-4′ (δH 0.90, d) to C-3′ (δC 33.8), C-2′ (δC 43.2) and C-5′, in addition to the COSY correlation from H-3′ (δH 1.78, m) to H2-5′ (δH 4.36), H3-4′ and H2-2′. The configuration of C-1′ was determined on the basis of the revised Mosher method.28 Firstly, the phenolic group at C-11 was protected by methylation with dimethyl carbonate (DMC),29 and then the esterification of 11-methylated 1 with (R)-MPA and (S)-MPA was accomplished. Calculation of the Δδ (δR − δS) data of the (R)- and (S)-MPA esters of 11-methylated 1 (Fig. 2) caused the configuration of C-1′ to be S. In addition, the absolute configuration at C-3′ was established according to the methodology reported by Riguera and coworkers for determining the absolute configuration of β-chiral primary alcohols.30 11-Methylated 1 was reacted with (R)- and (S)-2-(anthracen-9-yl)-2-methoxyacetic acid (9-AMA) to form 9-AMA esters. Calculation of Δδ (δR − δS) values obtained from the R- and S-ester derivatives 1c and 1d led to an S configuration assigned at C-3′. Thus, the structure of 1 was assigned to 5′-hydroxypenicillide.Inspection of spectroscopic data and the comparison of the NMR data and specific rotation resulted in the structures of 15 known phenolic metabolites to be found to be identical to penicillide (2),27 isopenicillide (3),31 dehydroisopenicillide (4),27 1-dehydroxypencillide (5),27 vermixocin B (6),32 methyl tenellate (7),33 secopenicillide A (8),34 talaromycin C (9),35 deacetyl talaromycin C (10),35 deoxyfunicone (11),36 funicone (12),37 3-O-methylfunicone (13),38 vermistatin (14),39 hydroxyvermistatin (15),40 and methoxyvermistatin (16).41 Based on their scaffolds, these compounds are classified as penicillide-type, tenellic acid-type, funicone-type, and vermistatin-type.
During the separation process, a spirolactone purpactin C41 was isolated as an unstable component, which was able to convert to secopenicillide A (8). The penicillide-type analogues such as vermixocin B (6) were likely derived from 8 via reduction of the aldehydic group to form an alcohol intermediate, and then esterification occurred. In addition, the structural relationship of the remaining diphenyl ethers was depicted by the occurrence of deacetylation, hydroxylation, methylation, and dehydration (Fig. 3).
3.2. Pharmacological activity
 | ||
| Fig. 5 Compounds inhibit lipid accumulation in oleic acid (OA)-elicited HepG2 cells. (A) and (B) Intracellular levels of total cholesterol (TC) and triglycerides (TGs). | ||
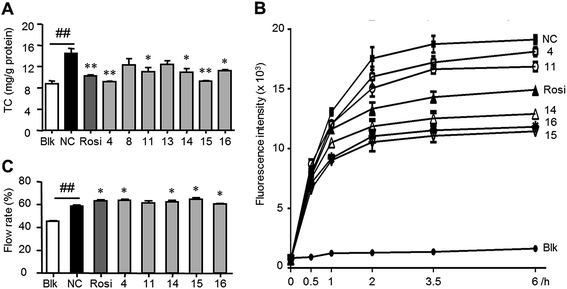
Lipid dysregulation is a key factor to induce atherosclerosis, a major risk of cardiovascular disease (peripheral arterial disease, coronary heart disease, stroke, and heart attack). Macrophage-derived foam cells are a major constituent of the fatty deposits characterizing the disease atherosclerosis. Foam cells are formed when certain immune cells (macrophages) take on oxidized low-density lipoproteins (oxLDL) through failed phagocytosis. High-density lipoproteins (HDL) are known to have a number of anti-atherogenic effects. One of these stems from their ability to remove excess cellular cholesterol for processing in the liver, a process called reverse cholesterol transport (RCT). HDL induced macrophage RCT by forming foam cells and removing excess lipids by efflux transporters.
4. Conclusions
In summary, this is the first report of diphenyl ethers and related natural products, which were potent for lipid-lowering effects and inhibition of foam cell formation. These findings suggested them to be potential leads against hyperlipidemia and atherosclerosis. Mechanistic study revealed the inhibition of intracellular total cholesterol (TC) levels and intracellular triglycerides (TGs) of compounds 4, 11, and 13–15 related to down-regulation of fatty acid synthase (FAS), acetyl-CoA carboxylase (ACC) and 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) and the up-regulation of the lipid catabolic gene carnitinepalmitoyl transferase-1 (CPT-1). The suppression of oxLDL-induced foam cell formation by compounds 4, 11, and 14–16 via inhibiting cholesterol influx was induced by the down-regulation of CD36 and SR-1 or promotion of cholesterol efflux by up-regulation of PPARγ, LXRα and ABCG1. The present work shows a group of new chemical entities to be promising for the development of anti-hyperlipidemic and anti-atherosclerosis agents.Acknowledgements
This work was supported by the National Basic Research Program 973 (2015CB755906), the NSFC-Shangdong Joint Fund for Marine Science (U1406402), the National Hi-Tech863-Projects (2011AA090701, 2013AA092902), COMRA (DY125-15-T-01), and National Natural Science Foundation of China (41376127, 81573436).Notes and references
- K. M. Patel, A. Strong, J. Tohyama, X. Jin, C. R. Morales, J. Billheimer, J. Millar, H. Kruth and D. J. Rader, Circ. Res., 2015, 116, 789–796 CrossRef CAS PubMed.
- J. S. Kishor, M. K. Kathivarin and S. Rahul, Bioorg. Med. Chem., 2007, 15, 4674–4699 CrossRef PubMed.
- S. M. Grundy, G. J. Balady and M. H. Criqui, Circulation, 1998, 97, 1876–1887 CrossRef CAS PubMed.
- W. F. Keane, J. Peter and B. L. Kasiske, Kidney Int. Suppl., 1992, 38, S134–S141 CAS.
- M. J. Veerkamp, J. Graaf, S. J. H. Bredie, J. C. M Hendriks, P. N. M. Demacker and A. F. H Stalenhoef, Arterioscler., Thromb., Vasc. Biol., 2002, 22, 274–282 CrossRef CAS.
- R. L. Satarasinghe, R. Ramesh, A. A. A. Riyaaz, P. A. K. G. Gunarathne and A. P. Silva, Drug Metab. Drug Interact., 2007, 22, 279–283 CAS.
- L. Fernandez-Friera, J. L. Penalvo, A. Fernandez-Ortiz, B. Ibanez, B. Lopez-Melgar, M. Laclaustra, B. Oliva, A. Mocoroa, J. Mendiguren, V. M. de Vega, L. García, J. Molina, J. Sánchez-González, G. Guzmán, J. C. Alonso-Farto, E. Guallar, F. Civeira, H. Sillesen, S. Pocock, J. M. Ordovás, G. Sanz, L. J. Jiménez-Borreguero and V. Fuster, Circulation, 2015, 131, 2104–2113 CrossRef PubMed.
- R. Ladeiras-Lopes, S. Agewall, A. Tawakol, B. Staels, E. Stein, R. J. Mentz, A. Leite-Moreira, F. Zannad and W. Koenig, Int. J. Cardiol., 2015, 192, 72–81 CrossRef PubMed.
- S. Mendis and O. Chestnov, Curr. Cardiol. Rep., 2014, 16, 486 CrossRef PubMed.
- K. M. Patel, A. Strong, J. Tohyama, X. Jin, C. R. Morales, J. Billheimer, J. Millar, H. Kruth and D. J. Rader, Circ. Res., 2015, 116, 789–796 CrossRef CAS PubMed.
- L. N. Rao, T. Ponnusamy, S. Philip, R. Mukhopadhyay, V. V. Kakkar and L. Mundkur, Lipids, 2015, 50, 785–797 CrossRef CAS PubMed.
- X. H. Yu, Y. C. Fu, D. W. Zhang, K. Yin and C. K. Tang, Clin. Chim. Acta, 2013, 424, 245–252 CrossRef CAS PubMed.
- S. O. Rahaman, W. Swat, M. Febbraio and R. L. Silverstein, J. Biol. Chem., 2011, 286, 7010–7017 CrossRef CAS PubMed.
- G. J. Zhao, K. Yin, Y. C. Fu and C. K. Tang, Mol. Med., 2012, 18, 149–158 CAS.
- J. Y. Lee, J. Karwatsky, L. Ma and X. Zha, Am. J. Physiol.: Cell Physiol., 2011, 301, C886–C894 CrossRef CAS PubMed.
- M. A. Kennedy, G. C. Barrera, K. Nakamura, A. Baldan, P. Tarr, M. C. Fishbein, J. Frank, O. L. Francone and P. A. Edwards, Cell Metab., 2005, 1, 121–131 CrossRef CAS PubMed.
- A. Ji, J. M. Wroblewski, L. Cai, M. C. de Beer, N. R. Webb and D. R. van der Westhuyzen, J. Lipid Res., 2012, 53, 446–455 CrossRef CAS PubMed.
- L. Yvan-Charvet, N. Wang and A. R. Tall, Arterioscler., Thromb., Vasc. Biol., 2010, 30, 139–143 CrossRef CAS PubMed.
- A. Chawla, W. A. Boisvert, C. H. Lee, B. A. Laffitte, Y. Barak, S. B. Joseph, D. Liao, L. Nagy, P. A. Edwards, L. K. Curtiss, R. M. Evans and P. Tontonoz, Mol. Cell, 2001, 7, 161–171 CrossRef CAS PubMed.
- G. Chinetti, S. Lestavel, V. Bocher, A. T. Remaley, B. Neve, I. P. Torra, E. Teissier, A. Minnich, M. Jaye, N. Duverger, H. B. Brewer, J. C. Fruchart, V. Clavey and B. Staels, J. Nat. Med., 2001, 7, 53–58 CrossRef CAS PubMed.
- A. Ravelli, Arthritis Rheum., 2012, 64, 33–36 CrossRef PubMed.
- K. Pahan, Cell. Mol. Life Sci., 2006, 63, 1165–1178 CrossRef CAS PubMed.
- A. Endo, M. Kuroda and Y. Tsujita, J. Antibiot., 1976, 29, 1346–1348 CrossRef CAS PubMed.
- A. Endo, J. Med. Chem., 1985, 28, 401–405 CrossRef CAS PubMed.
- C. J. Vaughan and A. M. Gotto Jr, Circulation, 2004, 110, 886–892 CrossRef CAS PubMed.
- E. S. Stroes, P. D. Thompson, A. Corsini, G. D. Vladutiu, F. J. Raal, K. K. Ray, M. Roden, E. Stein, L. Tokgozoglu, B. G. Nordestgaard, E. Bruckert, G. De Backer, R. M. Krauss, U. Laufs, R. D. Santos, R. A. Hegele, G. K. Hovingh, L. A. Leiter, F. Mach, W. März, C. B. Newman, O. Wiklund, T. A. Jacobson, A. L. Catapano, M. J. Chapman and H. N. Ginsberg, Eur. Heart J., 2015, 36, 1012–1022 CrossRef PubMed.
- K. Suzuki, K. Nozawa, S. Udagawa, S. Nakajima and K. Kawai, Phytochemistry, 1991, 30, 2096–2098 CrossRef CAS.
- T. Amagata, A. Amagata, K. Tenney, F. A. Valeriote, E. Lobkovsky, J. Clardy and P. Crews, Org. Lett., 2003, 5, 4393–4396 CrossRef CAS PubMed.
- R. Bernini, F. Crisante and M. C. Ginnasi, Molecules, 2011, 16, 1418–1425 CrossRef CAS PubMed.
- F. Freire, J. M. Seco, E. Quiñoá and R. Riguera, Chem. Commun., 2007, 1456–1458 RSC.
- D. Zhao, C. Shao, Q. Zhang, K. Wang, F. Guan, T. Shi and C. Wang, J. Nat. Prod., 2015, 78, 2310–2314 CrossRef CAS PubMed.
- B. Prokas, D. Uhrin, J. Adamcova and J. Fuska, J. Antibiot., 1992, 45, 1268–1272 CrossRef CAS.
- H. Oh, T. O. Kwon, J. B. Gloer, L. Marvanova and C. A. Shearer, J. Nat. Prod., 1999, 62, 580–583 CrossRef CAS PubMed.
- S. Komai, T. Hosoe, T. Itabashi, K. Nozawa, T. Yaguchi, K. Fukushima and K. Kawai, J. Nat. Med., 2006, 60, 185–190 CrossRef CAS.
- M. Chen, L. Han, C. Shao, Z. She and C. Wang, Chem. Biodiversity, 2015, 12, 443–450 CAS.
- T. Sassa, M. Nukina and Y. Suzuki, Agric. Biol. Chem., 1991, 55, 2415–2416 CrossRef CAS.
- L. Merlini, G. Nasini and A. Selva, Tetrahedron, 1970, 26, 2739–2749 CrossRef CAS PubMed.
- S. De Stefano, R. Nicoletti, A. Milone and S. Zambardino, Phytochemistry, 1999, 52, 1399–1401 CrossRef CAS.
- N. Murtaza, S. A. Husain, T. B. Sarfaraz, N. Sultana and S. Faizi, Planta Med., 1997, 63, 191 CrossRef CAS PubMed.
- Y. Liu, G. Xia, H. Li, L. Ma, B. Ding, Z. She, Y. Lu, L. He and X. Xia, Planta Med., 2014, 80, 912–917 CrossRef CAS PubMed.
- Z. Liu, G. Xia, S. Chen, Y. Liu, H. Li and Z. She, Mar. Drugs, 2014, 12, 3669–3680 CrossRef PubMed.
- C. Wu, Y. Guo, Y. Su, X. Zhang, H. Luan, X. Zhang, H. Zhu, H. He, X. Wang, G. Sun, X. Sun, P. Guo and P. Zhu, J. Cell. Mol. Med., 2014, 18, 293–304 CrossRef CAS PubMed.
- C. Wu, X. Zhang, X. Zhang, H. Luan, G. Sun, X. Sun, X. Wang, P. Guo and X. Xu, J. Nutr. Biochem., 2014, 25, 412–419 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra00033a |
| This journal is © The Royal Society of Chemistry 2016 |