Advances in click chemistry for silica-based material construction
Abstract
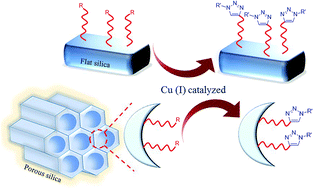
Click chemistry is undoubtedly the most powerful 1,3-dipolar cycloaddition reaction in organic synthesis. Because the click reaction can be used to straightforwardly attach two different molecules, this type of synthesis has attracted much attention from scientists. If the desired molecule is functionalized with azide or alkyne group(s), it can be readily attached to the target molecule or substrate through the click reaction. The potential of this reaction is very great; moreover, alkyne and azide groups can be incorporated into a widespread range of substituents. In addition, the resulting 1,2,3-triazoles have desirable features in electronic devices, solar cells and medicinal chemistry. In this study, different types of silica based surfaces were investigated for the click reaction, including flat silica materials, silica nanoparticles and porous silica compounds. Moreover, the advantages and applications of various triazole modified silica surfaces were compared.

 Please wait while we load your content...
Please wait while we load your content...