Preparation and electromagnetic wave absorption properties of FeNi3 nanoalloys generated on graphene–polyaniline nanosheets
Abstract
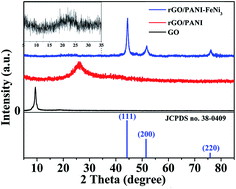
The ternary nanocomposites of rGO–PANI–FeNi3 were successfully synthesized by combining polymerization with hydrothermal reduction reaction. Graphene and polyaniline unite with FeNi3 to form ternary composite for the first time and the paraffin composite containing 20 wt% rGO–PANI–FeNi3 exhibit excellent electromagnetic wave absorption properties. The maximum reflection loss of −43.17 dB is obtained at 6.2 GHz and the absorption bandwidth (<−10 dB) of the reflection loss value can be obtained in the whole frequency range (2.96–18 GHz) with the sample thickness varying from 2.0 to 6.0 mm. The results demonstrate that rGO–PANI–FeNi3 nanocomposites containing magnetic loss and dielectric loss materials have potential applications in the electromagnetic wave absorbing area and can be widely used in developing electromagnetic wave absorption materials.

 Please wait while we load your content...
Please wait while we load your content...