Electrochemical detection of two tumor markers based on functionalized polypyrrole microspheres as immunoprobes†
Junqing Zhao,
Zilin Guo,
Jinjin Guo,
Junchun Wang and
Yuzhong Zhang*
College of Chemistry and Materials Science, The Key Laboratory of Functional Molecular Solids, Ministry of Education, Anhui Laboratory of Molecule-Based Materials and Anhui Key Laboratory of Chemo-Biosensing, Anhui Normal University, Wuhu 241000, People's Republic of China. E-mail: zhyz65@mail.ahnu.edu.cn; Fax: +86 553 3869303; Tel: +86 553 3869303
First published on 22nd March 2016
Abstract
In this study, a sandwich-type electrochemical immunosensor for the simultaneous detection of two tumor markers has been reported using functionalized polypyrrole microspheres as immunoprobes. The polypyrrole microspheres, which were synthesized through an one step chemical oxidative polymerization approach, showed high adsorption ability to redox probes such as thionine and adriamycin. Meanwhile, the polypyrrole microspheres functionalized with redox probes and gold nanoparticles (Au NPs) provided a larger surface area for the two different antibodies immobilization. The reduced graphene oxide sheet (rGO) was decorated with Au NPs as a substrate to immobilize two different antibodies. Carcinoembryonic antigen (CEA) and alpha-fetoprotein (AFP) were used as model analytes in this study. Two well resolved reduction peaks, one at about −0.26 V (corresponding to thionine), another at about −0.69 V (corresponding to adriamycin, both vs. SCE) can be obtained in differential pulse voltammograms. Under optimal conditions, CEA and AFP can be simultaneously detected in the range from 1.0 pg mL−1 to 50 ng mL−1. The limit of detection for CEA and AFP was 0.40 pg mL−1 and 0.33 pg mL−1 (at S/N = 3), respectively. Further, the immunosensor was evaluated using a human serum sample, and the recovery was within 99.5–107% for CEA and 96.3–99.9% for AFP. The analysis results with the serum sample using the immunosensor were in accordance with those of an enzyme linked immunosorbent assay (ELISA), indicating the immunosensor has a potential application in real sample analysis.
1. Introduction
Recently, the analytical methods for determination of multiple tumor markers simultaneously have attracted considerable attention as they can provide more information for early clinical diagnosis and treatment of malignancies. Therefore, various methods for multiple markers detection have been developed including optical and electrochemical methods.1–6 Among them, an electrochemical immunoassay has become a powerful analytical means due to its high sensitivity and selectivity. Up to now, various electrochemical immunoassay methods for the multiple tumor markers have been reported.7–11 To obtain high sensitivity, signal amplification strategies are often employed including enzymes, metal nanoparticles and quantum dots.12–15 For example, enzymes functionalized carbon nanotubes,16,17 metal nanoparticles functionalized polystyrene nanospheres18 and quantum dots coated silica nanosphere were used as immunoprobes for amplifying response signal.19 From literature, three main issues are need considered for high sensitive multiplexed immunoassay fabrication. The first important is to exploit a new nanomaterials with larger surface area for the redox probes and antibodies immobilization; second, it is suitable to search different redox probes which possess distinguishable and separate electrochemical signal; third, the peak potential should be controlled under 0.10 V (vs. SCE) to avoid the interferences of serum substance such as dopamine and ascorbic acid.Based on the reasons above, many attentions have been paid to the polymer nanomaterials due to their conductivity and larger surface area. Polypyrrole (PPy) microspheres obtained through simple oxidative polymerization possessed larger surface area and abundant active sites, indicating redox probes were easy to be immobilized on the surface of PPy microspheres. Thus, PPy microspheres have become one of ideal nanomaterials as immunoprobes.20 Previous, metal ions and redox probes were used as multiple immunosensor signal tags. Metal ions have narrow distinguishable voltammetric peak and immunosensor obtained had good sensing performance. However, the numbers of metal ions used for signal tags are limited (most reported metal ions Pb2+, Zn2+, Cu2+, Cd2+). In contrast to metal ions, the numbers of organic redox probes are abundant, and meanwhile it has well-defined redox peak. As a result, it enriched signal tags and types of immunosensors.
In this work, we selected adriamycin and thionine for signal tags and PPy microspheres were used for immobilized carriers. In design, PPy microspheres were first prepared to assemble the redox probes, and Au NPs were then assembled on the surface of functionalized PPy by dispersing the PPy microspheres in HAuCl4 solution and using NaBH4 reduced method in situ. The as-prepared functionalized PPy microspheres can load larger amounts of antibodies (carcinoembryonic antigen (CEA) and alpha-fetoprotein (AFP)). The immunoprobes obtained exhibited two separately voltammetric signals at potential of about −0.26 V (vs. SCE) (corresponding to thionine) and −0.69 V (vs. SCE) (corresponding to adriamycin) in differential pulse voltammogram, the peak to peak difference was about 430 mV. Moreover, peak potentials were controlled under 0.10 V (vs. SCE), indicating the cross and active substance interference in serum sample could be prevent. The electrochemical immunosensor was applied to real sample. The results obtained were well consistent with those of enzyme linked immunosorbent assay (ELISA).
2. Experimental
2.1. Reagents and materials
Monoclonal AFP antigen and antibodies, monoclonal CEA antigen and antibodies, prostate specific antigen (PSA) and humanchorionic gonadotropin (HCG) were purchased from Biocell Biotech. Co., Ltd (Zhengzhou, China). Standard solutions of CEA and AFP were from ELISA kits of CEA and AFP, respectively. Bovine serum albumin (BSA) was purchased from the Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Human serum albumin (HSA) and human immunoglobin G (IgG) were purchased from Sigma Co., Ltd. Reduced graphene oxide (rGO) (purity ≥ 98%) and sodium borohydride (NaBH4) were purchased from the Institute of Coal Chemistry, Chinese academy of sciences (Taiyuan, China). Adriamycin was purchased from Sangon Inc. (Shanghai, China). Thionine and pyrrole were purchased from Aldrich (St. Louis, USA). Chloroauric acid (HAuCl4·4H2O) (prior to use, it was distilled under reduced pressure), 30% hydrogen peroxide (H2O2) and ferrous chloride (FeCl2) were obtained from Shanghai Chemical Reagent Co., Ltd. (Shanghai, China). Phosphate buffered saline (PBS) with various pH values were prepared by mixing the stock solution of 0.1 M Na2HPO4 and 0.1 M NaH2PO4. The washing buffer was pH 7.0 PBS containing 0.05% (w/v) Tween (PBST). Clinical serum samples were from the clinical laboratory of the Yiji Shan Hospital (Wuhu, China). All solutions were prepared with twice-quartz-distilled water.2.2. Apparatus
Electrochemical experiments including cyclic voltammetry (CV) and different pulse voltammetry (DPV) were performed on CHI 650C electrochemical work station (Shanghai Chenhua Instrument, China). Three-electrode system consisting of a platinum wire as the auxiliary electrode, a saturated calomel electrode (SCE) as the reference electrode and bare glassy carbon electrode (GCE, 3 mm diameter, Shanghai Chenhua) or modified electrode as the working electrode was used in experiment. Energy dispersive X-ray spectrometer (EDS) and scanning electron microscope (SEM, S-4800, Hitachi, Japan) were employed in this work.2.3. Preparation of PPy microspheres
PPy microspheres were synthesized through a simple oxidative polymerization approach according to previous described with a little modification.21 In brief, 0.1 g FeCl2 and 0.50 mL 30% H2O2 were added into 60 mL water containing 1.0 mL pyrrole, and reacted for 12 h. During the process, pyrrole was oxidative polymerized to form microspheres. After that, the product was filtered, washed and freeze-dried treatment. Thus, PPy microspheres were obtained. SEM and EDS were employed to investigate the synthesized PPy microspheres.2.4. Preparation of immunoprobes
300 μL of 2 mg mL−1 thionine and adriamycin were injected into two equal parts of 2.0 mL PPy microspheres (suitable mount of PPy microspheres were dispersed in water under ultrasonic stirring) and ultrasonic stirred 12 h, respectively. During the process, signal tags (thionine and adriamycin) were attached on the surface of PPy microspheres, respectively. After centrifugation, the collected precipitation was re-dispersed in 10 mL of 10 mM HAuCl4, and 200 μL of 0.075% NaBH4 was gradually dropped into and reacted for 6 h. Thus, Au NPs was deposited on the PPy@signal tags due to the reduction interaction of NaBH4. The reaction solution was centrifugal settling, the precipitate obtained was denoted as PPy@thionine@Au NPs and PPy@adriamycin@Au NPs nanocomposites, respectively.150 μL anti-CEA (Ab2,CEA) and anti-AFP (Ab2,AFP) were injected into 2.0 mL of 0.50 mg mL−1 PPy@thionine@Au NPs or PPy@adriamycin@Au NPs solution, respectively. After 24 h, 100 μL of 1% BSA was then added into to block out the excess active sites. After 1 h, the solution was centrifuged and the product was washed, thus, the immunoprobes were obtained and denoted as PPy@thionine@Au NPs/Ab2,CEA or PPy@adriamycin@Au NPs/Ab2,AFP.
2.5. Fabrication of the immunosensor
The fabrication protocol of the immunosensor is shown in Scheme 1. First, 5 μL of 0.5 mg mL−1 rGO was dropped on the surface of clearly GCE (it was treated according to our previous reported22) and dried in air. Subsequently, the electrode modified with rGO was immersed in 1.0 mg mL−1 HAuCl4 and electrodeposited for 20 s at −0.20 V.23 Thus, Au NPs were deposited onto the surface of rGO.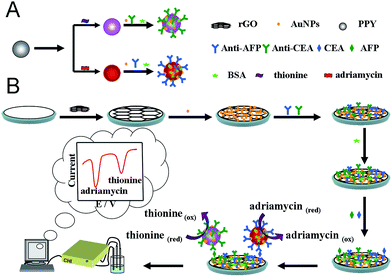 | ||
| Scheme 1 Schematic illustration of the preparation procedure of (A) PPy@signal tags@Au NPs/Ab2 bioconjugates and (B) the preparation of the sandwich type electrochemical immunosensor. | ||
Second, the electrode modified with Au NPs/rGO was incubated with the 2.0 mL mixture solution (200 ng mL−1 CEA + 200 ng mL−1 AFP) for 12 h at 4 °C. During the process, two antibodies were immobilized on the surface of Au NPs/rGO via Au–NH2 affinity. After washing with PBST solution, the modified electrode with antibodies/Au NPs/rGO was immersed in 0.25% BSA solution for 1 h to remove nonspecific adsorption. Thus, the immunosensor was obtained.
2.6. Electrochemical measurements
The immunosensor was first incubated with various concentrations of target antigens or serum samples for 50 min at 37 °C, followed by washing with PBST solution. It was then continuously incubated with two immunoprobes for 50 min at 37 °C. Finally, the immunosensor was transferred to 10 mL electrolytic cell with 3.0 mL pH 7.4 PBS for electrochemical measurement. Before experiment, high-purity nitrogen was injected into the solution for 10 min, and a blanket of nitrogen was maintained over the solution during the measurements. DPV was applied to record the responsive signal of immunosensor.3. Results and discussion
3.1. Investigation of the immunosensor assembled process
In this study, SEM was used to investigate the graphene and Au NPs decorated graphene, the results obtained are shown in Fig. 1S.† It can be seen that the graphene has irregularly crumpled and wrinkled sheet-like structure and Au NPs were uniformly loaded on the surface of graphene.CV technology was used to investigate the assembled procedure of immunosensor in 1.0 mM [Fe(CN)6]3−/4− solution and the results obtained are shown in Fig. 1A. In contrast to bare GCE (curve a), a sharply increased peak signal was observed at the rGO modified electrode (curve b) due to the excellent conductivity of rGO. And the peak current continuously increased after Au NPs were electrodeposited onto the surface of rGO (curve c). However, it could be seen that peak currents all decreased (curve d–f) after when the Ab1, BSA and antigens were successively immobilized on the surface of Au NPs/rGO, the reasonable explanation is that proteins are non-conductivity and block the electron transfer of [Fe(CN)6]3−/4−.
Fig. 1B showed the CVs of the immunosensor was employed to detect mixture of AFP and CEA. Two pairs of well-shape distinguishable voltammetric peaks were observed clearly, indicating the immunoprobes were captured on the surface of immunosensor via specific recognition interaction of antigen–antibody.
3.2. Characterization of immunoprobes
Fig. 2 displayed the morphologies of immunoprobes, a sphere and surface covering were observed. An average diameter of ppy microspheres was estimated about 260 ± 5 nm and Au NPs were well-distributed on PPy microspheres. The element analysis was accomplished using EDS and result obtained is shown in Fig. 2S.† It could be seen that the peaks of Au, C, N, O and S were appeared, indicating Au NPs and signal tags were assembled on the surface of PPy microspheres.3.3. Signal amplification
To investigate the signal amplification from Au NPs and PPy microspheres, various immunoprobes including (a) signal tags/Ab2, (b) signal tags@Au NPs/Ab2 and (c) PPy@signal tags@Au NPs/Ab2 (here, signal tags were thionine and adriamycin) were employed for this investigation. And the results are shown in Fig. 3. It could be observed that the signal was the strongest when PPy@signal tags@Au NPs/Ab2 bioconjugate was employed. The signal intensity was 1.8 fold and 1.4 fold respectively higher than that of signal tags/Ab2 and signal tags@Au NPs/Ab2 were used as immunoprobes, indicating PPy@Au NPs provided larger surface for the immobilization of lots of signal tags and amplified response signal.3.4. Optimization of experimental conditions
As is well known, the incubation time of the antigen–antibody and the acidity of the buffer solution are important factors for the sensing performance of the immunosensor. Therefore, we optimized experimental conditions of pH and incubation time in this study. The results obtained are shown in Fig. 3SA and B.† During 50 min, the signal intensities were clearly increased as incubation time increased. After that, signal intensities reached a stable value, so, 50 min was selected as the optimal incubation time. From Fig. 3SB,† it could be observed that the response signal was the strongest when pH was 7.4.3.5. Performance of the immunosensor
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C (ng mL−1) with a correlation coefficient of 0.9954. For AFP, the linear regression equation was I (μA) = 41.38 + 10.71
C (ng mL−1) with a correlation coefficient of 0.9954. For AFP, the linear regression equation was I (μA) = 41.38 + 10.71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C (ng mL−1) with a correlation coefficient of 0.9967. The limit of detection for CEA and AFP were 0.40 pg mL−1 and 0.33 pg mL−1 at a signal-to-noise ratio of 3, respectively. The low limit of detection was attributed to the immunoprobes containing lots of signal tags. Compared with previously reported in multiplexed immunoassay24–28 (seen Table 1S†), the immunosensor exhibited high sensitivity and wider linear range, the response signals were well-shaped and distinguished, and the peak potentials were lower than 0.1 V, so the interference from serum substance can be repelled.
C (ng mL−1) with a correlation coefficient of 0.9967. The limit of detection for CEA and AFP were 0.40 pg mL−1 and 0.33 pg mL−1 at a signal-to-noise ratio of 3, respectively. The low limit of detection was attributed to the immunoprobes containing lots of signal tags. Compared with previously reported in multiplexed immunoassay24–28 (seen Table 1S†), the immunosensor exhibited high sensitivity and wider linear range, the response signals were well-shaped and distinguished, and the peak potentials were lower than 0.1 V, so the interference from serum substance can be repelled.
The selectivity of immunosensor was evaluated in absence and presence of some interference including IgG, PSA, HCG and HSA. The results obtained are shown in Fig. 6. It can be observed that the signal intensities of CEA and AFP are kept constant in presence of interference, indicating the immunosensor had good selectivity.
Long-term storage stability was also examined. The average decrease value of peak current was less than 4.2% compared with freshly prepared immunosensor after stored in refrigerator for 7 days, and reached 9.7% for 20 days. And the results are exhibited in Fig. 7, indicating a good stability of the proposed immunosensor.
| Sample no. | This method (ng mL−1, n = 5) | ELISA (ng mL−1) | RSD (%, n = 5) | |||
|---|---|---|---|---|---|---|
| CEA | AFP | CEA | AFP | CEA | AFP | |
| 1 | 0.55 ± 0.04 | 0.42 ± 0.04 | 0.55 | 0.41 | +3.5 | +4.5 |
| 2 | 9.15 ± 0.25 | 2.12 ± 0.27 | 9.07 | 2.26 | +3.0 | −3.1 |
| 3 | 58.4 ± 0.60 | 59.6 ± 0.50 | 60.0 | 60.0 | −4.0 | +3.0 |
4. Conclusions
In summary, a sensitive multiplexed immunosensor has been reported using functionalized PPy nanospheres as immunoprobes. The immunosensor showed high sensitivity, selectivity and reproducibility. It could be applied to the detection of multiple tumor markers in real sample.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 20675002) and the Nature Science Foundation of Education Department of Anhui Province.Notes and references
- S. P. Song, B. Li, J. Hu and M. Q. Li, Anal. Chim. Acta, 2004, 510, 147–152 CrossRef CAS.
- D. P. Tang, J. Tang, B. L. Su, J. J. Ren and G. N. Chen, Biosens. Bioelectron., 2010, 25, 1658–1662 CrossRef CAS PubMed.
- P. S. Petrou, S. E. Kakabakos, I. Christofidis, P. Argitis and K. Misiakos, Biosens. Bioelectron., 2002, 17, 261–268 CrossRef CAS PubMed.
- Z. F. Fu, F. Yan, H. Liu, Z. J. Yang and H. X. Ju, Biosens. Bioelectron., 2008, 23, 1063–1069 CrossRef CAS PubMed.
- G. D. Liu, H. Wu, J. Lin and Y. H. Wang, Small, 2006, 2, 1139–1143 CrossRef CAS PubMed.
- K. Kojim, A. Hiratsuka, H. Suzuki, K. Yan, K. Ikebukuro and K. Isao, Anal. Chem., 2003, 75, 1116–1122 CrossRef.
- L. N. Feng, Z. P. Bian, J. Peng, F. G. Jiang, H. Yang, D. Zhu, L. P. Jiang and J. J. Zhu, Anal. Chem., 2012, 84, 7810–7815 CrossRef CAS PubMed.
- J. Han, Y. Zhuo, Y. Q. Chai, R. Yuan, W. Zhang and Q. Zhu, Anal. Chim. Acta, 2012, 746, 70–76 CrossRef CAS PubMed.
- Q. Zhu, Y. Q. Chai, R. Yuan and Y. Zhuo, Anal. Chim. Acta, 2013, 800, 22–28 CrossRef CAS PubMed.
- D. P. Tang, L. Hou, R. Niessner, M. D. Xu, Z. Q. Gao and D. Knopp, Biosens. Bioelectron., 2013, 46, 37–43 CrossRef CAS PubMed.
- T. Li, B. Shu, B. Jiang, L. Ding, H. Z. Qi, M. H. Yang and F. L. Qu, Sens. Actuators, B, 2013, 186, 768–773 CrossRef CAS.
- Y. Li, Z. Y. Zhong, Y. Q. Chai, Z. J. Song, Y. Zhuo, H. L. Su, S. M. Liu, D. Wang and R. Yuan, Chem. Commun., 2012, 48, 537–539 RSC.
- L. Li, W. P. Li, H. M. Yang, C. Ma, J. H. Yu, M. Yan and X. R. Song, Electrochim. Acta, 2014, 120, 102–109 CrossRef CAS.
- Y. Zhang, L. Li, J. J. Lu, L. Ge, S. G. Ge, M. Yan, X. R. Song and J. H. Yu, Sens. Actuators, B, 2013, 186, 545–549 CrossRef CAS.
- X. L. Jia, Z. M. Liu, N. Liu and Z. F. Ma, Biosens. Bioelectron., 2014, 53, 160–166 CrossRef CAS PubMed.
- J. F. Rusling, G. Sotzing and F. Papadimitrakopoulosa, Bioelectrochemistry, 2009, 76, 189–194 CrossRef CAS PubMed.
- G. S. Lai, F. Yan and H. X. Ju, Anal. Chem., 2009, 81, 9730–9736 CrossRef CAS PubMed.
- J. J. Shi, T. T. He, F. Jiang, E. S. Abdel-Halim and J. J. Zhu, Biosens. Bioelectron., 2014, 55, 51–56 CrossRef CAS PubMed.
- J. Qian, C. Y. Zhang, X. D. Cao and S. Q. Liu, Anal. Chem., 2010, 82, 6422–6429 CrossRef CAS PubMed.
- A. Ramanavicius, Y. Oztekin and A. Ramanaviciene, Sens. Actuators, B, 2014, 197, 237–243 CrossRef CAS.
- Z. Liu, Y. Liu, S. Poyraz and X. Y. Zhang, Chem. Commun., 2011, 47, 4421–4423 RSC.
- J. Wang, A. Q. Shi, X. Fang, X. W. Han and Y. Z. Zhang, Microchim. Acta, 2014, 181, 935–940 CrossRef CAS.
- Y. Z. Zhang and W. Jiang, Decorating graphene sheets with gold nanoparticles for the detection of sequence-specific DNA, Electrochim. Acta, 2012, 71, 239–245 CrossRef CAS.
- J. Tang, D. P. Tang, R. Niessner, G. N. Chen and D. Knopp, Anal. Chem., 2011, 83, 5407–5414 CrossRef CAS PubMed.
- J. H. Lin, Z. J. Wei and C. M. Mao, Biosens. Bioelectron., 2011, 29, 40–45 CrossRef CAS PubMed.
- X. Chen and Z. F. Ma, Biosens. Bioelectron., 2014, 55, 343–349 CrossRef CAS PubMed.
- Z. J. Song, R. Yuan, Y. Q. Chai, Y. Zhuo, W. Jiang, H. L. Su, X. Che and J. J. Li, Chem. Commun., 2010, 46, 6750–6752 RSC.
- D. X. Feng, L. H. Li, X. W. Han, X. Fang, X. Z. Li and Y. Z. Zhang, Sens. Actuators, B, 2014, 201, 360–368 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra01773h |
| This journal is © The Royal Society of Chemistry 2016 |







