Aqueous lubrication of poly(N-hydroxyethyl acrylamide) brushes: a strategy for their enhanced load bearing capacity and wear resistance
Jingjing Zhanga,
Shengwei Xiaoa,
Mingxue Shen*b,
Li Sunc,
Feng Chena,
Ping Fana,
Mingqiang Zhonga and
Jintao Yang*a
aCollege of Materials Science & Engineering, Zhejiang University of Technology, Hangzhou 310014, P. R. China. E-mail: yangjt@zjut.edu.cn
bSchool of Mechanical Engineering, Zhejiang University of Technology, Hangzhou 310014, P. R. China. E-mail: shenmx@zjut.edu.cn
cCollege of Education Science & Engineering, Zhejiang University of Technology, Hangzhou 310014, P. R. China
First published on 18th February 2016
Abstract
Surface friction is a very important property for biomaterials, especially for those with implantation purposes. Recently, a new hydrophilic polymer, namely, poly(N-hydroxyethyl acrylamide) (PHEAA) has shown significant potential as biocompatible material. Herein, the surface friction of PHEAA brushes was studied. First, PHEAA brushes with well-controlled thickness were prepared via surface-initiated atomic transfer radical polymerization (SI-ATRP). Then, the surface friction coefficients of these brushes in water were measured aiming to better understand the thickness dependence of the surface lubrication properties. The results showed that the surface lubrication of PHEAA brushes highly depends on surface morphology and hydration state. With an optimal polymer film thickness of ∼21.5 nm, PHEAA-grafted surfaces show an ultralow friction coefficient of ∼0.013. To enhance the load bearing and wear resistance of the surface lubrication, a strategy of cross-linking was proposed. Cross-linked gel brushes were prepared by adding cross-linker N,N′-methylene bis(acrylamide) during the SI-ATRP. It was shown that the presence of a cross-linker in the ATRP resulted in high surface roughness, leading to a significant increment of surface friction to ∼2, albeit the water contact angle was lower. However, the strategy of cross-linking showed great success in enhancing the load bearing and wear resistance of polymer brushes. Differing from uncross-linked polymer brushes, cross-linked gel brushes showed decreased friction coefficients when increasing the compression load. Moreover, the wear resistance of the polymer brushes was also improved, as evidenced by the fact that the friction coefficient of cross-linked gel brushes was stable at 2000 s, while the friction coefficient of uncross-linked brushes increased quickly (at ∼700 s).
Introduction
Efficient aqueous lubrication systems are very common but important in nature. For example, human joints such as hip, knee, shoulder, ankle, and finger joints show very low friction, with friction coefficients in the range of 0.001–0.01, helping people walk, run, and do other complicated exercises easily.1–3 However, loss or even deterioration of the lubrication will cause serious health problems. With the increased ageing global population, more and more people are suffering from this problem. Although artificial joints show great potential to solve this problem, it is still hard for them to fully satisfy some certain properties required for human joints, such as ultralow friction, high load bearing, and high wear resistance.4–7 Besides artificial joints, some medical devices such as soft contact lenses, gastroscopes and urinary catheters also have urgent needs for surface lubrication to enhance their performance.8,9An enormous research effort has been devoted to develop surfaces that have ultralow friction and high wear resistance.10–12 This effort includes many methods or techniques to design such surfaces and studies on lubrication mechanism.13–16 Recently, polymer brushes in which polymer chains are tethered at one end to the surface have emerged as potential coatings showing low friction in water.17,18 In aqueous media, water will form hydrated layer on the surface of these polymer brushes, mainly hydrophilic polymer brushes, leading to the low friction.11,19 Generally, the superior hydration is formed, the lower surface friction is obtained. However, the precise mechanisms of aqueous lubrication and relationship between aqueous lubrication and hydration layer structure are still not well understood, albeit many hydrophilic polymers including zwitterionic polymers, polyelectrolyte, and non-charged polymers have been fabricated into low friction surfaces.11,18,20–24 From structure view of polymer brushes, not surprisingly, it can be found that this structure intrinsically have some problems as lubrication surface, two of the most serious ones among these problems are the low compression bearing and poor wear resistance.
In our group, we recently reported a new hydrophilic polymer, namely, poly(N-hydroxyethyl acrylamide) (PHEAA), which showed excellent hydration properties in water and consequent antifouling properties.25–29 Its excellent biocompatibility also provides great potentials as surface modification materials for implanted biomaterials.30 However, the aqueous lubrication of this polymer has not been reported yet. Can the superior hydration be transferred to ultralow surface friction? To answer this question, herein, we prepared PHEAA brushes with well controlled thickness via surface-initiated atom transfer radical polymerization (SI-ATRP). Macro friction coefficient measurements were used to evaluate the aqueous of PHEAA brushes in water. Effect of film thickness as a key factor on friction coefficient was particularly examined. To enhance the compression and wear resistances of polymer brushes during friction, cross-linked gel brushes were prepared by introducing cross-linker during ATRP. Surface morphologies, hydrophilicity, and surface friction of such cross-linked gel brushes were characterized. The results showed that the friction coefficient was greatly enhanced due to the high surface roughness of the gel brushes. However, the strategy of cross-linking showed success in improving the load bearing and wear resistance of polymer brushes.
Experimental section
Materials
Copper(I) bromide (purchased from Sigma-Aldrich (Shanghai)) was purified by successive washing with acetic acid and ethanol and was dried under vacuum. Tris[2-(dimethylamino)ethyl]amine (Me6TREN, 99%) was purchased from Tokyo Chemical Inc. (TCI). The initiator for grafting polymer brushes on silica wafer, 3-(2-bromoisobutyramido)propyl(trimethoxy)silane, was purchased from Gelest, Inc. (Morrisville, PA). N-Hydroxyethyl acrylamide (HEAA) and N,N′-methylene bis(acrylamide) were purchased from Sigma-Aldrich (Shanghai). Methanol was purchased from Shanghai Lingfeng Chemical Reagent Co. Ltd. Water used in these experiments was purified by a Millipore water purification system with a maximum resistivity of 18.0 MΩ cm. All other reagents and solvents were commercially obtained at extra-pure grade and were used as received without any purification.Preparation of polymer brushes
All the brush samples were prepared from silicon wafers immobilized with surface initiator by SI-ATRP method. A few sheets of silicon wafer (20 mm × 15 mm) were placed into a fresh piranha solution (H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 = 3
H2O2 = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 120 °C for ∼0.5 h. The wafers were repeatedly washed with deionized water and dried with N2 flow. Subsequently were treated with plasma (CORONA Lab. CTP-2000, Nanjing, China) for 1.5 min to enhance hydrophilicity. The cleaned silica wafers were immediately immersed into 1 mM dehydrate toluene solution containing 3-(trimethoxysilypropyl)-2-bromo-2-methypropionate for 12 h at room temperature. The initiator-grafted surfaces were thoroughly rinsed with toluene, ethanol, and water to remove physically absorption of initiator molecules and then dried with N2 flow. The hydrophilic polymer brushes PHEAA were synthesized through SI-ATRP as follows.
1) at 120 °C for ∼0.5 h. The wafers were repeatedly washed with deionized water and dried with N2 flow. Subsequently were treated with plasma (CORONA Lab. CTP-2000, Nanjing, China) for 1.5 min to enhance hydrophilicity. The cleaned silica wafers were immediately immersed into 1 mM dehydrate toluene solution containing 3-(trimethoxysilypropyl)-2-bromo-2-methypropionate for 12 h at room temperature. The initiator-grafted surfaces were thoroughly rinsed with toluene, ethanol, and water to remove physically absorption of initiator molecules and then dried with N2 flow. The hydrophilic polymer brushes PHEAA were synthesized through SI-ATRP as follows.
The HEAA monomer (4.35 mmol) and Me6TREN (0.14 mmol) were dissolved in water (2.5 mL), and the mixture was completely deoxygenated by three cycles of freeze–pump–thaw, then flowing a stream of nitrogen for ∼20 min. Methanol was also degassed in the same manner. A 10 mg portion of CuBr (0.07 mmol) and silica wafer with the initiator-coated SAM surface were placed in a well-dried reaction tube with a rubber stopper and then removed oxygen as previous step. The degassed methanol (2.5 mL) and water solution containing the monomer and ligand were added using a syringe to the reaction tube under nitrogen protection. The tube was then subjected to two evacuation-nitrogen purging cycles and kept at room temperature for the SI-ATRP reaction. After the pre-specified reaction time, the solution was exposed to air to terminate the reaction. The silicon wafer was collected, washed with methanol and deionized water using a ultrasound machine for 10 min to remove the free polymer absorbed on its surface and dried with N2 flow at room temperature for further characterizations.
The PHEAA brushes and cross-linked brushes were tuned by adjusting the cross-linker concentration, synthesized in a similar manner using CuBr, Me6TREN and N,N′-methylenebis(acrylamide) (molar ratio: 2.5%, 5.0%, and 10%) in methanol at RT for 24 h. The scheme for preparation of PHEAA and cross-linked PHEAA brush layers are illustrated in Scheme 1.
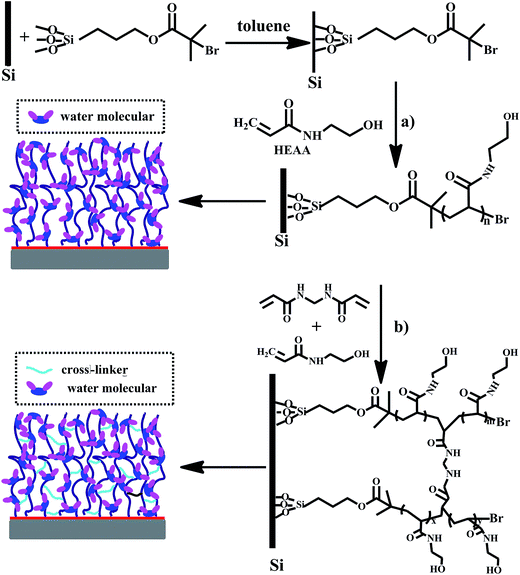 | ||
| Scheme 1 Schematic of SI-ATRP method for preparing PHEAA brushes on silica wafer coated with immobilized initiators. (a) PHEAA and (b) cross-linked PHEAA. | ||
Film thickness measurement
The film thickness of polymer brushes was measured using a ellipsometric measurements performed on an α-SE ellipsometer (J. A. Woollam Co., Lincoln, NE) with a He–Ne laser (λ = 632.8 nm) and a fixed angle of incidence of 70°. Bare silicon wafer was used to determine the thickness of SiO2 layer.Surface composition measurement
The surface composition of the modified silicon wafer was determined by X-ray photoelectron spectroscopy (XPS). The binding energy (BE) scale is calibrated by C1s of the hydrocarbon peak at 284.8 eV.Surface morphology measurement
Atomic force microscopy (AFM) measurements were performed on an Bruker Dimension AFM (Bruker Daltonics Inc., USA) to characterize the surface morphology and roughness. All images were acquired in tapping mode as 256 × 256 pixel images at a typical scan rate of 0.6 Hz with a scan range of 4.0 μm. All the measurements are made in ambient conditions.Contact angle measurement
The static contact angle (CA) was acquired on a OCA 15EC Video-based Optical Contact Angle Measuring System (Eastern-Dataphy Instruments Co., Ltd., Beijing) at room temperature, equipped with a sessile drop shape analysis system and a video camera. A droplet of 2 μL of deionized water was used as probe liquid. Three different positions were measured for each sample to get the average CA. All the contact angles were measured with about 200 s of residence time of water droplet on the surface.Friction measurement
A macroscopic friction test was made at different friction conditions using a Universal Micro-Tribometer (UMT-2, CETR) by sliding a elastomeric poly(dimethylsiloxane) (PDMS) hemisphere (ϕ 6 mm) on the substrates in water along a distance of 20 mm at a sliding velocity of 2 × 103 m s−1 at room temperature. The loading was increased from 0.5 N to 3 N at intervals of 0.5 N. The friction coefficient for each sample was acquired from three independent measurements on virgin surface area. Each measurement included 20 sliding cycles, and 60 data points were used to obtain average value and standard deviation.Results and discussion
Silicon wafer with ∼2 nm silica layer was cleaned and grafted with ATRP initiator, followed by ATRP to obtain the PHEAA brushes. To confirm the achievement of the multistep-modified surfaces, XPS was employed to analyze the surface chemistry composition. The XPS spectra of the surfaces grafted with initiator and polymer brushes are shown in Fig. 1. For the surface grafted with initiators, the appearance of peaks at 70.3 eV and 285 eV can be assigned to Br3d and C1s (Fig. 1a), indicating that the initiator was successfully immobilized on the surface. In the case of PHEAA grafted surface, a peak of N1s appeared in its XPS spectrum. Furthermore, high-resolution O1s (as shown in Fig. 1c) spectra showed two distinct peaks, in which the peak at ∼531.2 eV was attributed to amide carbon O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N and the peak at ∼532.4 eV is related to the O in C–O. These results improve the success of grafting PHEAA on silica surface by SI-ATRP.
C–N and the peak at ∼532.4 eV is related to the O in C–O. These results improve the success of grafting PHEAA on silica surface by SI-ATRP.
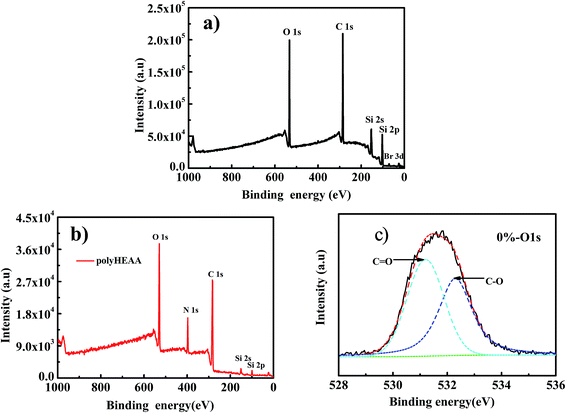 | ||
| Fig. 1 XPS survey spectra for the silicon surfaces grafted with (a) initiators, (b) PHEAA brushes, (c) high-resolution survey scans of O1s for PHEAA brushes. | ||
For polymer brushes prepared by SI-ATRP, their thickness can be readily tuned by controlling the reaction time. Herein, PHEAA brushes with four different film thicknesses ranging from ∼10 nm to ∼83 nm were synthesized with the aim to study the thickness dependence of hydrophilicity and surface friction. The morphology of these surfaces was first characterized using AFM, as shown in Fig. 2. For polymer brushes with a film thickness of ∼9.5 nm, the surface is covered by isolated, instead of uniform, polymer brushes, resulting in a high roughness. When the film thickness increases to ∼21.5 and ∼48.4 nm, the grafted polymer brushes become denser and more uniform. As a result, the surfaces of these brushes become smoother. When the thickness of brushes is further increased to ∼83.0 nm, some holes are found on the surface, leading to a high roughness value. Due to high dependence of surface friction on surface roughness, it seems like that the thicknesses of ∼21.5 and ∼48.4 nm are optimal thicknesses to obtain low friction coefficient.
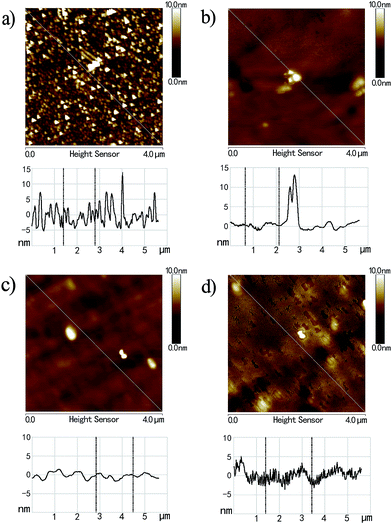 | ||
| Fig. 2 AFM characterization of PHEAA brushes on silica wafers with film thicknesses of (a) 9.5 nm, (b) 21.5 nm, (c) 48.4 nm, and (d) 83.0 nm. | ||
The hydrophilicity of the films with various thicknesses was evaluated by water contact angle measurement. Fig. 3a shows the water contact angles on the PHEAA-grafted surfaces with thickness of ∼9.5 nm, ∼21.5 nm, ∼48.4 nm, and ∼83.0 nm. It is found that the hydrophilicity is decreased as increasing the thickness. Water contact angle on the film of ∼9.4 nm is ∼30°, while that on the film of ∼83.0 nm is increased to ∼55°. The decreased hydrophilicity of thick film might be resulted from the high molecular weight and dense structure which consequently causes the high dense of intramolecular hydrogen bond. Fig. 3b shows the friction coefficients of the PHEAA-grafted surfaces with different thickness. Strong roughness and hydration dependences of aqueous lubrication were observed. The films of ∼9.5 nm and ∼83.0 nm show relatively high surface friction coefficients due to their high roughness and low hydrophilicity, respectively. It is noteworthy that the surface friction coefficient of ∼9.5 nm film is low at the beginning of the measurement and increases with time. This result indicates that the isolated structure might be easily destroyed, leading to the high surface friction. With optimal surface roughness and hydration property, ∼21.5 nm film exhibits the lowest friction coefficient of ∼0.013, which is in the range of high lubrication.
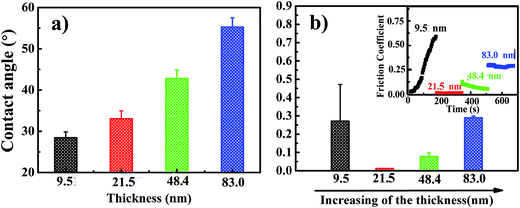 | ||
| Fig. 3 (a) Contact angles of water and (b) surface friction coefficients of PHEAA brushes with various thicknesses. | ||
To enhance the load bearing and wear resistance of polymer brushes, cross-linked gel structure was prepared by adding cross-linker during SI-ATRP. Fig. 4b shows the high resolution of O1s spectrum of the cross-linked gel PHEAA brushes. The higher area ratio of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C–O as compared to that of PHEAA brushes indicates the existence of cross-linker in the brushes.
O/C–O as compared to that of PHEAA brushes indicates the existence of cross-linker in the brushes.
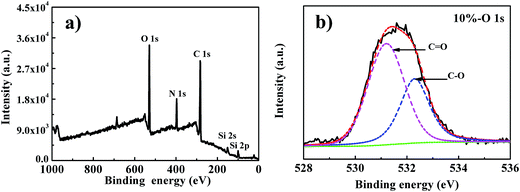 | ||
| Fig. 4 (a) XPS survey spectra and (b) high-resolution survey scans of O1s for the silicon surfaces grafted with cross-linked PHEAA. | ||
The presence of cross-linker also shows influence on the film thickness (Fig. 5). With the addition of 2.5 mol% and 5.0 mol% cross-linker, the film thickness increases from ∼25.0 nm to ∼32.2 and ∼46.0 nm. Whereas, when 10 mol% cross-linker is added, the film thickness slightly decreased to ∼18.8 nm. Although the film thickness of the gel brushes changes with the content of cross-linker, all of these brushes are in optimal thickness range. The effect of cross-linker on surface morphology is investigated by the morphology characterizations of the gel brushes with various cross-linker contents using AFM, as shown in Fig. 6, it is clear that the addition of cross-linker leads to the coverage of clusters on the surface. The clustering and roughening effect might be resulted from the spatial variations in cross-linking density during polymerization.
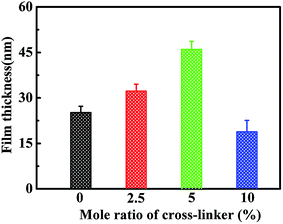 | ||
| Fig. 5 Film thickness of cross-linked PHEAA brushes as a function of cross-linker mole ratios of 0%, 2.5%, 5.0%, and 10%. | ||
 | ||
| Fig. 6 Tapping-mode AFM images for cross-linked PHEAA brushes with different cross-linkers of (a) 0 mol%, (b) 2.5 mol%, (c) 5.0 mol%, and (d) 10 mol%. | ||
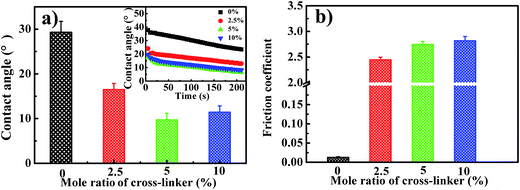
To investigate the influence of cross-linking on the hydrophilicity of the polymer brushes, water contact angles on the gel brushes from various cross-linker concentrations were measured (Fig. 7a). The water contact angle gradually decreased from ∼30° on uncross-linked brushes to ∼10° on the cross-linked brushes of 5.0 mol% cross-linker. Adding more cross-linker, such as 10 mol%, leads to a slight enhancement of water contact angle to ∼15°. Given the higher hydrophilicity of cross-linked gel brushes, we measured their surface friction coefficient with expectation to obtain high lubrication. First, friction coefficients of the elastomeric PDMS hemisphere with load of 0.5 N against the cross-linked surfaces of various cross-linker concentrations were measured and compared with that on uncross-linked brushes, as shown in Fig. 7b. However, surface coefficients sharply increases to 2.5–2.8 for the cross-linked surfaces. The higher surface friction is believed to result from higher surface roughness.
To test whether the cross-linked structure can improve the compression resistance of polymer brushes, higher loads up to 3.0 N were applied on the PDMS hemisphere, and the resultant surface friction coefficients were then measured (Fig. 8). For uncross-linked PHEAA brushes, surface friction increases as increasing load, likely due to conformation change of the brushes under compression (Fig. 8a). As for the cross-linked gel brushes, for instance, brushes of 5.0 mol% cross-linker, however, the relationship between surface friction coefficient and compression load shows an opposite trend. The surface friction coefficient decreases from ∼2.8 to ∼1.3 when the load increases from 0.5 N to 3.0 N (Fig. 8b). Regardless cross-linker concentration, all the gel brushes show the decreased surface friction coefficient as increasing compression load. The absorbed water in the gel brushes can be used to interpret this phenomenon (Fig. 8c). Under high load, the water inside gel brushes will be squeezed out, and forms a fluid-like layer on the surface, which consequently increases the lubricity in the contact region. The schematic representing this speculation is shown as Fig. 8d.
Long term friction measurement was carried out to evaluate the wear resistance of polymer brushes during friction. Fig. 9 shows the friction force as a function of time for the uncross-linked brushes and cross-linked brushes with 5 mol% cross-linker under normal loads of 0.5 N (a and a′) and 2.0 N (b and b′). It is clear that the cross-linked PHEAA brushes show a much higher stability of the friction coefficient against long friction time. Under normal load of 0.5 N, both uncross-linked brushes and cross-linked brushes show stable surface friction coefficient within the period of 2000 s. However, when the normal load is increased to 2.0 N, the aqueous lubrication of uncross-linked PHEAA brushes deteriorates at ∼700 s. On the contrary, the surface friction coefficient of cross-linked brushed keeps consistent in the whole friction measurement of 2000 s, indicating the high wear resistance of gel brushes.
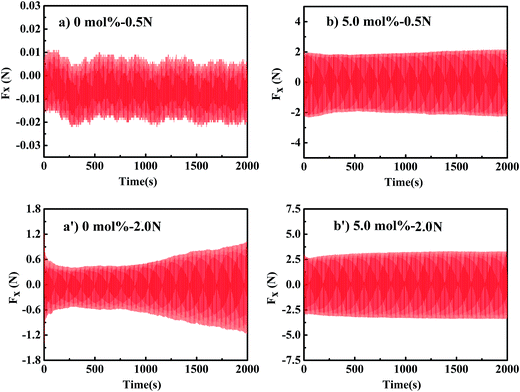 | ||
| Fig. 9 Long term friction measurements (Fx (N) versus time) for (a) PHEAA brushes and (b) cross-linked PHEAA brushes with 5.0 mol% cross-linker under loads of 0.5 N and 2.0 N (a′ and b′). | ||
Conclusions
In summary, we studied the aqueous lubrication behavior of PHEAA brushes. With optimal thickness, PHEAA showed an ultralow surface friction coefficient of ∼0.01. The addition of cross-linker during SI-ATRP could produce cross-linked gel brushes, which induced the increase of surface roughness and hydrophilicity. The high surface roughness leaded to high surface friction coefficients of 2–3. However, the cross-linked structure greatly improved the load bearing capacity and wear resistance of the brushes. Under high load, the water inside the gel brushes could be squeezed out and form a fluid-like layer, consequently, an even lower surface coefficient as compared to that under low compression load could be achieved. Moreover, cross-linked gel brushes also showed high wear resistance, as evidenced by the stable friction coefficient even after a continuous friction of 2000 s.Acknowledgements
This material is based upon work funded by Natural Science Foundation of China under Grant (No. 21274131, 51273178 and 51203139), Natural Science Foundation of Zhejiang Province (LY14E030005 and LY16E030012), and Zhejiang Top Priority Discipline of Textile Science and Engineering (2015KF06).References
- J. Klein, Proc. Inst. Mech. Eng., Part J, 2006, 220, 691–710 CrossRef CAS.
- D. A. Swann, K. J. Bloch, D. Swindell and E. Shore, Arthritis Rheum., 1984, 27, 552–556 CrossRef CAS PubMed.
- C. W. McCutchen, Wear, 1962, 5, 1–17 CrossRef.
- A. M. Forster, J. W. Mays and S. M. Kilbey, J. Polym. Sci., Part B: Polym. Phys., 2006, 44, 649–655 CrossRef CAS.
- J. Klein, E. Kumacheva, D. Mahalu, D. Perahia and L. J. Fetters, Nature, 1994, 370, 634–636 CrossRef CAS.
- O. Ben and D. Vavylonis, J. Phys.: Condens. Matter, 2005, 17, R63 CrossRef.
- P. A. Schorr, T. C. Kwan, S. M. Kilbey, E. S. Shaqfeh and M. Tirrell, Macromolecules, 2003, 36, 389–398 CrossRef CAS.
- Z. Zhou and Z. Jin, Biosurface and Biotribology, 2015, 1, 3–24 CrossRef.
- S. Nagaoka and R. Akashi, J. Bioact. Compat. Polym., 1990, 5, 212–226 CrossRef CAS.
- U. Raviv and J. Klein, Science, 2002, 297, 1540–1543 CrossRef CAS PubMed.
- W. H. Briscoe, S. Titmuss, F. Tiberg, R. K. Thomas, D. J. McGillivray and J. Klein, Nature, 2006, 444, 191–194 CrossRef CAS PubMed.
- K. Ishihara, Polym. J., 2015, 47, 585–597 CrossRef CAS.
- K. Glinel, A. M. Jonas, T. Jouenne, J. Leprince, L. Galas and W. T. S. Huck, Bioconjugate Chem., 2009, 20, 71–77 CrossRef CAS PubMed.
- J. Xu and K. Kato, Wear, 2000, 245, 61–75 CrossRef CAS.
- W.-F. Kuo, Y.-C. Chiou and R.-T. Lee, Wear, 1996, 201, 217–226 CrossRef CAS.
- S. H. Kim, C. Marmo and G. A. Somorjai, Biomaterials, 2001, 22, 3285–3294 CrossRef CAS PubMed.
- M. Chen, W. H. Briscoe, S. P. Armes and J. Klein, Science, 2009, 323, 1698–1701 CrossRef CAS PubMed.
- M. Kobayashi and A. Takahara, Chem. Rec., 2010, 10, 208–216 CrossRef CAS PubMed.
- M. Kobayashi, Y. Terayama, H. Yamaguchi, M. Terada, D. Murakami, K. Ishihara and A. Takahara, Langmuir, 2012, 28, 7212–7222 CrossRef CAS PubMed.
- Y. Wu, Q. Wei, M. Cai and F. Zhou, Adv. Mater. Interfaces, 2015, 2(1–15), 1400392 Search PubMed.
- J. Klein, Friction, 2013, 1, 1–23 CrossRef.
- H. Sakata, M. Kobayashi, H. Otsuka and A. Takahara, Polym. J., 2005, 37, 767–775 CrossRef CAS.
- Q. Wei, M. Cai, F. Zhou and W. Liu, Macromolecules, 2013, 46, 9368–9379 CrossRef CAS.
- J. Yang, H. Chen, S. Xiao, M. Shen, F. Chen, P. Fan, M. Zhong and J. Zheng, Langmuir, 2015, 31, 9125–9133 CrossRef CAS PubMed.
- C. Zhao and J. Zheng, Biomacromolecules, 2011, 12, 4071–4079 CrossRef CAS PubMed.
- C. Zhao, K. Patel, L. M. Aichinger, Z. Liu, R. Hu, H. Chen, X. Li, L. Li, G. Zhang, Y. Chang and J. Zheng, RSC Adv., 2013, 3, 19991–20000 RSC.
- J. Yang, M. Zhang, H. Chen, Y. Chang, Z. Chen and J. Zheng, Biomacromolecules, 2014, 15, 2982–2991 CrossRef CAS PubMed.
- H. Chen, M. Zhang, J. Yang, C. Zhao, R. Hu, Q. Chen, Y. Chang and J. Zheng, Langmuir, 2014, 30, 10398–10409 CrossRef CAS PubMed.
- H. Chen, Q. Chen, R. Hu, H. Wang, B. Newby, Y. Chang and J. Zheng, J. Mater. Chem. B, 2015, 3, 5426–5435 RSC.
- H. X. Wu, L. Tan, Z. W. Tang, M. Y. Yang, J. Y. Xiao, C. J. Liu and R. X. Zhuo, ACS Appl. Mater. Interfaces, 2015, 7, 7008–7015 CAS.
| This journal is © The Royal Society of Chemistry 2016 |