Optically transparent electrodes for spectroelectrochemistry fabricated with graphene nanoplatelets and single-walled carbon nanotubes†
Abstract
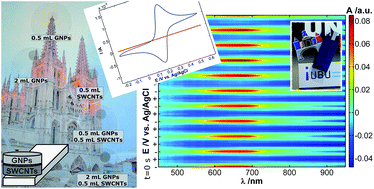
Optically transparent electrodes (OTEs) are needed for a wide range of applications such as solar cells, printable electronics, touch screens, light emitting diodes or flexible displays. Furthermore, OTEs are required for normal transmission spectroelectrochemistry measurements to obtain simultaneously electrochemical and spectroscopic responses. The search for new materials with a good transparency and conductivity, the basic requirements for an OTE, is outstanding. For this reason, carbon allotropes, such as graphene nanoplatelets (GNPs) and single-walled carbon nanotubes (SWCNTs), have been used in the present work in order to fabricate GNPs/SWCNTs-OTEs. The methodology used to fabricate these hybrid electrodes, based on vacuum filtration techniques, has several advantages such as the use of commercial nanomaterials, an easy cleaning of the final electrode and the availability of the process to almost any laboratory. The optimization of transparency and conductivity of these new electrodes has been achieved by design of experiments, showing that a percolation threshold of SWCNTs needs to be reached to ensure a minimum conductivity. The suitable performance of the GNPs/SWCNTs-OTEs has been validated by studying a film of poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) by spectroelectrochemistry.

 Please wait while we load your content...
Please wait while we load your content...