DOI:
10.1039/C5RA27942A
(Paper)
RSC Adv., 2016,
6, 27301-27312
pHEMA hydrogels with pendant triazinyl-β-cyclodextrin as an efficient and recyclable reservoir for loading and release of plant-based mosquito repellents: a new aqueous mosquito repellent formulation†
Received
29th December 2015
, Accepted 7th March 2016
First published on 10th March 2016
Abstract
Plant-based insect repellents are environmentally-friendly and play a practical and economic role in preventing the transmission of diseases to humans. Preformed 2-hydroxyethyl methacrylate-co-ethylene glycol dimethacrylate (pHEMA) hydrogels were functionalized with pedant triazinyl-β-cyclodextrin (T-β-CD) with the aim of improving the ability to host and release plant-based repellents. The structural and thermal properties were investigated by FTIR and thermogravimetric analysis, respectively. The most potent mosquito repellents, geraniol, methyl salicylate and trans-cinnamaldehyde, were screened from 35 plant-based compounds through Y-tube olfactometer assay. Pendant T-β-CDs did not affect the swelling of the hydrogels and exhibited low cytotoxicity towards mouse embryo fibroblasts, and no skin irritation or allergy was observed on human skin treated with modified hydrogels loaded with repellents. The physicochemical properties of the repellent and its affinity to form complexes determined the role played by the CDs in the loading and release. Methyl salicylate possessed an optimal stability constant KS (232.19 M−1) and thus it got a longer protection duration with higher repellent activity. Increasing the crosslink density resulted in decreases in the degree of swelling, the amount of T-β-CDs bonded to hydrogels, and the duration of complete repellency. Additionally, pHEMA hydrogels with pendant T-β-CDs showed good recyclability. The new hydrogel formulation loaded with plant-based repellents may provide a suitable, eco-friendly and safe approach to achieve protection against mosquitoes.
1. Introduction
Yearly, millions of people die or are seriously debilitated as a consequence of vector-borne disease such as malaria, dengue fever, yellow fever and several forms of viral encephalitis.1 Mosquitoes are the most medically important arthropod vectors for transmitting the causative agents of these diseases.2–5 As a creature having been honed through 170 million years of evolution, the mosquito is particularly difficult or impossible to control effectively due to its evolutionary biology, such as its great population and reproductive capacity, genomic flexibility and metamorphosis.6–9 The metamorphosis means that they are locomotive from aquatic environments to atmosphere after molt and thus entirely adaptable to their own ecological role in the life cycle. In spite of these facts, many pyrethroid insecticides, such as permethrin and deltamethrin, have been applied to control mosquitoes in the past.10–12 However, the abuse and misuse of these insecticides is responsible for the aggravating resistance.13–17 Meanwhile, with successively intensive applications of synthetic insecticides to manage the resistant population, there occur undesirable effects on non-target organisms, on human health and on the environment.18–21 In recent decades, it has been demonstrated that the use of repellents is a practical and economical means of preventing the transmission of the diseases to human, since they could provide a vapor barrier masking human scent or transmit a scent repelling insects when they were applied to human skin.22–24 The most common and widely used insect repellent is DEET (N,N-diethyl-3-methylbenzoylamide).25 However, extensive use of DEET has been associated with reproductive and developmental toxicity in animals.26,27 Other disadvantages of DEET include toxic reaction and damage to plastic and synthetic fabrics.28 In light of these problems, alternatives to synthetic insecticides and new vector control tools are urgently needed. Botanical essential oils are promising alternatives since they are effective, environmentally friendly, easily biodegradable, often inexpensive, and easily volatile to maintain an effective vapor concentration.24,28–33 Therefore, botanical essential oils as insect repellents have been widely investigated.22,23,29,34,35
Historically, aerosol formulations serve as an important tool in the control of mosquitoes.36–39 However, due to the inflammable property of compressed propellants and potential danger of explosion when the container suffers from heat, it is potentially unsafe for users. Currently, the water-based formulations, mostly familiar with topically aqueous-alcohol tinctures dissolving the active ingredients,40–43 are widely used. For these formulations, it is superior to spray indoors than outdoors because of their short-term duration and the effective concentration threshold could be easily achieved by spraying repeatedly in a relatively confined space. To pursue a long-lasting repellent or insecticidal activity, the new approach of controlled release formulation is focused intensively over past decades.38 Generally, according to the impetus to control release, there are three different kinds of formulations. The first one is mosquito repellent coil driven by burning to reach an 8–9 hours lasting release.44,45 Though effective to kill mosquitoes, its burning might be responsible for some fires. More importantly, many studies have determined lung cancer risks and other adverse effects on human health posed by excessive exposure to coils' smoke.46–48 The second one gives off bioactive ingredient motivated by electric heating. Commonly, electric mosquito liquid or electric mosquito mat is enclosed in a vaporizer and they are commercially available. Obviously, application of vaporizer is restricted to the places with convenient electricity supply. Lastly, the promising formulations have been utilizing microcapsule, natural fabric, polymer, and micro-particle systems designed to provide continuous long-term release of bioactive ingredients, which is safe and free of electric power. Among these formulations, extensive researches have focused on the β-cyclodextrin over the last years due to its most accessibility and lowest-priced property. Functionalization of formulations with β-cyclodextrin can be used for improving the solubility,49 masking unpleasant smells,50 UV protection,51 and stabilization of essential oils.52 However, β-cyclodextrin-encapsulated and other micro-encapsulated formulations occur mostly in the form of solution or solid powder,53 being lack of manipulation and recyclability.
Hydrogel is a three-dimensional network of hydrophilic polymers in which a large amount of water is present.54,55 The synthesis of biocompatible, comfortable and durable hydrogels to be used for sustained drug delivery has been increasingly pursued in the field of biomedical applications over the years.56,57 We envision that hydrogels capable of swelling or de-swelling reversibly can be designed as reusable vehicles with essential oils loading or release—charge/discharge cycles. To achieve this goal, chemically cross-linked hydrogel is prior to physical gel because chemically cross-linked networks have permanent rather than transient junctions. 2-Hydroxyethyl methacrylate-co-ethylene glycol dimethacrylate (pHEMA) is one of the first components of chemically cross-linked hydrogels and is still widely used due to its biomimetic properties.58 Cross-linked pHEMA gels can resist high temperature and acid or alkaline hydrolysis so pHEMA hydrogel is still a strong candidate for the development of flexible hydrogel for recyclable delivery systems and, particularly, for surface modification.59 Due to their high water content, the hydrophobic nature of essential oils or drugs could lead to poor loading and controlled release qualities. These limitations could be at least partially overcome by incorporation of β-cyclodextrin into polymeric network, which can regulate drug uptake and release through an affinity-driven mechanism as previously reported.60 Nevertheless, there have scarce reports about the hydrogels used as essential oils delivery systems in mosquito control.
Hydrogels modified by β-CD acting as topical mosquito repellent formulation exhibit superior advantages, since they gather the hydrogels attributes (higher degree of swelling and easy manipulation) and the encapsulation ability of cyclodextrins. Moreover, it has been reported that hydrogels with pendant CDs could impregnate drugs in a great proportion compared with the hydrogels with built-in CDs.60 Inspired by surface modification of natural fibers with monochlorotriazinyl-β-cyclodextrin (MCT-β-CD),61–67 we firstly fabricated the pHEMA hydrogel networks and bonded MCT-β-CD to the networks. Meanwhile, preliminary screening of the most potent mosquito repellents was performed. Geraniol, methyl salicylate and trans-cinnamaldehyde had the most potent repellent activity and were preferably used for further study. Next, the work was to explore the possibilities of using MCT-β-CD for modulating the loading and release properties (mosquito repellent profiles) of the three potent plant-based mosquito repellents from pHEMA hydrogels. The recyclable property of this aqueous-based controlled release formulation was also determined.
2. Materials and methods
2.1 Materials
Analytical grade 2-hydroxyethyl methacrylate (HEMA), ethyleneglycol dimethacrylate (EGDMA) and 2,2-azo-bis(isobutyronitrile) (AIBN) were supplied by Macklin Inc. (Shanghai, China). Chemical grade β-cyclodextrin (β-CD) and monochlorotriazinyl-β-cyclodextrin (MCT-β-CD) with the degree of substitution of 0.51 per anhydrous glucose unit were generously provided by Jilin SANBANG Chemical Co., Ltd. in China. Thirty-five plant-based mosquito repellents (see Table 1) investigated in this study were purchased from Shanghai Aladdin Reagent co., Ltd. (Shanghai, China). These essential oils were stored in a refrigerator at 4 °C until use. Bidistilled water was purified by using a Super Q Millipore System, with conductivity lower than 1.8 μS cm−1. All other reagents were analytical grade. Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), trypsin and penicillin-streptomycin were purchased from Sangon Biotech Shanghai Co., Ltd., China. Culex pipiens pallens were obtained from Shaanxi Provincial Disease Prevention and Control Center and reared in our laboratory at 28 ± 1 °C and 75 ± 5% relative humidity (r. h.) under a 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 h (light/dark) photoperiod for over 20 generations free of exposure to pathogens or insecticides. Adult mosquitoes were maintained on a 10% glucose solution and blood fed on live mice. Non-blood-fed female adults (3 to 4 days old) were used in this study.
10 h (light/dark) photoperiod for over 20 generations free of exposure to pathogens or insecticides. Adult mosquitoes were maintained on a 10% glucose solution and blood fed on live mice. Non-blood-fed female adults (3 to 4 days old) were used in this study.
Table 1 Repellency of 35 compounds against C. pipiens pallens females
| Test compounds |
Mean repellency ± SEM (%) |
| 5 min |
10 min |
15 min |
20 min |
| Values are expressed as the mean of three replicates ± SEM. Data not being observed. |
| Farnesol |
22.53 ± 1.58a |
—b |
— |
— |
| Geraniol |
100.00 ± 0.00 |
100.00 ± 0.00 |
100.00 ± 0.00 |
100.00 ± 0.00 |
| Eucalyptol |
43.18 ± 4.21 |
— |
— |
— |
| Citronellol |
58.67 ± 3.07 |
46.38 ± 2.29 |
40.58 ± 4.01 |
30.92 ± 2.85 |
| Linalool |
38.41 ± 2.56 |
— |
— |
— |
| α-Terpineol |
27.62 |
— |
— |
— |
| L-Menthol |
43.68 ± 2.54 |
— |
— |
— |
| Terpinen-4-ol |
93.81 ± 3.14 |
58.33 ± 4.21 |
33.52 ± 2.45 |
<20.00 |
| Phenylethyl alcohol |
62.70 ± 3.12 |
46.36 ± 2.56 |
27.68 ± 2.98 |
<20.00 |
| L-Carvone |
95.24 ± 2.75 |
54.20 ± 3.25 |
<20.00 |
<20.00 |
| D-Carvone |
100.00 ± 0.00 |
64.94 ± 2.45 |
48.79 ± 2.56 |
<20.00 |
| L-Menthone |
48.33 ± 4.12 |
— |
— |
— |
| α-Pinene |
44.09 ± 3.12 |
— |
— |
— |
| β-Pinene |
31.36 ± 1.23 |
— |
— |
— |
| β-Myrcene |
27.89 ± 3.02 |
— |
— |
— |
| β-Caryophyllene |
61.38 ± 5.18 |
43.90 ± 2.54 |
<20.00 |
<20.00 |
| D-Limonene |
21.67 ± 1.98 |
— |
— |
— |
| Citral |
63.18 ± 2.98 |
53.64 ± 3.12 |
<20.00 |
<20.00 |
| trans-Cinnamaldehyde |
100.00 ± 0.00 |
100.00 ± 0.00 |
100.00 ± 0.00 |
100.00 ± 0.00 |
| Cinnamic alcohol |
30.24 ± 2.59 |
— |
— |
— |
| p-Cymene |
60.32 ± 2.56 |
54.21 ± 3.26 |
51.11 ± 2.84 |
<20.00 |
| Carvacrol |
52.96 ± 3.02 |
24.92 ± 2.22 |
<20.00 |
<20.00 |
| Thymol |
56.05 ± 3.11 |
39.43 ± 3.56 |
<20.00 |
<20.00 |
| Cuminaldehyde |
100.00 ± 0.00 |
96.67 ± 1.92 |
84.34 ± 5.26 |
74.90 ± 5.32 |
| Cumic alcohol |
52.45 ± 3.05 |
28.27 ± 2.15 |
<20.00 |
<20.00 |
| Eugenol |
55.61 ± 2.64 |
<20.00 |
<20.00 |
<20.00 |
| Isoeugenol |
58.27 ± 2.15 |
<20.00 |
<20.00 |
<20.00 |
| Vanillin |
65.44 ± 2.98 |
32.89 ± 3.55 |
<20.00 |
<20.00 |
| L-Perillaldehyde |
90.83 ± 3.39 |
83.04 ± 4.56 |
72.50 ± 4.12 |
46.89 ± 2.35 |
| trans-Anethole |
<20.00 |
— |
— |
— |
| Anisaldehyde |
30.42 ± 1.99 |
— |
— |
— |
| Anisole |
<20.00 |
— |
— |
— |
| Methyl laurate |
<20.00 |
— |
— |
— |
| Methyl salicylate |
100.00 ± 0.00 |
100.00 ± 0.00 |
100.00 ± 0.00 |
100.00 ± 0.00 |
| 2-Phenylethyl propionate |
46.21 ± 13.47 |
— |
— |
— |
2.2 Y-tube olfactometer assay
A modified Y-tube olfactometer was used to test the repellent activity of 35 compounds. As shown in the Fig. 1, the Y-tube olfactometer consists a decision chamber and two observation legs which terminated into attached test chambers. A consistent air stream from the laboratory's pressurized air system was purified with a filter of activated charcoal, heated up to 28 ± 1 °C and humidified to a relative humidity of 70 ± 5% before it was transported into the tube system and the observation leg, with wind velocities of 0.33 mL s−1 in the observation leg. The test procedure was carried out according to the reported method.68 Typically, all test solutions of 35 compounds were prepared at the concentration of 0.1 μL per 1.0 μL ethanol. A 2.0 μL test solution was applied on a filter paper (1 × 2 cm) and then the filter paper was quickly attached to the inner side of test chamber. In the control chamber, 2.0 μL ethanol was applied on a same filter paper. For only the female mosquitoes attack on humans, only female insects were used in the assays. In each assay, 15–20 female C. pipiens pallens adults (3 to 4 days old) were used for each time. After waiting for 30 s to allow the scents of the essential oils to reach the main arm, mosquitoes were introduced into the apparatus and the pressurized air system was operated after the introduction hole was closed with a rubber stopper. Tests with each time were replicated 3 times, and the experiments were repeated twice, thus a total of six replicates were used. Repellency of the compounds was determined for 4 various exposure times ranging from 5 min to 20 min. Preliminary tests done by changing the treated and control legs showed that there was no position bias between the arms of the olfactometer. After each experiment, the Y-tube and connections were thoroughly washed with ethanol and dried in 110 °C in order to avoid any possible interference of the test compound's scents. Tests were conducted at the same temperature and r. h. as those in an insectary under a light intensity of 135 lux. The mosquitoes in each arm were counted and percent repellency (PR) was calculated by eqn (1).| |
 | (1) |
where Ct = number of mosquitoes in control arm; Tr = number of mosquitoes in treated arm. Mosquitoes that remained in the main arm were not taken into account.
 |
| | Fig. 1 The illustration (top) and apparatus photo (bottom) of Y-tube. | |
2.3 Phase solubility diagram
Phase solubility studies were carried out following the reported method.69–72 The three most potent compounds geraniol, methyl salicylate and trans-cinnamaldehyde were chosen as the test targets. An excess amount of test compound was added to screw-capped vials containing 10 mL aqueous solution of β-CD ranging from 0 to 14 mmol L−1 in concentration. Samples were protected from light using tin foil and shaken at 28 °C in a water bath until reaching equilibrium. Then samples were filtered using 0.45 μm PTFE filters and diluted. The concentration of dilute samples were spectrophotometrically determined at 280 nm (trans-cinnamaldehyde), 302 nm (methyl salicylate), and 222 nm (geraniol) using a Shimadzu UV-160A spectrophotometer with temperature control. Standard curves were developed from the absorbance of 5 different standard concentrations. All studies were carried out in triplicate. The stability constants, KS (L mol−1), were calculated by eqn (2).| |
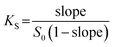 | (2) |
where S0 (mol L−1) is the intercept (solubility in water without β-CD).
2.4 Synthesis of hydrogels and post-functionalization with MCT-β-CD
The pHEMA hydrogels were prepared according to the reported method.60 Typically, the polymerization mixture was composed of HEMA (35 mL), EGDMA (8 mmol) (2.7% mol/mol with respect to the monomer), and AIBN (10 mmol). The mixture was well stirred in a flask to get a homogeneous solution. The solution was injected into hydrogel moulds. The mould supplied by native producer comprised two glass plates attached internally to a polypropylene film and separated by silicone frame of 2 mm, 4 mm or 6 mm thick. The moulds were then placed in an oven at 50 °C and after 12 h they were heated for 24 h at 70 °C. Once polymerization was completed, each gel sheet was immersed in boiling water for 15 min to remove unreacted monomers and this facilitated the cutting of discs (1 cm × 1 cm, 2 cm × 2 cm, 3 cm × 3 cm, 4 cm × 4 cm) (Fig. 2). These square hydrogels were presented with their size (height–length) in the context. To investigate the effect of various EGDMA densities upon the potential application of pHEMA hydrogels in topical formulation, other two types of hydrogel with 6 mm thick and 4 cm length were synthesized with different concentrations of EGDMA (I0.6–4, 1.0% EGDMA and 10 mmol AIBN in 35 mL HEMA; II0.6–4, 6.0% EGDMA and 10 mmol AIBN in 35 mL HEMA). MCT-β-CD is a reactive cyclodextrin capable of forming covalent bonds with nucleophilic groups. The following procedure for permanent bonding MCT-β-CD to the pHEMA hydrogel was developed in this study, based on the previously reported method.63,67 Typically, in a 100 mL beaker, hydrogel discs were dipped into 0.14 g mL−1 MCT-β-CD aqueous solution. After addition of 0.1 g mL−1 Na2CO3 solution with equal volume, it was stirred at 40 °C for 5 min. The hydrogels were added to the vessel and shaken at 60 °C for 4 h until water absorption reached equilibrium. Thereafter, the wetted hydrogels were dried at 60 °C and cured in hot air oven at 130 °C for 5 min at atmospheric pressure. Finally hydrogels were repeatedly rinsed with deionized water to remove the excess alkali and unreacted MCT-β-CD and dried to get pHEMA-T-β-CD hydrogels. All the dried gels were stored at room temperature in dried state until use in further tests. The quantity of MCT-β-CD bonded to hydrogels was estimated by the weight difference of the hydrogels before and after the fixing process.
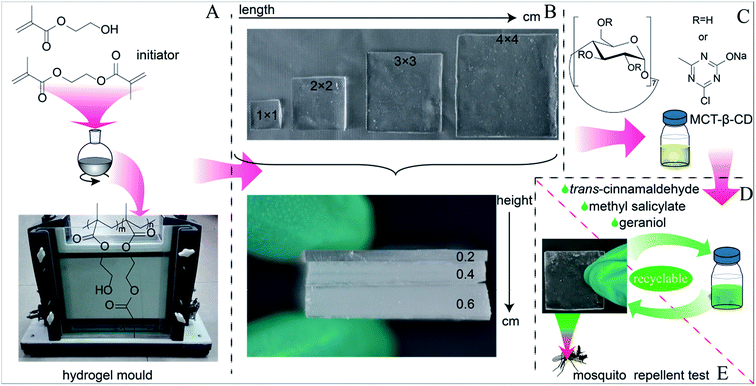 |
| | Fig. 2 The synthesis illustration for pHEMA and pHEMA-T-β-CD (A–C); (D) and (E) represent the loading of repellents and recyclability of pHEMA-T-β-CD hydrogels. | |
2.5 Fourier transform infrared spectroscopy (FT-IR) and thermogravimetric analysis
Fourier transform infrared spectroscopy (Thermo Nicolet 380) spectra of samples were recorded using a KBr disc for scans with a resolution of 2 cm−1 in the range of 4000 to 400 cm−1. Thermal stability was investigated by thermogravimetric analysis (TGA) performed on a TA Q500 thermal analysis system. Nitrogen was used as a purge gas for all testing. A heating rate of 20 °C min−1 with a flow rate of 100 mL min−1 was used for all tests.
2.6 Swelling kinetics
The swelling property of dried hydrogel discs (three replicates) was estimated as the relative weight gain when immersed in water at 28 °C. The samples were weighted at various times t after carefully wiping its surface with a soft tissue. The swelling ratio was estimated according to eqn (3).| |
 | (3) |
where W0 is the weight of dry sample and Wt is the weight at time t.
2.7 Surface contact angle measurement
Contact angles were measured at 5 different positions on dried hydrogel discs in static mode using a XG-CAMB1 contact angle meter (Shanghai Xuanyichuangxi Industrial Equipment Co., Ltd.). A drop of 10 μL of water was deposited on the hydrogel disc using a 22-gauge needle. The angle was measured using the imaging software provided by the supplier.
2.8 Cytocompatibility
According to ISO-10993 standard, in vitro cell viability in the presence of pHEMA hydrogel and modified pHEMA hydrogels extract was investigated by the MTT assay using mouse embryo fibroblasts (MEFs) obtained through primary culture. Fibroblasts were cultured in DMEM containing 10% FBS at extraction ratio of 0.1 g mL−1 and then incubated at 37 °C for 24 h. At the end of this period, the hydrogels were removed and the extracts were obtained. The culture medium was used as blank control. The cells were seeded into 96-well plates at a density of about 1 × 105 cell per well and incubated at 37 °C in 5% CO2 overnight. Then the culture medium (100 μL) was replaced with extraction solutions and MTT assays were performed at 24 h. The cell viability was calculated as eqn (4).| |
 | (4) |
2.9 Compounds loading and Y-tube olfactometer assay
Dried MCT-β-CD functionalized hydrogels were added to screw capped vials containing geraniol (methyl salicylate or trans-cinnamaldehyde) aqueous solution (0.3 mmol mL−1) and the vials were shaken at 28 °C for 48 h until reaching equilibrium. The amount of testing compounds loaded by each hydrogel was calculated as the difference between the initial amount of compound in the solution and the amount remaining after loading. Compound-loaded discs were rinsed with water and their surface was carefully wiped. Then the discs were immediately placed in the test chamber of Y-tube olfactometer. Repellency of the compound was recorded at hourly intervals until 12 h.
2.10 Arm in cubical cage test and recyclable test
Repellent efficacy against C. pipiens pallens females was carried out in controlled laboratory conditions according to arm in cage methods.73 Sugar-fed 5- to 10-day old females of these mosquitoes were used in laboratory repellent test. Before testing, the mosquitoes were starved for 24 h. Evaluations were carried out using volunteers in a chamber maintained at 28 ± 1 °C and humidified to a relative humidity of 70 ± 5%. The light intensity was regulated at 135 lux. For testing, repellent impregnated hydrogels were applied in the center of one forearm of each volunteer (Fig. 3). The exposed area of forearm was about 4 × 10 cm2. Tests were carried out in cubic cages (45 × 45 × 45 cm) containing 200 female mosquitoes. At the beginning of each test, the readiness of the mosquito to bite was confirmed by inserting the untreated forearm into the cage for up to 30 s. The mosquitoes were blown from the arm before any blood was taken. If at least 2 mosquitoes landed or bite the arm, the repellency test was carried out; otherwise the test was not conducted. After treatment, every 60 min, the number of biting mosquitoes on the exposed area was recorded for 3 min. At the same time, the control arm was inserted into another similar cage containing 200 female mosquitoes and the number of biting mosquitoes was recorded over 3 min. Control and treatment arm were interchanged between experimental sessions to eliminate bias. The tests for each hydrogel were conducted on separate days. All tests were replicated 3 times. Repellency data were expressed as protective efficacy (PE) and were calculated using the eqn (5).| |
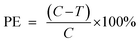 | (5) |
where C is the number of mosquitoes countered from control and T is the number countered from the treated areas of volunteers. For the recyclable test, 0.6–4 and II0.6–4 pHEMA-T-β-CD hydrogel discs were kept at oven at 50 °C until no scent was smelt before the next cycle.
 |
| | Fig. 3 The photo for the arm in cubic cage test. | |
3. Results and discussions
3.1 Y-tube olfactometer assay
Olfactometer test is a quick and efficient way to evaluate the behavioral response of mosquitoes toward volatile stimuli. Thirty-five testing compounds are ingredients or major ingredients of different botanical essential oils and are commercially available. Preliminary screening of the most potent repellent of these compounds against C. pipiens pallens were performed with a modified Y-tube olfactometer (Fig. 1). As shown in Table 1, the results showed that all the compounds exhibited repellency in varying degrees against C. pipiens pallens adult females at the testing dosage. For all the compounds except for geraniol, methyl salicylate and trans-cinnamaldehyde, the repellent activity exhibited time dependent and gradually decreased or diminished with duration increasing. Geraniol, methyl salicylate and trans-cinnamaldehyde had the most repellent activity in this preliminary screening study and were preferably used in the following studies.
3.2 Phase solubility studies
Stoichiometries and stability constants of the inclusion complexes of testing compounds and β-CD were estimated from the phase solubility diagrams (Fig. 4), which were obtained by plotting solubility of testing compounds as a function of β-CD concentration. The solubilities of trans-cinnamaldehyde, geraniol and methyl salicylate apparently increased linearly with the growing concentration of β-CD due to the formation of inclusion complexes. Solubilities of geraniol and methyl salicylate increased 4-fold and 3-fold in 0.014 M β-CD compared with their aqueous solubilities (intercept) respectively (see Table S1 in ESI†). The effect of the β-CD on the solubility of trans-cinnamaldehyde was smaller but still relevant and its increment was 1.4-fold. The phase solubility plots were AL-type,69 which indicated that in a complexation reaction solubility of ligand agents increases as the concentration increases and subscript L refers to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molecular ratio formation of soluble complexes.
1 molecular ratio formation of soluble complexes.
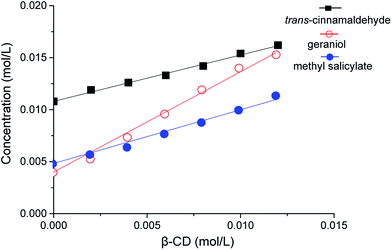 |
| | Fig. 4 Phase solubility diagrams of geraniol, methyl salicylate and trans-cinnamaldehyde with β-CD in water at 28 °C. | |
Table 2 showed the coefficients of determination (R2), slopes, intercepts (solubility when β-CD concentration is 0) and KS. The stability constant (KS) is a useful index to estimate the binding strength of the ligand and the host.53 A small KS value indicates a higher amount of free ligand due to a weak interaction between ligand and β-CD, which results in premature release of the ligand. On the other hand, a large KS indicates that the equilibrium is displaced towards the complex formation, which leads to a retarded or an incomplete release of the ligand and consequently absorption is hindered. Usually, complexes with KS from 100 to 5000 M−1 seem to be suitable for practical applications. In the light of these considerations, methyl salicylate is an ideal candidate compound.
Table 2 Phase solubility coefficients (KS) as well as the slope and intercept values for complex formation between β-CD and testing compounds at 28 °C
| Compounds |
R2 |
Intercept |
Slope |
KS (M−1) |
| trans-Cinnamaldehyde |
0.998 |
0.0108 |
0.446 |
74.54 |
| Geraniol |
0.998 |
0.00380 |
0.966 |
7476.78 |
| Methyl salicylate |
0.998 |
0.00461 |
0.517 |
232.19 |
3.3 Synthesis of pHEMA hydrogels with pendant β-CD
The synthesis of the pHEMA hydrogels was carried out in bulk by free radical polymerization of 2-hydroxyethyl methacrylate in the presence of crosslinker ethylene glycol dimethacrylate. Typically, the application profiles of pHEMA hydrogels in biomedical field are mostly determined by the hydrogel mechanical and swelling properties which are directly related to the crosslink density. The variation of crosslinker content in pHEMA hydrogels is helpful to raise the selectivity of water content and mechanical strength of hydrogel for different application.74 It has been found that the strength of hydrogels could be improved by incorporating crosslinking agents and increasing the degree of crosslinking. Arima et al. have demonstrated that strength tends to increase as the EGDMA concentration is increased, up to about 50%, after which the values level out or begin to fall.75 Particularly, water absorption drops with increasing crosslinking agent over the whole range of concentrations.75 These trends observed with EGDMA were in agreement with other crosslinkers.75 In present study, the effect of crosslinking density was evaluated with three different EGDMA concentrations and discussed in the next sections.
It has been well known that MCT-β-CD is a reactive cyclodextrin capable of forming covalent bonds with nucleophilic groups, such as –OH in the natural fibers. Inspired by these surface modifications of natural fibers with MCT-β-CD, we demonstrated that it is a practical method to fix the MCT-β-CD to pHEMA hydrogel. The quantity of MCT-β-CD fixed to pHEMA hydrogel was determined by gravimetric measurements. The amount of MCT-β-CD bonded on pHEMA was dependent on the height of hydrogel, crosslink density, and the heating temperature at the concentration of 0.14 g mL−1.
The results in Fig. 5 illustrated the influence of the height on the amounts of MCT-β-CD fixed on the surface of hydrogels with 2.7% EGDMA. However, the surface area, as a result, had insignificant influence on the average fixing amount at the same height. With the height of hydrogels increasing from 0.2, 0.4 cm to 0.6 cm, the average amounts decreased from 0.116 mmol g−1, 0.091 mmol g−1 to 0.080 mmol g−1, respectively (Table S2†). Although the average amount decreased, the total amount still increased due to the growing weight. When the concentration of EGDMA increased to 6.0%, the fixing amount of the II0.6–4 hydrogel significantly decreased to 0.065 mmol g−1. The effect of temperature of the heat treatment is shown in Fig. 6. The hydrogels were immersed in the MCT-β-CD aqueous solution at the concentration of 0.14 g mL−1. Temperature had positive influence on enhancing the fixing amount of MCT-β-CD which was nearly proportional to the heating temperature between 70 and 130 °C. Nevertheless, the higher temperature above than 150 °C would give a color change from colorless to yellow. The duration of heating treatment was also investigated. Ten minutes was sufficient to obtain a constant fixing amount.
 |
| | Fig. 5 (A) The average β-CD content (mmol per gram hydrogel) in pHEMA-T-β-CD hydrogels with different sizes. (B) The total β-CD content in pHEMA-T-β-CD hydrogels with different sizes. The square hydrogels were presented with their sizes (height–length). | |
 |
| | Fig. 6 Influence of reaction temperature on the content of β-CD in 0.6–4 pHEMA-T-β-CD hydrogels. | |
3.4 Swelling study and contact angle measurements
After immersing the dried discs in water, all of them reached saturable water absorption capacity within approximate 4 hours (see Table S3 in ESI†). The hydrogels with 2.7% EGDMA had good mechanical strength in hydrated state after extraction of initiators, reaction by-products, residual monomer, and impurities with boiling water. Hydrogels functionalized with β-CD possessed slightly lower swelling degrees of 63–66% than non-functionalized ones with swelling degrees of 65–68%. The degrees of swelling were similar for hydrogels with different sizes and the influence of the amount of β-CD was minor. When prepared with the lower EGDMA concentration of 1.0%, the I0.6–4 hydrogels were too fragile after extraction with boiling water to be handled and, therefore, were not suitably used for the further studies. This could be attributed to the lower crosslinking density and the higher water content after swelling. Mabilleau et al. have demonstrated that the Young's modulus of dried hydrogels sharply decreased after hydration.76 With EGDMA concentration increasing to 6.0%, hydrated II0.6–4 hydrogels showed good mechanical strength, but the swelling degree significantly decreased to 55%. The decrease in swelling degree is mainly due to two factors: first a matrix with higher crosslink density has less free space to be occupied by water; and second the crosslink degree generates a more compact network structure which limits the chains' mobility and increases the elastic force opposing to the expansion of the hydrogel's internal space.77 The swelling degree of II0.6–4 hydrogel was 53% after they were modified by MCT-β-CD. There was not much difference in the swelling degree of II0.6–4 hydrogels with and without being modified by MCT-β-CD. According to these results, the mechanical and swelling properties of pHEMA hydrogels were strongly influenced by the crosslink density, as it was reported by Arima et al.75 In addition, these results indicated that grafting of the MCT-β-CD did not significantly increase the degree of cross-linking of the network, as expected for MCT-β-CD to have more than one active arm.
To gain an insight into how the hydrophilicity of hydrogels varied after incorporation of hydrophilic β-CD and variation of the content of hydrophobic crosslinker, the contact angle measurements were made. The measurements of 0.6–4 hydrogels before and after modification with MCT-β-CD displayed theta values (θ°) of 71.9° ± 2.2 and 68.2° ± 1.8, respectively. For the II0.6–4, the θ° decreased to 64.3° ± 1.2 and 62.5° ± 1.5, respectively. This decrease could be ascribed to the more content of hydrophobic crosslinker and a more compact network structure restraining the wetting process of water. Nevertheless, the observed contact angle values less than 90° implied the good wettability of the hydrogels. Moreover, there was no significant difference of contact angles in pHEMA hydrogels before and after modification with MCT-β-CD, which could be attributed to the insignificant change in the degree of crosslinking as discussed above.
3.5 Fourier transform infrared spectroscopy (FT-IR) and thermogravimetric analysis
Fig. S1 (in ESI†) showed the FT-IR spectra of MCT-β-CD, pHEMA, and pHEMA-T-β-CD. MCT-β-CD exhibited a broad –OH stretching peak around 3300–3347 cm−1. The stretching vibration of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N around 1570 cm−1 were overlapped with those of the –OH bending of CDs at 1630 cm−1. C–Cl stretching vibration occurred at 690 cm−1. pHEMA showed a broad peak around 3300–3600 cm−1 and a sharp peak at 1720 cm−1 corresponding to –OH and C
N around 1570 cm−1 were overlapped with those of the –OH bending of CDs at 1630 cm−1. C–Cl stretching vibration occurred at 690 cm−1. pHEMA showed a broad peak around 3300–3600 cm−1 and a sharp peak at 1720 cm−1 corresponding to –OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration respectively. Asymmetric stretching of C–H is observed at 2950 cm−1. After modification, the pHEMA-T-β-CD showed an obviously increasing absorption in 1630 cm−1 ascribed to the –OH bending of CDs. Meanwhile, the presence of MCT-β-CD became evident by a broadening of –OH band around 3300–3600 cm−1 and disappearance of C–Cl stretching vibration at 690 cm−1.
O stretching vibration respectively. Asymmetric stretching of C–H is observed at 2950 cm−1. After modification, the pHEMA-T-β-CD showed an obviously increasing absorption in 1630 cm−1 ascribed to the –OH bending of CDs. Meanwhile, the presence of MCT-β-CD became evident by a broadening of –OH band around 3300–3600 cm−1 and disappearance of C–Cl stretching vibration at 690 cm−1.
Fig. S2 (in ESI†) showed the thermographs of pHEMA hydrogel and 0.6–4 pHEMA-T-β-CD hydrogel. The pHEMA hydrogel had obvious loss of weight starting from 280 to 440 °C. The maximum thermal decomposition at 370 °C was attributed to the depolymerization and decomposition of the backbone of pHEMA hydrogel. The pHEMA-T-β-CD hydrogel showed obvious loss of weight starting at 190 °C and a maximum thermal decomposition at 300 °C related to the degradation of CDs.78 These results indicated that functionalization of pHEMA hydrogel at 130 °C was stable enough to give pHEMA-T-β-CD hydrogels.
3.6 Cytocompatibility
The use of hydrogel for biomedical applications dates back to 1960 when Wichterle and Lim developed the first synthetic pHEMA hydrogels with EGDMA as crosslinker.79 Due to their absence of cytotoxicity or stimulatory effects, pHEMA-based hydrogels were widely used for production of topical contact lenses which were later developed as drug delivery systems to cure ophthalmic diseases.80 In this study, in vitro cytotoxicity against MEFs for pHEMA hydrogels was first performed by MTT assay as described. The cell viability (Fig. 7) was found to be higher than 95%, indicating very high biocompatibility. Hossein et al. has also demonstrated that cells in the presence of pHEMA hydrogels show high viability.58 Then, in vitro cytotoxicity of pHEMA-β-CD hydrogels was assessed to investigate their potential application in topical formulations. Fig. 7 showed that MEFs viability was over 95% after treated with extracts of MCT-β-CD modified pHEMA hydrogels for 24 h. According to GB/T 16886.5-2003 (ISO 10993-5: 1999), samples with cell viability larger than 75% can be considered as non-cytotoxic. Meanwhile, no skin irritation or allergy was observed on the treated skin of the human after application of MCT-β-CD modified pHEMA hydrogels loaded with repellents. These results suggested that the pHEMA hydrogels modified by MCT-β-CD were highly biocompatible and therefore readily available for topical formulations.
 |
| | Fig. 7 Cytotoxicity test of pHEMA and pHEMA-T-β-CD hydrogels. | |
3.7 Compounds loading and Y-tube olfactometer assay
In the preliminary screening assay, geraniol, methyl salicylate and trans-cinnamaldehyde exhibited the most repellent activity (see 3.1) and were chosen as the ones for intensive study. Loading of them into the pHEMA-T-β-CD hydrogels was carried out by immersion in a testing compound suspension followed by shaking at 28 °C for 48 h until reaching equilibrium. In such a way, the pendant CDs could fulfill their capability to form complexes. When a hydrogel is immersed in an aqueous drug solution, the amount loaded mainly depends on both the drug concentration in the loading solution and the drug's affinity to the hydrogel network. To gain an insight into the role of interaction between loading compounds and the pendant CDs, the partition coefficient K between the polymer network and the drug loading solution was estimated from the eqn (6).81,82| |
 | (6) |
where Vs is the volume of water absorbed by the hydrogel; Wp is the dried hydrogel weight; Vp is the volume of dried hydrogel; and C0 is concentration of the drug in the loading solution. The K value is an index of the affinity of the drug to hydrogel network. The amounts of loading compounds by 0.6–4 and II0.6–4 hydrogels and K values were shown in Table 3. Geraniol and methyl salicylate showed higher KT and KW values than trans-cinnamaldehyde, probably due to the strength of unspecific hydrophobic interactions with the hydrogel networks with and without pedant CDs. Similar trends of loading compounds were observed for II0.6–4 hydrogels (Table 3). The higher KT/KW value of geraniol was mainly attributed to its higher KS. Moreover, II0.6–4 hydrogels with higher crosslink density resulted in decrease in loading amounts of compounds, KW, and KT. Sun Yiming et al. have demonstrated that increasing the EGDMA content in pHEMA hydrogels resulted in decrease in the drug loading which was mainly due to a more compact network structure that restrained the drug diffusion and the interaction of macromolecular chains with drugs.83 In our case, the decrease of loading amounts could be caused by the decrease of CD content and the increase of crosslink density. How the physicochemical properties of the repellent and its affinity to form complexes affected its release profile was evaluated by Y-tube olfactometer assay (in vitro) and arm in cubical cage test assay (in vivo).
Table 3 Amount of trans-cinnamaldehyde, geraniol and methyl salicylate loaded by hydrogels, network/water partition coefficient KW and KT and relative values of KT/KW
| Compounds |
Hydrogel sample |
Loading amount in pHEMA hydrogel (mmol g−1) |
KW |
Loading amount in pHEMA-T-β-CD hydrogel (mmol g−1) |
KT |
KT/KW |
| trans-Cinnamaldehyde |
0.6–4 |
0.164 |
8.28 |
0.206 |
10.51 |
1.27 |
| II0.6–4 |
0.136 |
7.01 |
0.187 |
9.79 |
1.39 |
| Geraniol |
0.6–4 |
0.148 |
21.98 |
0.220 |
32.96 |
1.50 |
| II0.6–4 |
0.122 |
18.48 |
0.171 |
26.06 |
1.41 |
| Methyl salicylate |
0.6–4 |
0.178 |
21.78 |
0.227 |
27.91 |
1.28 |
| II0.6–4 |
0.153 |
19.12 |
0.193 |
24.22 |
1.26 |
Unlike application of hydrogels in aqueous phase, the well swollen hydrogels with the loading compounds were put in air to get the mosquito repellent profile. From the point of application, what we ultimately concern is the duration and percent repellency. Therefore, their release profile could be directly evaluated by the duration and percent repellency, as well as the reported topical repellent formulations.84–87 Theoretically, the more repellents hydrogels load, the better repellent activity and the longer duration hydrogels will exhibit. Therefore, 0.6–4 pHEMA-T-β-CD hydrogels were used for studying the repellents loading and release profile by a Y-tube olfactometer assay. Hydrogels without modification were used as controls. The results were shown in Fig. 8. It could be revealed that the control 0.6–4 pHEMA hydrogels loaded with geraniol, methyl salicylate and trans-cinnamaldehyde gave complete repellency for 4 h, 6 h, and 7 h, respectively. Geraniol had shorter lasting complete repellency hours and its percent repellency decreased quickly, which is mainly due to its intrinsic difference of repellency and relative higher values of KT and KS. Irrespective of the intrinsic difference of repellency, methyl salicylate got a longer increment of complete repellency for 3 h than geraniol and trans-cinnamaldehyde. Due to the lower loading amounts in II0.6–4 pHEMA hydrogels, methyl salicylate and trans-cinnamaldehyde showed shorter complete repellency durations of 5 h and 6 h than their loading in 0.6–4 hydrogels (see Fig. S4†). After modification of II0.6–4 pHEMA hydrogels with T-β-CD, methyl salicylate had a longer increment of complete repellency for 2 h than geraniol (1 h) and trans-cinnamaldehyde (1 h). The proper KT and KS values of methyl salicylate determined its superiority to the other two repellents. These facts revealed that the physicochemical properties of the repellent and its affinity to form complexes made a critical contribution to its application. At the same time, increasing the crosslink density resulted in a decrease in repellents loading and thus reduced the complete repellency duration.
 |
| | Fig. 8 Percent repellency of trans-cinnamaldehyde (A), geraniol (B), and methyl salicylate (C) loaded in 0.6–4 pHEMA-T-β-CD hydrogels and pHEMA hydrogels (as control). | |
3.8 Arm in cubical cage test and recyclability
Arm in cubic cage test is a quick and efficient method to investigate the efficacy of mosquito repellent formulation in laboratory. This method is recommended by WHO and suitable to evaluate topical repellents, such as creams, lotions and liquid formulations. Repellent-impregnated fabrics, polymer patches and gels also can be evaluated within cubic cage test.88,89 The 0.6–4 pHEMA-T-β-CD hydrogels loaded with geraniol, methyl salicylate and trans-cinnamaldehyde were applied onto the forearm and they were evaluated by an arm in a cage. The performances of geraniol, methyl salicylate and trans-cinnamaldehyde differed under in vivo and in vitro conditions, since in vivo experiments take the response of mosquitoes to human into consideration. The results of in vivo tests were shown in Fig. 9. In the case of without considering the intrinsic difference in repellency, the increment of complete repellency for methyl salicylate (2 h) was larger than the other two repellents (1 h). This mainly owed to the proper KT and KS values of methyl salicylate. Thus 0.6–4 pHEMA-T-β-CD hydrogels loaded with methyl salicylate gave a 10 h complete protection efficacy interval which was enough for an overnight protection. For the II0.6–4 hydrogels, geraniol, methyl salicylate and trans-cinnamaldehyde had similar trends compared with their release profiles in 0.6–4 hydrogels (see Fig. S5†). Methyl salicylate loaded in II0.6–4 pHEMA-T-β-CD hydrogels had an 8 h complete efficacy interval. Therefore, 0.6–4 hydrogels had an obvious superiority over II0.6–4 hydrogels. Besides, pHEMA-T-β-CD hydrogels showed very good recyclability at different crosslinker concentrations. Fig. 10 showed a 10 h average of repellency with each loading and release cycle of 0.6–4 pHEMA-T-β-CD hydrogels after 5 times and Fig. S6† showed an 8 h average of repellency of II0.6–4 pHEMA-T-β-CD hydrogels after 5 times. The average repellency had no significantly difference with the fresh cycle.
 |
| | Fig. 9 Protection efficacy of trans-cinnamaldehyde (A), geraniol (B), and methyl salicylate (C) loaded in 0.6–4 pHEMA-T-β-CD hydrogels and pHEMA hydrogels (as control). | |
 |
| | Fig. 10 Recycle numbers over 0.6–4 pHEMA-T-β-CD hydrogels loaded with methyl salicylate. | |
4. Conclusions
pHEMA hydrogels were preformed and functionalized with pendant T-β-CD. Influence of different conditions on the amount of T-β-CD bonded to pHEMA hydrogels was studied. Water absorption, cytocompatibility and thermal property of modified hydrogels were evaluated as well. The loading and release properties of geraniol, methyl salicylate and trans-cinnamaldehyde were investigated. The physicochemical properties of the repellent and its ability to form complexes determined the role played by the CDs in the loading and release. Methyl salicylate possessed an optimal stability constant KS and thus it got a longer protection duration with higher repellent activity. Additionally, increasing the crosslink density resulted in decreases in degree of swelling, the amount of T-β-CDs bonded to hydrogels, and the duration of complete repellency. pHEMA hydrogel with pendant T-β-CD is a suitable and recyclable formulation to load and delivery plant-based repellents into the surrounding environment efficiently. Current study lays down some preliminary works for further optimization of the performance of the plant-based repellents to provide a higher protection efficacy for a longer time.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (No. 31272074 and 31471800). The authors were thankful to Chen hongmei for cytocompatibility experiments.
References
- WHO fact sheet N117, 2015, http://www.who.int/mediacentre/factsheets/fs117/en/.
- F. Dube, K. Tadesse, G. Birgersson, E. Seyoum, H. Tekie, R. Ignell and S. Hill, Malar. J., 2011, 10, 375 CrossRef CAS PubMed.
- K. Karunamoorthi, Clin. Microbiol. Infect., 2011, 17, 1608–1616 CrossRef CAS PubMed.
- L. Roberts, Science, 2002, 298, 82–83 CrossRef PubMed.
- M. Enserink, Science, 2007, 317, 1485 CrossRef CAS PubMed.
- A. Wilke and M. Marrelli, Parasites Vectors, 2015, 8, 342 CrossRef PubMed.
- D. E. Neafsey, R. M. Waterhouse, M. R. Abai, S. S. Aganezov, M. A. Alekseyev and J. E. Allen, et al., Science, 2014, 347, 1258522 CrossRef PubMed.
- C. F. Oliva, D. Damiens, M. J. B. Vreysen, G. Lemperière and J. Gilles, PLoS One, 2013, 8, e78884 Search PubMed.
- J. T. Nishiura, P. Ho and K. Ray, J. Med. Entomol., 2003, 40, 498–507 CrossRef CAS PubMed.
- R.-D. Xue, W. A. Qualls, G. C. Muller and T.-Y. Zhao, J. Am. Mosq. Control Assoc., 2011, 27, 168–169 CrossRef CAS PubMed.
- T. O. Alimi, W. A. Qualls, D. D. Roque, D. P. Naranjo, D. M. Samson, J. C. Beier and R.-D. Xue, J. Am. Mosq. Control Assoc., 2013, 29, 49–53 CrossRef CAS PubMed.
- F. W. Mosha, I. N. Lyimo, R. M. Oxborough, J. Matowo, R. Malima, E. Feston, R. Mndeme, F. Tenu, M. Kulkarni, C. A. Maxwell, S. M. Magesa and M. W. Rowland, Ann. Trop. Med. Parasitol., 2008, 102, 367–376 CrossRef CAS PubMed.
- M. Betson, M. Jawara and T. Awolola, Malar. J., 2009, 8, 187 CrossRef PubMed.
- D. Thongwat and N. Bunchu, Asian Pac. J. Trop. Med., 2015, 8, 14–18 CrossRef CAS PubMed.
- N. Aizoun, R. Aikpon, G. Padonou, O. Oussou, F. Oke-Agbo, V. Gnanguenon, R. Osse and M. Akogbeto, Parasites Vectors, 2013, 6, 223 CrossRef CAS PubMed.
- N. Protopopoff, J. Matowo, R. Malima, R. Kavishe, R. Kaaya, A. Wright, P. West, I. Kleinschmidt, W. Kisinza, F. Mosha and M. Rowland, Malar. J., 2013, 12, 149 CrossRef PubMed.
- A. Ayorinde, B. Oboh, A. Oduola and O. Otubanjo, J. Insect Sci., 2015, 15, 75 CrossRef PubMed.
- F. Cantalamessa, Arch. Toxicol., 1993, 67, 510–513 CrossRef CAS PubMed.
- M. Kale, N. Rathore, S. John and D. Bhatnagar, Toxicol. Lett., 1999, 105, 197–205 CrossRef CAS PubMed.
- C. Wang, F. Chen, Q. Zhang and Z. Fang, J. Environ. Sci., 2009, 21, 1710–1715 CrossRef CAS.
- M. I. Yousef, Food Chem. Toxicol., 2010, 48, 1152–1159 CrossRef CAS PubMed.
- M. Maia and S. Moore, Malar. J., 2011, 10, S11 CrossRef CAS PubMed.
- J. C. Dickens and J. D. Bohbot, Pestic. Biochem. Physiol., 2013, 106, 149–155 CrossRef CAS.
- L. S. Nerio, J. Olivero-Verbel and E. Stashenko, Bioresour. Technol., 2010, 101, 372–378 CrossRef CAS PubMed.
- T. M. Katz, J. H. Miller and A. A. Hebert, J. Am. Acad. Dermatol., 2008, 58, 865–871 CrossRef PubMed.
- D. M. Wright, B. D. Hardin, P. W. Goad and D. W. Chrislip, Fundam. Appl. Toxicol., 1992, 19, 33–42 CrossRef CAS PubMed.
- F. B. Antwi, L. M. Shama and R. K. D. Peterson, Regul. Toxicol. Pharmacol., 2008, 51, 31–36 CrossRef CAS PubMed.
- Y. G. Gillij, R. M. Gleiser and J. A. Zygadlo, Bioresour. Technol., 2008, 99, 2507–2515 CrossRef CAS PubMed.
- M. B. Isman, Crop Prot., 2000, 19, 603–608 CrossRef CAS.
- F. Bakkali, S. Averbeck, D. Averbeck and M. Idaomar, Food Chem. Toxicol., 2008, 46, 446–475 CrossRef CAS PubMed.
- V. K. Bajpai, K.-H. Baek and S. C. Kang, Food Res. Int., 2012, 45, 722–734 CrossRef CAS.
- H. A. E. Shaaban, A. H. El-Ghorab and T. Shibamoto, J. Essent. Oil Res., 2012, 24, 203–212 CrossRef CAS.
- G. Fournier, M. Leboeuf and A. Cavé, J. Essent. Oil Res., 1999, 11, 131–142 CrossRef CAS.
- T. Burfield and S. L. Reekie, Int. J. Aromather., 2005, 15, 30–41 CrossRef CAS.
- J. U. Rehman, A. Ali and I. A. Khan, Fitoterapia, 2014, 95, 65–74 CrossRef CAS PubMed.
- A. D. Gonzalez, A. H. Pechko and R. E. Kalafsky, US Pat., US6969521, 2005.
- M. S. Fradin and J. F. Day, N. Engl. J. Med., 2002, 347, 13–18 CrossRef CAS PubMed.
- M. Brown and A. A. Hebert, J. Am. Acad. Dermatol., 1997, 36, 243–249 CrossRef CAS PubMed.
- B. D. Lett and H. S. Kraus, US Pat., US 5298250, 1994.
- N. P. Yadav, V. K. Rai, N. Mishra, P. Sinha, D. U. Bawankule, A. Pal, A. K. Tripathi and C. S. Chanotiya, BioMed Res. Int., 2014, 2014, 11 Search PubMed.
- C. E. Webb and R. C. Russell, J. Trav. Med., 2011, 18, 282–283 CrossRef PubMed.
- K. L. Lawrence, N. L. Achee, U. R. Bernier, K. D. Mundal and J. P. Benante, J. Med. Entomol., 2014, 51, 980–988 CrossRef PubMed.
- A. Wilson, V. Chen-Hussey, J. Logan and S. Lindsay, Malar. J., 2014, 13, 446 CrossRef PubMed.
- S. W. Avicor, M. F. F. Wajidi and Z. Jaal, Asian Pac. J. Trop. Biomed., 2015, 7, 386–389 Search PubMed.
- S. Ogoma, S. Moore and M. Maia, Parasites Vectors, 2012, 5, 287 CrossRef PubMed.
- J. Dubey, A. Banerjee, R. K. Meena, K. M. Kumari and A. Lakhani, Bull. Environ. Contam. Toxicol., 2014, 92, 650–654 CrossRef CAS PubMed.
- J. Zhang, H. W. Qi, Y. P. Sun, H. K. Xie and C. C. Zhou, Oncol. Lett., 2015, 9, 1667–1671 Search PubMed.
- R. Kodgule, S. Limaye, D. Salvi, S. Madas, V. Murlidharan and S. Salvi, Respirology, 2014, 19, 27 Search PubMed.
- C. Samperio, R. Boyer, W. N. Eigel, K. W. Holland, J. S. McKinney, S. F. O'Keefe, R. Smith and J. E. Marcy, J. Agric. Food Chem., 2010, 58, 12950–12956 CrossRef CAS PubMed.
- L. Szente and J. Szejtli, Trends Food Sci. Technol., 2004, 15, 137–142 CrossRef CAS.
- K. El-Tahlawy, K. El-Nagar and A. G. Elhendawy, J. Text. Inst., 2007, 98, 453–462 CrossRef CAS.
- F. Kayaci, Y. Ertas and T. Uyar, J. Agric. Food Chem., 2013, 61, 8156–8165 CrossRef CAS PubMed.
- H. M. C. Marques, Flavour Fragrance J., 2010, 25, 313–326 CrossRef.
- K. Pal, A. K. Banthia and D. K. Majumdar, Des. Monomers Polym., 2009, 12, 197–220 CrossRef CAS.
- E. M. Ahmed, J. Adv. Res., 2015, 6, 105–121 CrossRef CAS PubMed.
- A. S. Hoffman, Adv. Drug Delivery Rev., 2012, 64, 18–23 CrossRef.
- E. Caló and V. V. Khutoryanskiy, Eur. Polym. J., 2015, 65, 252–267 CrossRef.
- H. Omidian, K. Park, U. Kandalam and J. G. Rocca, J. Bioact. Compat. Polym., 2010, 25, 483–497 CrossRef CAS.
- L. Ferreira, M. M. Vidal and M. H. Gil, Int. J. Pharm., 2000, 194, 169–180 CrossRef CAS PubMed.
- J.-F. R. dos Santos, J.-J. Torres-Labandeira, N. Matthijs, T. Coenye, A. Concheiro and C. Alvarez-Lorenzo, Acta Biomater., 2010, 6, 3919–3926 CrossRef CAS PubMed.
- W. Sricharussin, C. Sopajaree, T. Maneerung and N. Sangsuriya, J. Text. Inst., 2009, 100, 682–687 CrossRef CAS.
- D. Knittel and E. Schollmeyer, J. Text. Inst., Part 1, 2000, 91, 151–165 CrossRef.
- A. Hebeish, A. Higazy, A. El-Shafei and S. Sharaf, Polym.-Plast. Technol. Eng., 2006, 45, 1163–1173 CrossRef CAS.
- M. Sundrarajan and A. Rukmani, J. Text. Inst., 2013, 104, 188–196 CrossRef CAS.
- R. de Bergamasco, G. Zanin and F. de Moraes, J. Inclusion Phenom. Macrocyclic Chem., 2007, 57, 75–78 CrossRef.
- A. Vyas, S. Saraf and S. Saraf, J. Inclusion Phenom. Macrocyclic Chem., 2008, 62, 23–42 CrossRef CAS.
- T. Furuta, Y. Kusuya, T.-l. Neoh, L. Rehmann, S.-h. Beak and H. Yoshii, J. Inclusion Phenom. Macrocyclic Chem., 2006, 56, 107–111 CrossRef CAS.
- F. Erler, I. Ulug and B. Yalcinkaya, Fitoterapia, 2006, 77, 491–494 CrossRef CAS PubMed.
- T. Higuchi and K. A. Connors, Adv. Anal. Chem. Instrum., 1965, 4, 117–212 CAS.
- M. Filippa, M. I. Sancho and E. Gasull, J. Pharm. Biomed. Anal., 2008, 48, 969–973 CrossRef CAS PubMed.
- F. Tao, L. E. Hill, Y. Peng and C. L. Gomes, LWT--Food Sci. Technol., 2014, 59, 247–255 CrossRef CAS.
- I. Mourtzinos, N. Kalogeropoulos, S. E. Papadakis, K. Konstantinou and V. T. Karathanos, J. Food Sci., 2008, 73, S89–S94 CrossRef CAS PubMed.
- C. E. Schreck, Annu. Rev. Entomol., 1977, 22, 101–119 CrossRef CAS PubMed.
- A. K. Bajpai and M. Shrivastava, J. Macromol. Sci., Part A: Pure Appl.Chem., 2002, 39, 667–692 CrossRef.
- T. Arima, T. Hamada and J. F. Mccabe, J. Dent. Res., 1995, 74, 1597–1601 CrossRef CAS PubMed.
- G. Mabilleau, I. C. Stancu, T. Honoré, G. Legeay, C. Cincu, M. F. Baslé and D. Chappard, J. Biomed. Mater. Res., Part A, 2006, 77, 35–42 CrossRef CAS PubMed.
- P. A. Faccia, eXPRESS Polym. Lett., 2015, 9, 554–566 CrossRef CAS.
- R. L. Abarca, F. J. Rodríguez, A. Guarda, M. J. Galotto and J. E. Bruna, Food Chem., 2016, 196, 968–975 CrossRef CAS PubMed.
- O. Wichterle and D. Lim, Nature, 1960, 185, 117–118 CrossRef.
- M. Tomar, N. Tomar, N. Gulati and U. Nagaich, International Journal of Health & Allied Sciences, 2012, 1, 224–230 Search PubMed.
- C. Rodriguez-Tenreiro, C. Alvarez-Lorenzo, A. Rodriguez-Perez, A. Concheiro and J. Torres-Labandeira, Pharm. Res., 2006, 23, 121–130 CrossRef CAS PubMed.
- S. Kim, Y. Bae and T. Okano, Pharm. Res., 1992, 9, 283–290 CrossRef CAS.
- Y. M. Sun and J. N. Chang, J. Polym. Res., 1995, 2, 71–82 CrossRef CAS.
- A. Masetti and S. Maini, Bulletin of Insectology, 2006, 59, 157–160 Search PubMed.
- J. Logan, N. Stanczyk, A. Hassanali, J. Kemei, A. Santana, K. Ribeiro, J. Pickett and A. J. Mordue, Malar. J., 2010, 9, 239 CrossRef PubMed.
- J. K. Yoon, K.-C. Kim, Y. D. Cho, H. S. Cho, Y.-W. Lee, M. Kim, B.-K. Choi, Y.-K. Oh and Y. B. Kim, J. Med. Entomol., 2014, 51, 182–188 CrossRef CAS PubMed.
- J. K. Yoon, K.-C. Kim, Y. Cho, Y.-D. Gwon, H. S. Cho, Y. Heo, K. Park, Y.-W. Lee, M. Kim, Y.-K. Oh and Y. B. Kim, J. Parasitol. Res., 2015, 2015, 6 Search PubMed.
- W.-J. Lee, H.-S. Lee, Y.-J. Ahn and D.-K. Lee, Entomol. Res., 2004, 34, 37–42 CrossRef.
- P. Chattopadhyay, S. Dhiman, S. Borah, B. Rabha, A. K. Chaurasia and V. Veer, Acta Trop., 2015, 147, 45–53 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra27942a |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 h (light/dark) photoperiod for over 20 generations free of exposure to pathogens or insecticides. Adult mosquitoes were maintained on a 10% glucose solution and blood fed on live mice. Non-blood-fed female adults (3 to 4 days old) were used in this study.
10 h (light/dark) photoperiod for over 20 generations free of exposure to pathogens or insecticides. Adult mosquitoes were maintained on a 10% glucose solution and blood fed on live mice. Non-blood-fed female adults (3 to 4 days old) were used in this study.

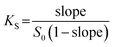



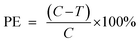
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molecular ratio formation of soluble complexes.
1 molecular ratio formation of soluble complexes.

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N around 1570 cm−1 were overlapped with those of the –OH bending of CDs at 1630 cm−1. C–Cl stretching vibration occurred at 690 cm−1. pHEMA showed a broad peak around 3300–3600 cm−1 and a sharp peak at 1720 cm−1 corresponding to –OH and C
N around 1570 cm−1 were overlapped with those of the –OH bending of CDs at 1630 cm−1. C–Cl stretching vibration occurred at 690 cm−1. pHEMA showed a broad peak around 3300–3600 cm−1 and a sharp peak at 1720 cm−1 corresponding to –OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration respectively. Asymmetric stretching of C–H is observed at 2950 cm−1. After modification, the pHEMA-T-β-CD showed an obviously increasing absorption in 1630 cm−1 ascribed to the –OH bending of CDs. Meanwhile, the presence of MCT-β-CD became evident by a broadening of –OH band around 3300–3600 cm−1 and disappearance of C–Cl stretching vibration at 690 cm−1.
O stretching vibration respectively. Asymmetric stretching of C–H is observed at 2950 cm−1. After modification, the pHEMA-T-β-CD showed an obviously increasing absorption in 1630 cm−1 ascribed to the –OH bending of CDs. Meanwhile, the presence of MCT-β-CD became evident by a broadening of –OH band around 3300–3600 cm−1 and disappearance of C–Cl stretching vibration at 690 cm−1.









