Pd nanoparticles modified rod-like nitrogen-doped ordered mesoporous carbons for effective catalytic hydrodechlorination of chlorophenols
Abstract
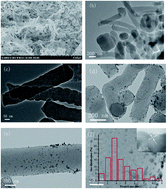
The preparation of rod-like nitrogen-doped ordered mesoporous carbons (NOMCs) through a facial aqueous soft-template self-assembly route in one-pot is presented in this work. After calcination at 800 °C under a nitrogen atmosphere, NOMCs with large specific surface area (∼419.3 m2 g−1), high pore volume (∼0.33 cm3 g−1), highly ordered mesostructure, and rich nitrogen content of 5.4 wt% were obtained. When NOMCs were used as the catalyst support, Pd nanoparticles (Pd NPs) could be well dispersed on the surface and in the mesopores, possibly owing to the coordination between the Pd NPs and the nitrogen atoms. The Pd modified NOMCs nanocatalyst exhibited higher catalytic performance for the hydrodechlorination of chlorophenols under mild conditions, compared with other Pd supported catalysts.

 Please wait while we load your content...
Please wait while we load your content...