Electrocatalytic oxidation of ethanol on a glassy carbon electrode modified with a gold nanoparticle-coated hydrolyzed CaFe–Cl layered double hydroxide in alkaline medium
Masoumeh Taei*,
Elahe Havakeshian,
Hossein Salavati and
Fardin Abedi
Chemistry Department, Payame Noor University, 19395-4697 Tehran, I. R. of Iran. E-mail: m.taei@ch.iut.ac.ir
First published on 11th March 2016
Abstract
The Ca–Fe–Cl LDH was synthesized by a co-precipitation method in alkaline medium. Then, Ca–Fe-LDH was shaken in deionized water to form the hydrolyzed structure (H–CaFe–Cl LDH). After that, the hydrolyzed form was immobilized on the AuNPs electrodeposited on the GCE (LDH/AuNPs/GCE) and the electrocatalytic activity of this modified electrode was investigated for the ethanol oxidation in alkaline medium. The characterizations and electrocatalytic activity of the H–CaFe-LDH structure and the prepared electrodes were investigated using X-ray diffraction (XRD), field emission scanning electron microscopy (FE-SEM), Fourier transform infrared spectroscopy (FT-IR), cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). The results indicated that the LDH/AuNPs/GCE exhibits a higher current density and lower onset potential for ethanol oxidation in comparison with the LDH/GCE, AuNPs/GCE and AuNPs/LDH/GCE. The high active surface area and the strong adsorption of ethanol into LDH flakes were known as the main factors enhancing the catalytic activity of the LDH/AuNPs/GCE. Moreover, the chronoamperometric and cyclic voltammetric results showed that the LDH flakes immobilized on the AuNPs/GCE play a critical role in the increase of the catalyst endurance against poisonous species. As a result, the proposed electrode can be used as a suitable anode in direct alcohol fuel cells.
1. Introduction
Fuel cells are a family of technologies that generate electricity through electrochemical processes, rather than combustion. There are many fuel cell types, but the principal ones include alkaline fuel cells (AFCs), proton exchange membrane (PEM) fuel cells, direct fuel cells (DFCs), molten carbonate fuel cells (MCFCs), phosphoric acid fuel cells (PAFCs), and solid oxide fuel cells (SOFCs). The DFCs use liquid fuels without the reforming step, have a compact design and potentially can offer up to ten times the energy density of rechargeable batteries. In addition, DFCs can operate at ambient temperature, which significantly reduces the thermal management challenges for small systems. These advantages make the technology attractive to the rapidly growing need for portable power sources.Ethanol emerges as an attractive fuel in DFCs since it is less toxic, possesses a higher energy density than methanol and can be produced from biomass.1 However, direct ethanol fuel cells are still far from attaining acceptable levels of power output, since their performance is affected by the slow electrochemical ethanol oxidation and water and ethanol crossover.
Due to stable chemical properties, good biocompatibility, high catalytic activity and, more importantly, no observable surface poisoning during the electrochemical process compared with other metals,2 supported gold nanoparticles are used as efficient catalysts for application in pollution control, chemical processing and fuel cells. The nature of the support, the Au precursor, the preparation procedure, the pretreatment conditions and the reaction conditions are some factors influencing the catalytic activity of supported gold nanoparticles.3 Metal oxides, metal salts, carbon materials and clays4 are supports which have been studied for AuNPs, so far. Among them, layered double hydroxides (LDHs), known as anionic clays or hydrotalcite-like compounds, have attracted growing interest for use in numerous fields such as catalyst, photocatalysts, absorbents, drug delivery and fuel cells5–8 owing to their desirable properties, including low cost, high thermal stability and good catalytic activity.9 At the same time, the lamellar structure and anion exchange properties of LDHs make them attractive for technological applications as host structures for nanocomposite materials.9,10 It is well-known that by incorporating a guest species into/on the layered host, the properties of the structure are modified, which leads to the improvement of physical and chemical properties of the host lattice. Moreover, the way used for incorporating the components is also critical and effective on the composite properties.
In this work, AuNPs modified GCE was fabricated by cyclic voltammetry (CV) in HAuCl4 solution and the prepared electrode was referred as AuNPs/GCE. Then, AuNPs/GCE was coated with H–CaFe–Cl LDH by casting the brown suspension of H–CaFe–Cl LDH solution, and dried at room temperature. Finally, the electrocatalytic activity of the modified electrode, that was named LDH/AuNPs/GCE, was investigated for ethanol oxidation in alkaline medium.
2. Experimental
2.1. Apparatus and reagents
All electrochemical experiments were performed using a Metrohm instrument, Model 797 VA processor or Autolab potentiostat-galvanostat, Model PGSTAT302. A conventional three-electrode electrochemical system was used for all the electrochemical experiments, which consisted of a working electrode (the modified or unmodified GCE), a platinum wire counter electrode, and Ag/AgCl (3.0 mol L−1 KCl) as a reference electrode. A GCE with a geometric surface area of 0.0314 cm2 was used as the basal working electrode. All the reported potentials are vs. Ag/AgCl.The X-ray diffraction pattern was obtained by Holland Philips Xpert, X-ray diffractometer with Cu-Kα radiation. Fourier transformed infrared (FT-IR) spectra of sample in a pressed KBr matrix was recorded on a FT-IR, Jasco 4200 spectrometer in the range 4000–400 cm−1.
All chemicals used were of analytical grades and doubly distilled water was used throughout. Reagent grade CaCl2, FeCl3, ethanol, HAuCl4·3H2O, K3Fe(CN)6, K4Fe(CN)6 and NaOH were purchased from Aldrich Chemicals (Milwaukee, USA). All solutions were prepared just prior to use with deionized and double distilled water.
2.2. Synthesis of Ca–Fe-LDHs
The procedure used for the synthesis of the CaFe-LDH followed a coprecipitation method reported previously.11,12 In this synthesis, 0.05 mol of (CaCl2·2H2O) and 0.02 mol of (FeCl3·10H2O) were dissolved in 50 mL of deionized water. Then, above solution was slowly added into the 100 mL of alkaline solution which containing 0.12 mol of NaOH under vigorous magnetic stirring. The suspension was aged under magnetic stirring for 18 h at 25 °C. The product was washed with deionized water several times and dried at 50 °C.2.3. LDH/AuNPs/GCE preparation
A glassy carbon electrode was polished with alumina powder (5 μm) until a mirror-like surface was obtained. Then, GCE was sonicated in a mixture of ethanol/distilled water solution (50% v/v) for 10 min and dried in the air. Afterwards, the electrode was immersed into 4.0 × 10−3 mol L−1 HAuCl4·3H2O and 0.10 mol L−1 KNO3 for electrodeposition of AuNPs using cyclic voltammetry in the potential range of −1.50 to 0 V for 40 continuous cycles with a scan rate of 50 mV s−1. The Ca–Fe-LDHs were dispersed in distilled water (0.10 mg LDHs per 10 mL) using ultrasonic agitation to obtain a relative stable suspension. Finally, about 5.0 μL of hydrolyzed LDH suspension (H–CaFe-LDHs) (0.8% wt%) was dropped on the surface of the electrode and allowed to dry.The above procedure was also used to prepare the AuNPs/GCE, LDH/GCE and AuNPs/LDH/GCE. To load the same Au amounts on the electrodes, the scan number of cyclic voltammetry was controlled during AuNPs electrodeposition.
3. Result and discussion
3.1. Characterization of the synthesized Ca–Fe-LDH
The H–CaFe–Cl-LDH and CaFe–Cl-LDH samples were characterized using XRD as shown in Fig. 1a. The XRD patterns are in good agreement with that previously reported by G. Qian and co-workers.11,12 Furthermore, the FT-IR spectra of hydrolyzed form of LDH was also investigated in Fig. 1b. Two splitting bands in FT-IR at around 1480 cm−1 (consistent with the XRD pattern of CaCO3 at 2θ of 29.4°) was assigned to the stretching vibration of CO32− indicating CO2 contamination in the sample.13 FT-IR spectra indicates that vibrations characteristic of the LDHs structure, including the band of H–O–H bending in water molecule at 1627 cm−1, the vibrations of metal–O or metal OH band at 500–750 cm−1 and vibration of M–O–M lattice at 428–433 cm−1.14 As shown in Fig. 1b, the broad band of OH vibration at 3397 cm−1 was recorded by FT-IR spectrum of the H–CaFe-LDH. Furthermore, the band vibrations at 711, 585 and 473 cm−1 were assigned to the Fe hydroxide structure. The morphology of the synthesized CaFe–Cl-LDH powder (Fig. 1c) shows that the CaFe–Cl-LDH is an agglomerated sample that consists of fine nanoparticles. | ||
| Fig. 1 XRD pattern of CaFe-LDH and H–CaFe-LDH (a), FT-IR spectra (b), and SEM image (c) of the H–CaFe-LDH sample. | ||
3.2. Surface characterizations of the prepared electrodes
The surface morphology of the GCE after each modification step was investigated using FE-SEM. Fig. 2A shows that gold nanoparticles (AuNPs) with an average diameter of 50 nm are successfully electrodeposited and distributed uniformly on the GCE surface. As seen in Fig. 2B, a nearly smooth film covers the surface of the AuNPs/GCE after the immobilization of the H–CaFe–Cl LDH. Some cracks and many macropores are also seen on the surface. The FE-SEM image with high magnification of the LDH/AuNPs/GCE surface (Fig. 2C) shows that the film is composed of many connected LDH flakes and macropores. This structure can provide paths for the access of the electrolyte to the inner surfaces and, thereby, can improve the electrochemical behavior of the LDH/AuNPs/GCE. It is remarkable that the LDH/AuNPs/GCE is morphologically similar to the synthesized LDH sample shown in Fig. 1c, indicating that the morphology of the LDH remains unchanged after deposition on the AuNPs/GCE. The observation of the peaks related to Fe, Ca and Au elements in EDS spectrum, shown in Fig. 2E, demonstrates the presence of both the CaFe-LDH flakes and AuNPs on the GCE surface. The FE-SEM image of the AuNPs/LDH/GCE surface was also obtained (Fig. 2D), which displays a smooth film, which is decorated with AuNPs. | ||
| Fig. 2 FE-SEM images of the (A) AuNPs/GCE, (B and C) LDH/AuNPs/GCE at different magnifications, and (D) AuNPs/LDH/GCE; (E) EDS spectrum of the LDH/AuNPs/GCE. | ||
3.3. Electrochemical characterization of the prepared electrodes
Fig. 3A displays the Nyquist plots obtained from the prepared electrodes in 5.0 mM [Fe(CN)6]3−/4− solution. A linear part is seen for LDH/AuNPs/GCE, while the plots of the other electrodes consist of a semicircle at higher frequencies and a line with the angle of about 45° at lower frequencies. The diameter of semicircle corresponds to the charge transfer resistance (Rct) and the linear part is related to the diffusion of [Fe(CN)6]3−/4− species. The electrochemical behavior of LDH/AuNPs/GCE and the other electrodes can be explained using the electrical model circuits shown in Fig. 3B and C, respectively. Here, Rs, Rct, CPE and W (Warburg impedance) are related to the electrolyte solution resistance, charge transfer resistance of the electrode, constant phase element and mass transport process via diffusion, respectively. The obtained values are given in Table 1. Rct for AuNPs/GCE is 487 Ω, which reduces significantly after the deposition of LDH on its surface (curve c), so that the semicircle part for LDH/AuNPs/GCE doesn't exhibit in the Nyquist plot. Curve (b) shows that LDH itself has a high Rct, implying the LDH flakes inhibit charge transfer. Therefore, it is concluded that AuNPs immobilized under the LDH flakes facilitate the charge transfer process at the electrode. Moreover, it is found that Rct for AuNPs/LDH/GCE is higher than that for the LDH/AuNPs/GCE. Indeed, AuNPs electrodeposited on GCE can act as bridges between LDH and GCE and thereby improve electron transfer kinetics at the LDH/AuNPs/GCE.| Electrode | Rs (Ω) | Rct (Ω) | CPE | W × 10−2 (sn Ω−1) | |
|---|---|---|---|---|---|
| Y0 × 10−5 (sn Ω−1) | n | ||||
| LDH/GCE | 248 | 1428 | 0.91 | 0.77 | 0.13 |
| AuNPs/GCE | 238 | 487 | 4.20 | 0.62 | 0.20 |
| AuNPs/LDH/GCE | 233 | 198 | 4.79 | 0.78 | 0.29 |
| LDH/AuNPs/GCE | 239 | — | — | — | 0.03 |
The cyclic voltammograms of the unmodified and modified GCE in 0.50 M KOH solution are shown in Fig. 4. No distinctive peak is observed for GCE, while the LDH/GCE exhibits a pair of redox peak at about 0.40 and 0.10 V. These peaks are related to the conversion of Fe2+/Fe3+ associated with OH−.15 For the AuNPs/GCE, three anodic peaks at the peak potentials of −0.10, 0.18 and 0.50 V and two cathodic peaks at the peak potentials of −0.20 and 0.10 V are observed. These anodic and cathodic peaks, which correspond to the formation and reduction of Au oxides and hydroxides, respectively,16 demonstrate the electrodeposition of AuNPs on the GCE surface.
 | ||
| Fig. 4 Cyclic voltammograms of the (a) GCE, (b) LDH/GCE, (c) AuNPs/GCE, and (d) LDH/AuNPs/GCE in deaerated 0.50 M KOH at the scan rate of 50 mV s−1. | ||
All of the peaks related to the Au oxides/hydroxides formation and reduction with higher current density are observed for the LDH/AuNPs/GCE. Since the peaks related to the CaFe-LDH oxidation/reduction overlaps with some formation and reduction peaks of Au oxides/hydroxides, no distinctive peak is observed for the LDH in cyclic voltammogram of the LDH/AuNPs/GCE. It is remarkable that depositing LDH on the AuNPs/GCE results in an increase in the current density and a decrease in the overpotential of the Au oxide reduction peak presented at 0.10 V. This indicates that Au oxide species become unstable in the presence of Fe hydroxides.
3.4. Ethanol oxidation
To study the electrocatalytic activity of the LDH/AuNPs/GCE, its cyclic voltammogram in 0.50 M KOH solution containing 0.50 M ethanol was recorded and compared with those of the other prepared electrodes. As seen in Fig. 5, in the potential range of −0.40 to 0.40 V, the well-known peaks related to the ethanol oxidation at gold nanostructures appear in the forward and reverse scans for LDH/AuNPs/GCE, AuNPs/LDH/GCE and AuNPs/GCE. Compared with the AuNPs/GCE, the LDH/AuNPs/GCE exhibits higher current density (about 2 times) and 0.07 V lower onset potential (Eonset). Therefore, it is concluded that the LDH deposited on the AuNPs/GCE has a significant role in the increase of catalytic activity of the LDH/AuNPs/GCE. On the other hand, curve (b) in Fig. 5 shows that the catalytic activity of H–CaFe–Cl LDH for ethanol oxidation is so low that it is negligible against the catalytic activity of AuNPs. For comparison, the cyclic voltammogram of AuNPs/LDH/GCE in ethanol solution was also obtained (curve d in Fig. 5). The current density of AuNPs/LDH/GCE is about 1.4 times lower than that for the LDH/AuNPs/GCE. Moreover, Eonset for the AuNPs/LDH/GCE is 0.07 V more positive than that for the LDH/AuNPs/GCE. All of the results indicate that the LDH/AuNPs/GCE has higher catalytic activity and represents that the assembling arrangement of the catalyst components on the electrode surface affects mainly the electrocatalytic behavior. | ||
| Fig. 5 Cyclic voltammograms of (a) GCE, (b) LDH/GCE, (c) AuNPs/GCE, (d) AuNPs/LDH/GCE, and (e) LDH/AuNPs/GCE in deaerated 0.50 M KOH solution containing 0.50 M ethanol at the scan rate of 50 mV s−1. | ||
To find how the LDH/AuNPs/GCE has a better catalytic performance than the other electrodes, the electroactive surface area (SEASA) of the catalyst loaded on each electrode was obtained by applying cyclic voltammetry at different scan rates (ν) in 5.0 mM [Fe(CN)6]3−/4− solution. Then, Ip was plotted versus ν1/2 and SEASA was calculated using Randles–Sevcik equation. Since [Fe(CN)6]3−/4− species react at both AuNPs and LDH sites, SEASA obtained in [Fe(CN)6]3−/4− solution is related to SEASA(total) provided using both of AuNPs and LDH, i.e. SEASA(total) = SEASA(AuNPs) + SEASA(LDH). The obtained SEASA(total) values are equal to 0.032, 0.067 and 0.044 cm2 for the AuNPs/GCE, LDH/AuNPs/GCE and AuNPs/LDH/GCE, respectively. On the other hand, SEASA(AuNPs) is usually calculated using SEASA(AuNPs) = Q/390, where 390 μC cm−2 is the charge required to reduce AuO monolayer on unit surface area of the electrode and Q is the total charge corresponding to the reduction of AuO monolayer formed on the electrode surface and is obtained from the area of the surface oxides reduction peak in acidic solution. Cyclic voltammograms obtained from the above electrodes in 0.10 M H2SO4 solution showed that the area of the surface oxides reduction peak for AuNPs/LDH/GCE is higher than those for the AuNPs/GCE and LDH/AuNPs/GCE. This represents that SEASA(AuNPs) for the AuNPs/LDH/GCE is higher than that of the AuNPs/GCE and LDH/AuNPs/GCE. Moreover, SEASA(AuNPs) was 0.028 and 0.019 cm2 for AuNPs/GCE and LDH/AuNPs/GCE, respectively, which shows that SEASA(AuNPs) decreases slightly when LDH covers the AuNPs/GCE. Accordingly, the high value of SEASA(total) for LDH/AuNPs/GCE (i.e. 0.067 cm2) mainly results from the LDH flakes loaded on the electrode surface.
For a better comparison of the catalytic activity of different electrodes, the current peak, which each electrode exhibits for ethanol oxidation was normalized to its related SEASA(total) (IPf SEASA−1). As listed in Table 2, the normalized current value for LDH/AuNPs/GCE is lower than those for the AuNPs/GCE and AuNPs/LDH/GCE. Accordingly, the increase of SEASA(total) by the LDH flakes is the main factor enhancing the catalytic activity of the LDH/AuNPs/GCE.
| Electrode composition | Electrolyte (M) + ethanol (M) | Eonset (V) | EPfc (V) | If (mA) | SEASA(total) (cm2) | IPf/SEASA (mA cm−2) | Ib (mA) | If/Ib | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| a NMCC: nanoparticle modified carbon ceramic.b CNTs: carbon nanotubes.c EPf: forward peak potential. | |||||||||
| AuNPs/GCE | KOH (0.5) + 0.5 | −0.25 | 0.15 | 2.76 | 0.032 | 86.35 | 1.74 | 1.59 | This work |
| AuNPs/LDH/GCE | KOH (0.5) + 0.5 | −0.25 | 0.14 | 3.51 | 0.044 | 79.68 | 2.10 | 1.67 | This work |
| LDH/AuNPs/GCE | KOH (0.5) + 0.5 | −0.32 | 0.24 | 4.87 | 0.067 | 72.64 | 1.36 | 3.57 | This work |
| MgFe-LDH/GCE | KOH (1.0) + 1.0 | ∼0.4 | 0.45 | 48.7 | — | — | — | — | 14 |
| NiAlLDH/NMCCa | NaOH (0.1) + 0.05 | 0.40 | 0.70 | 0.92 | — | — | — | — | 18 |
| Pt/CNTsb/NixMg1−xAl2O4/C | KOH (0.5) + 0.5 | −0.55 | −0.10 | 29.0 | — | — | 34.5 | 0.84 | 19 |
The investigation of the scan rate effect on the peak current of ethanol oxidation (Ip) shows that for the LDH/AuNPs/GCE the plot of Ip/ν is more linear than Ip/ν1/2 plot (Fig. 6A), which indicates that the ethanol oxidation is under adsorption control. However, Ip for the AuNPs/GCE and AuNPs/LDH/GCE increases linearly with the square root of the potential scan rate (Fig. 6B and C, respectively), suggesting the ethanol oxidation at theses electrodes are controlled by the diffusion of ethanol. Accordingly, the adsorption of ethanol into LDH flakes of the LDH/AuNPs/GCE and the increase of ethanol concentration at the AuNPs centers (which catalyze the oxidation of ethanol) can be another factor which increases the catalytic activity of LDH/AuNPs/GCE. In the case of AuNPs/LDH/GCE, as it can be seen from SEM images in Fig. 2D, AuNPs cover the LDH surface, thereby block many LDH sites and consequently, prevent or/and reduce ethanol adsorption into LDH flakes. As a result, being lower ethanol concentration at AuNPs sites leads to the lower catalytic activity of the AuNPs/LDH/GCE in comparison with the LDH/AuNPs/GCE.
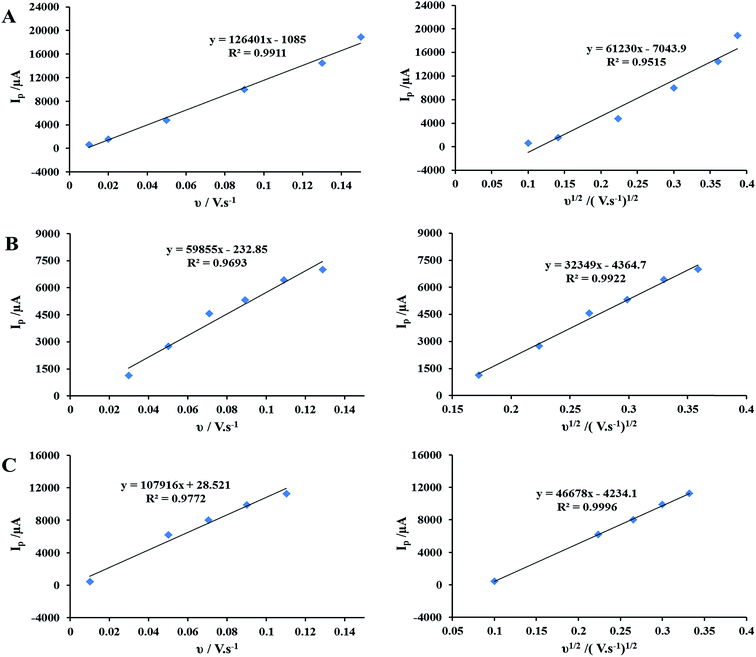 | ||
| Fig. 6 The scan rate effect on the peak current density of ethanol oxidation for (A) LDH/AuNPs/GCE, (B) AuNPs/GCE, and (C) AuNPs/LDH/GCE. | ||
Another important performance parameter is the ratio of the forward anodic peak current density (If) to the backward anodic peak current density (Ib). This parameter characterizes the tolerance of an electrocatalyst to carbonaceous oxidative intermediates accumulation on the electrocatalyst surface.17 As listed in Table 2, the value of If/Ib for LDH/AuNPs/GCE is 3.57, which is about 2.1 times higher than those for the AuNPs/LDH/GCE and AuNPs/GCE. This indicates that the oxidation of ethanol to the end products is performed effectively and/or the accumulation of poisoning species is low on the LDH/AuNPs/GCE surface.18
Some electrochemical performance parameters of the proposed electrode (LDH/AuNPs/GCE) and LDH composite-based electrodes studied for ethanol oxidation in other literature are also compared in Table 2.15,19,20 The results indicate that the LDH/AuNPs/GCE exhibits a better electrochemical performance than NiAl-LDH/NMCC. Although MgFe-LDH/GCE has a higher current density in comparison with the LDH/AuNPs/GCE, ethanol oxidation starts at lower potentials at the LDH/AuNPs/GCE. In the case of Pt/CNTs/NixMg1−xAl2O4/C, the higher current density and lower onset potential initially imply that the catalytic performance of Pt/CNTs/NixMg1−xAl2O4/C is better than the LDH/AuNPs/GCE. However, it is remarkable that If/Ib for the LDH/AuNPs/GCE is about 4.2 times higher than that for Pt/CNTs/NixMg1−xAl2O4/C, indicating the better tolerance of the LDH/AuNPs/GCE to carbonaceous oxidative intermediates.
3.5. Stability studies
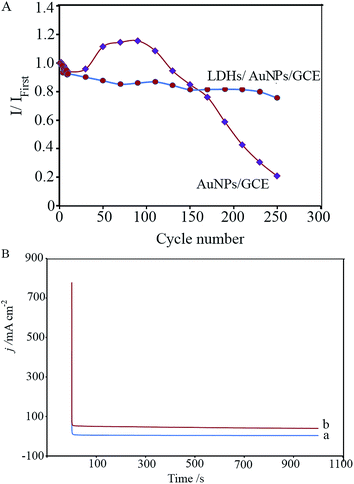
Poisoning species that are formed during the ethanol oxidation reaction can adsorb on the electrode surface and block its active sites, and thereby lead to a decrease in the catalytic activity of the electrode. Therefore, the endurance of the LDH/AuNPs/GCE against the poisoning species was investigated using cyclic voltammetry and chronoamperometry methods.250 successive cyclic voltammetry for the LDH/AuNPs/GCE and AuNPs/GCE were performed in the potential range of −0.40 to 0.80 V with a scan rate of 50 mV s−1 in a deaerated solution of 0.50 M ethanol. Then, the changes of the normalized forward anodic peak current of ethanol oxidation (I/I1st) were followed over 250 cycles. As shown in Fig. 7A, I/I1st decreases gradually for the LDH/AuNPs/GCE with cycle number and reaches to 78% of its value in the first scan after 250 potential cycles. In the case of the AuNPs/GCE, the current increases after 40th cycle. However, it declines sharply after 90th cycle, so that it reaches to about 20% of its value in the first scan. The results indicate that the poisonous species has a lower effect on the catalytic activity of the LDH/AuNPs/GCE in comparison with the AuNPs/GCE.
Moreover, the chronoamperometric curves for the LDH/AuNPs/GCE and AuNPs/GCE were obtained at the constant potential of 0 V for 1000 s in 0.50 M ethanol solution. As clearly seen in Fig. 7B, the LDH/AuNPs/GCE exhibits higher steady state current density than the AuNPs/GCE, demonstrating the greatly improved stability of the LDH/AuNPs/GCE against poisoning. The results suggest that the H–CaFe-LDH plays a critical role in the enhancement of the long-term stability of the electrode.
4. Conclusion
In this study, the H–CaFe–Cl layered double hydroxide synthesized using the co-precipitation method was immobilized on a glassy carbon decorated with AuNPs (LDH/AuNPs/GCE) and then, its electrocatalytic performance was studied for the ethanol oxidation in alkaline medium. The LDH/AuNPs supported on GCE was characterized by FE-SEM and electrochemical experiments, that showed a high active surface area and high level activity for the electrochemical oxidation of ethanol. Moreover, the proposed electrode exhibits satisfactory stability, which makes it attractive as an anode in fuel cells. The results reported here imply that the CaFe-LDH/AuNPs supported on a substrate can be further investigated and developed for the application in the fuel cells.References
- J. P. Pereira, D. S. Falcão, V. B. Oliveira and A. M. F. R. Pinto, J. Power Sources, 2014, 256, 14–19 CrossRef CAS.
- J. Zhao, X. Kong, W. Shi, M. Shao, J. Han, M. Wei, D. G. Evans and X. Duan, J. Mater. Chem., 2011, 21, 13926–13933 RSC.
- I. Dobrosz, K. Jiratova, V. Pitchon and J. M. Rynkowski, J. Mol. Catal. A: Chem., 2005, 234, 187–197 CrossRef CAS.
- B. Ballarin, A. Mignani, E. Scavetta, M. Giorgetti, D. Tonelli, E. Boanini, C. Mousty and V. Prevot, Langmuir, 2012, 28, 15065–15074 CrossRef CAS PubMed.
- J. Sun, Y. Zhang, J. Cheng, H. Fan, J. Zhu, X. Wang and S. Ai, J. Mol. Catal. A: Chem., 2014, 382, 146–153 CrossRef CAS.
- P. Kumar, K. Gill, S. Kumar, S. K. Ganguly and S. L. Jain, J. Mol. Catal. A: Chem., 2015, 401, 48–54 CrossRef CAS.
- J. M. Oh, M. Park, S. T. Kim, J. Y. Jung, Y. G. Kang and J. H. Choy, J. Phys. Chem. Solids, 2006, 67, 1024–1027 CrossRef CAS.
- L. G. Yan, K. Yang, R. R. Shan, T. Yan, J. Wei, S. J. Yu, H. Q. Yu and B. Du, J. Colloid Interface Sci., 2015, 448, 508–516 CrossRef CAS PubMed.
- M. Taei, F. Hasanpour and E. Dehghani, J. Taiwan Inst. Chem. Eng., 2015, 54, 183–190 CrossRef CAS.
- M. S. Auerbach, K. A. Carrado and P. K. Dutta, Hand Book of Layered Material, Marcel Dekker. NewYork, 2004, pp. 1–38 Search PubMed.
- J. Zhou, Z. P. Xu, S. Qiao, Q. Liu, Y. Xu and G. Qian, J. Hazard. Mater., 2011, 189, 586–594 CrossRef CAS PubMed.
- W. Wu, Y. Yu, J. Z. Zhou, J. Liu, Y. Chi, Z. P. Xu and G. Gian, Chem. Eng. J., 2012, 179(2012), 72–79 CrossRef.
- R. L. Frost, H. J. Spratt and S. J. Palmer, Spectrochim. Acta, Part A, 2009, 72, 984–988 CrossRef PubMed.
- S. J. Palmer, R. L. Frost and T. Nguyen, Coord. Chem. Rev., 2009, 253, 250–267 CrossRef CAS.
- M. Shao, F. Ning, J. Zhao, M. Wei, D. G. Evans and X. Duan, Adv. Funct. Mater., 2013, 23, 3513–3518 CrossRef CAS.
- J. Zhao, F. Wang, J. Yu and S. Hu, Talanta, 2006, 70, 449–454 CrossRef CAS PubMed.
- M. Hasan, S. B. Newcomb, J. F. Rohan and K. M. Razeeb, J. Power Sources, 2012, 218, 148–156 CrossRef CAS.
- R. Manoharan and J. B. Goodenough, J. Mater. Chem., 1992, 2, 875–887 RSC.
- G. Karim-Nezhad, S. Pashazadeh and A. Pashazadeh, Chin. J. Catal., 2012, 33, 1809–1816 CrossRef CAS.
- L. Zhang and F. Li, Appl. Clay Sci., 2010, 50, 64–72 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |