Fibrous AuPt bimetallic nanocatalyst with enhanced catalytic performance
Elvy Rahmia,
Akrajas Ali Umar*a,
Mohd Yusri Abd Rahmana,
Muhamad Mat Salleha and
Munetaka Oyamab
aInstitute of Microengineering and Nanoelectronics, Universiti Kebangsaan Malaysia, 43600 UKM Bangi, Selangor, Malaysia. E-mail: akrajas@ukm.edu.my; Fax: +60 389 250 439; Tel: +60 389 118 547
bDepartment of Material Chemistry, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, 615-8520, Japan
First published on 11th March 2016
Abstract
The nature of a physicochemical process and the surface reactivity properties involving charge transfer, catalysis, adsorption, surface segregation, etc., depend on the nanocatalyst morphology, available surface area and atomic composition. In this paper, we demonstrate a simple method to synthesize AuPt bimetal nanoparticles with an exotic morphology, namely fibrous nanoparticles (FNPs), directly on an ITO substrate surface via a liquid-phase deposition approach. The AuPt FNPs are constructed with a networked nanorod (diameter ca. 3–5 nm), and its morphology resembles a cauliflower. The lattice-mismatching effect between Au and Pt is assumed as the key factor for the formation of such an exotic structure. High-resolution transmission electron microscopy analysis shows that the nanorods are bounded by (001), a high-energy facet in the fcc crystal of Au or Pt. XPS analysis revealed that the AuPt FNPs show signs of possessing a highly unstable d-electron system at the top surface of the nanocrystals, which renders a highly reactive surface for efficient catalytic and surface reaction. Examination of the catalytic properties of the AuPt FNPs in a model catalytic reaction, namely acetone hydrogenation to produce isopropyl alcohol under microwave irradiation, reveals that the AuPt FNPs effectively and selectively converted the acetone to isopropyl alcohol with TON and TOF as high as 67 × 102 and 3.5 × 102 s−1, respectively. It was also found that the kinetic rate of the conversion linearly increases with increasing Au concentration in the AuPt FNPs. The mechanism of nanostructure growth and the detailed physicochemistry and catalytic properties of the AuPt FNPs will be discussed.
1. Introduction
Metallic nanoparticles have been widely explored as potential catalysts in a wide range of homogeneous and heterogeneous catalytic reactions due to their unique catalytic and surface functionalities that strongly depend on their shape and size.1–6 It has been witnessed that the catalytic and the selectivity properties can also be further expanded via compounding of two or more metallic systems.7 For example, the rate and the selectivity of the catalytic reaction of olefins hydrogenation on Pd8 can be enhanced for several order higher when 20% of Au was introduced into the Pd nanostructure or bimetallic system structure. Bimetallic nanostructure seems to be the most promising system in many cases for catalytic reaction not only because of its unusual catalytic properties but also due to its simple preparation procedure and straightforward structure and properties control.7,9,10 Platinum (Pt) based noble metal bimetallic system has been attracting a great deal of interest in this context due to its distinguish optical, magnetic, electrical and catalytic properties resulting from outstanding intrinsic catalytic properties of individual Pt nanostructure.11,12 In catalysis applications, particularly, enhanced selectivity and efficiency have been obtained in broad range of organic reaction, driving a great deal of effort to further explore the relationship between the atomic composition and the surface chemistry and physicochemical properties of these Pt-based bimetallic system. AuPt bimetal nanostructure has been used a potential model for bimetallic nanostructure synthesis and for the study of the relationship between the atomic composition on the structural growth and the catalytic properties of bimetal.13–16 Thank to significant lattice mismatch and different immiscibility between Au and Pt, unique crystal growth with large-area of high-energy surface can be realized. Peculiar catalytic properties have been obtained.13In this study, we demonstrate the synthesis of fibrous nanoparticles of AuPt bimetal (FNPs) directly on an ITO substrate with a high catalytic performance using a liquid-phase deposition method. The bimetallic AuPt FNPs is constructed by a networking of small nanorods with diameter and length of approximately 3–5 nm and 10–25 nm, respectively, and hence produce nanoparticles with large-surface area. By simply controlling the concentration of initial precursor concentration, AuPt bimetal FNPs with different Au to Pt atomic ratio can be realized. AuPt bimetal FNPs is expected to offer outstanding catalytic performance due to its unique surface chemistry properties resulting from being constituted by excellent catalytic properties of individual Au and Pt as well as its large surface area that promotes facile reactant and product diffusion onto the nanocatalyst surface.17 Acetone hydrogenation to produce isopropyl alcohol was used in this study as a model to evaluate the catalytic properties of the AuPt bimetal FNPs. Catalytic acetone hydrogenation is known as a benign method to produce isopropyl alcohol,18 a widely used chemical in broad range of scientific and technological process with function as a solvent for many organic reaction or potential fuel cell materials precursor.19 It was found that the AuPt FNPs may accelerate the acetone hydrogenation under microwave irradiation and can degrade the acetone up to 96% within only 40 second of reaction time. This is equivalent to TON and TOF (calculated at 40 seconds of reaction time) of as high as 96 × 102 and 2.4 × 102 s−1 respectively. It was also found that the rate of the catalytic hydrogenation of acetone increases with the increase of the Au content in the AuPt FNPs. The present acetone hydrogenation efficiency result is impressively high in the view of heterogeneous catalytic process. Peculiar surface atom high d-electron instability is assumed as the main reason for such high catalytic performance. Owing to its unique morphology with fibrous structure and simple preparation method, the AuPt bimetal fibrous nanoparticle should find extensive use in heterogeneous catalysis applications.
2. Experimental
2.1 Preparation of fibrous nanoparticle of AuPt (AuPt FNPs)
The AuPt FNPs was prepared on an ITO substrate by using our previous reported method,12,20,21 i.e. a liquid phase deposition. Briefly, a clean ITO substrate was immersed into a 15 mL aqueous solution that contains 1.0 mM potassium hexachloro platinate (Fluka), 0.67 mM chloroauric acid trihydrate (Fluka), 10 mM sodium dodecyl sulfate (Fluka) and 1.0 mM formic acid (Fluka). If we use this reaction condition, the concentration of Au and Pt ions should be approximately 0.28 and 0.40 mM, respectively. The solution was stirred at 400 rpm during the growth process and kept at a temperature of 40 °C. The growth time was fixed for 4 h. During the synthetic process, the growth solution gradually changes color from light yellow to dark gray. At the same time, a dark gray thin layer was also formed on the ITO substrate. Upon completion of the growth process, the ITO substrate was then removed from the solution, rinsed with a copious amount of deionized water and finally dried with a nitrogen gas flow. In order to obtain the effect of Au ion concentration on the structural growth of AuPt and its catalytic performance, the Au ion concentration was varied from 0.028 to 0.28 mM. Meanwhile, Pt ion concentration as well as other reagent were fixed. A pure water obtained from a Milli-Q water purification system was used for the preparation of aqueous solution throughout the study. All chemicals were used as received without any further purification process. ITO substrate (sheet resistance of 9–22 Ω per square was purchased from a VinKarola instrument, USA) was cleaned by a consecutive ultrasonication in acetone and ethanol for 30 min, respectively, prior to the growth process.2.2 Nanostructures characterization
Field Emission Scanning Electron Microscope (FESEM) Hitachi S-4800 operated at an accelerating voltage of 2 kV and high-resolution transmission electron microscopy (HRTEM) FEI Tecnai G2 F20 operated at accelerating voltage of 200 kV and 10–6 Pa with X-twin objectives lens were used to characterize the morphology of the AuPt FNPs. Energy-Dispersive X-ray (EDX), X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) analysis, were carried out to obtain the atomic composition, structural properties and chemical state of AuPt FNPs using EDX apparatus equipped with X-maxN 80T detector, XRD BRUKER D8 Advance system with CuKα irradiation (λ = 1.541 Å) and operated at a scan rate of 10° min−1 and Ulvac-PHI XPS Quantera II, with Al Kα – 1486.6 eV mono-chromated scanning X-ray source, respectively.For XPS analysis, the results were fitted by a pair of Gaussian–Lorentzian (GL) curves overlapping (70% and 30% for Gaussian and Lorentzian components, respectively) with a Shirley-type background. The calculations were calibrated against C1s element by setting the neutral C1s binding energy to 284.8 eV.
2.3 Heterogeneous catalytic characterization
The catalytic property of AuPt FNPs was examined in the hydrogenation of acetone to isopropyl alcohol under a microwave irradiation. The reaction procedure follows our recently reported method.20 Briefly, a Teflon sealed glass vial containing 0.1 M aqueous acetone and AuPt FNPs is placed in a microwaves oven (Panasonic home appliances microwave oven model NN-GDS577M) and exposed with a microwave irradiation power of 110 W. The kinetic hydrogenation of acetone was evaluated by recording the optical absorption spectrum of the solution every 10 s of the reaction using a Perkin Elmer Lambda 900 UV-VIS spectrometer. The temperature of the reaction was constantly monitored during the reaction using K-type thermocouple (FLUKE 50D). The acetone concentration during the catalytic hydrogenation process was calculated using a calibration curve, namely the extrapolation curve of the acetone absorption peak at 265 nm versus the concentration of acetone from 0.01 mol L−1 to 0.1 mol L−1.A high-performance liquid chromatography (HPLC) analysis was also carried out in this study to verify the formation of isopropyl alcohol in this process using an Agilent 1200 Series Rapid Resolution HPLC apparatus with an auto sampling system and a reflective index detector. HPLC pump was carried out under isocratic elution conditions using a Phenomenex RoA 300 × 7.8 mm column with 0.005 N H2SO4 as mobile phase that was operated at a flow rate of 0.6 mL min−1. Column temperature was kept at 60 °C during the process.
To evaluate the efficiency of the catalyst in converting the acetone to isopropyl alcohol, the turnover number (TON) and turnover frequency (TOF) were calculated based on the mole of catalyst involved and the product produced in the reaction. The catalyst mass on the ITO substrate was calculated via gravimetric method. At present, the active site on the catalyst surface cannot be obtained due to limited apparatus of chemisorption analysis. In order to approximate the value of TON and TOF, we simply used the entire catalyst mass on the ITO substrate for TON and TOF calculation,22 even though some portion of catalyst mass are confirmed to be not involved in the reaction due to grown on a substrate surface. From the gravimetric method, the mass of AuPt FNPs on single ITO slide (dimension of 1.2 × 1.5 cm) was found to be approximately 35.5 μg. By considering the atomic weight of Au (196.97 g mol−1) and Pt (195.08 g mol−1), the number of mole of AuPt catalyst could be approximately 95 nmol. For simplicity, the reduced mass concept was not applied and the atomic ratio of Au and Pt was considered as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 during the TON and TOF calculation. Besides the TON and TOF value, we also re-determined the conversion efficiency of the catalytic hydrogenation reaction respecting the yield of isopropyl alcohol, the mass of catalyst and the power of the microwave irradiation used, using the relation of η = (% yield of isopropyl alcohol)/(Cat.mass (μg) × microwave power (W)), to eliminate the effect of microwave irradiation power and to compare the result to the recent report.
1 during the TON and TOF calculation. Besides the TON and TOF value, we also re-determined the conversion efficiency of the catalytic hydrogenation reaction respecting the yield of isopropyl alcohol, the mass of catalyst and the power of the microwave irradiation used, using the relation of η = (% yield of isopropyl alcohol)/(Cat.mass (μg) × microwave power (W)), to eliminate the effect of microwave irradiation power and to compare the result to the recent report.
3. Results and discussion
3.1 AuPT FNPs synthesis and characterization
Fig. 1A illustrates a typical top-view FESEM image of AuPT FNPs growth on an ITO substrate. A well distributed AuPt FNPs are found to be effectively realized on an ITO substrate. As can be seen from the Fig. 1A, the AuPt FNPs exhibited a quasi-spherical morphology or cauliflower shape with diameter approximately 225 ± 44 nm. The nanostructure was found to cover nearly 92% of the ITO substrate. Actually the present reaction is a modification to our previous method in preparing fibrous Pt nanocubes.21 By introducing the Au ion into the growth solution, a drastic change in the morphology was obtained. Au ion in the reaction is a decisive factor in changing the nanoparticle morphology from the fibrous cubes (PtNCs) to fibrous cauliflower AuPt FNPs. It may be caused by the differences in the miscibility of the individual element, promoting a new morphology. The effect of lattice-mismatch as high as 4% between Pt and Au is also considered as a driving factor for the formation of new morphology of fibrous nanoparticles with a cauliflower like geometry.23 In this case we assumed that the presence of Au ion in the reaction promote a new crystal growth due to a unique selective interaction between Pt and Au. AuPt FNPs has extremely porous and fibrous and may provide a high active surface area for enhanced surface reaction.To reveal the crystalline phase of the AuPt FNPs, XRD analysis was carried out on the sample. Typical XRD spectrum of AuPt FNPs on an ITO substrate is shown on the Fig. 1B. As can be seen from the spectrum, by referring to the standard diffraction data provided for Pt and Au, JCPDS no. 70-2057 and 65-2868, respectively (see Table 1). The peaks at 2θ of 39.51°, 45.66°, and 66.59° can be associated with the AuPt FNPs with Bragg planes of (111), (200) and (220) respectively. Others peaks are attributed to the ITO substrate. As seen from the Table 1, the three prominent peaks of AuPt FNPs are fell in between those of the two pure metal elements (Pt and Au), namely slightly at the lower angle for about 0.25° to 0.86° if compared to the individual Pt and at the higher angle for about 1.28 to 2.02 if compared to Au. This result could be a strong indication of the successful formation of AuPt bimetal.
| Element | (111) | (200) 2θ (°) | (220) | JCPDS file no. |
|---|---|---|---|---|
| AuPt FNPs | 39.51 | 45.66 | 66.59 | — |
| Pt | 39.76 | 46.23 | 67.45 | 70-2057 |
| Au | 38.18 | 44.38 | 64.57 | 65-2868 |
The TEM images of the AuPt FNPs (Fig. 1C) shows a clear morphology of the bulk structure of the cauliflower AuPt FNPs. Surprisingly, it is shown that the AuPt FNPs is constructed by overlapping of large number of nanorods of length in the range of 10 to 20 nm. The diameter of the nanorod is about 3–5 nm. The structure may provide a high surface area that may accelerate the catalytic reaction due to a facile reactant and product diffusion onto the surface of the catalyst. High-resolution TEM image revealed that the nanorod that constructs the AuPt FNPs is single crystal in nature and grows along the [111] direction as judged from the d-spacing value of approximately 0.22 nm (see Fig. 1D). If compared to the d-spacing value of (111) facet of PtNCs and AuPt FNPs, the value is not changed significantly. However, the XRD results in Fig. 1B provides strong evidence that the AuPt FNPs truly constructed by the combination of Au and Pt elements. However, the detailed effect of specific chemical interaction between of Au and Pt atoms in bimetallic nanocrystals growth is not clear at the moment. Nevertheless, based on the EDX mapping analysis (will be discussed), it is indicated that the Au atom is effectively substituted into the Pt crystal, instead of interstitially grown. As can also be seen from the Fig. 1E, by considering the symmetry of the single nanorod, the nanorod wall faces should belong to the (001) Bragg plane, namely one of the highest surface energy of fcc crystal of Au and Pt. Actually, by considering the symmetry of the nanorod of most noble metals, including Au and Pt, they also comprise of (110) facets,24 the highest energy facet. However, it has a less portion compared to the (001) facet on the surface of individual nanorods. Thus, the AuPt FNPs structure provides a high energy surface, which is potential for enhanced catalytic properties.
Fig. 2 shows EDX elemental mapping of AuPt FNPs with different Au atoms concentration. As can be seen from the figure, the AuPt FNPs is confirmed to be constructed by two main elements, namely Pt and Au. One important fact can be noted here that the Au atomic distribution in the AuPt bimetallic FNPs depends on the concentration of Au ion precursor in the reaction. At low concentration (0.028 mM), the nanostructure exhibits to contain the Au atoms at the center region of the nanostructure (see Fig. 2A). While the Pt is homogeneously distributed on the entire of the structure. If the Au ion concentration increased to ten times higher, namely 0.28 mM, the nanostructure has the Au as well as the Pt atoms homogenously distributed throughout the structure (Fig. 2B). Certainly, such unique fact may generate peculiar optical, electrical and catalytic properties, of which depend on the atomic distribution in the nanostructure. This also infers that the properties can be simply tuned by preparing the nanostructure with different Au ion concentrations during the reaction. This therefore promise unusual performance in applications.
 | ||
| Fig. 2 Energy-dispersive X-ray (EDX) mapping of AuPt FNPs. (A) AuPt FNPs prepared using Au ion concentration of 0.028 mM and (B) 0.28 mM. | ||
In accordance with the dependency of Au atomic distribution in the nanocrystal, the dynamic growth (indicated by the nanostructures' size and density) of the nanostructure is also determined by the concentration of Au ion in the reaction. This is certainly due to the effect of surface segregation, the change in the growth kinetics or due to direct interactions between Pt and Au ion in growth solution. It may then drive kinetics exchange and the binding competition between Pt and Au that favor the anisotropic growth of AuPt FNPs.25 For example, at low Au ion concentration (0.028 mM), it was found that the density of AuPt FNPs grown on the ITO substrate is low with surface coverage less than 20%. The surface coverage is calculated by estimating the effective area occupied by FNPs within a 1 μm × 1 μm of ITO substrate surface obtained from the FESEM analysis. While the average diameter is only as high as 116 ± 39 nm. The density significantly increase to 40% and 100% if concentration augmented to 0.084 and 0.28 mM, respectively. Similarly, the size of the nanostructure drastically increased to 151 ± 61 nm and 225 ± 44 nm if using Au ion concentration of 0.084 and 0.28 mM, respectively. The results are shown in Fig. 3. Regarding the morphology, in the absence of Au ion, the morphology of the fibrous structure is cube. It gradually change with the increasing of Au ions concentration in the reaction to cauliflower shape.
Actually, the present novel structure is synthesized following our previous method for the formation of fibrous Pt nanocubes.21 It has been predicted that, while nanorod structure is formed via effective directed-growth of the nanocrystal by sodiumdodecyl sulphate surfactant, the fibrous structure resulted from the networked-nanorod is formed via a long-range surface interaction amongst the nanorods.26 In the absence of Au ion in the reaction, the networked-nanorods formed fibrous nanocubes morphology. Due to a lattice-mismatching that does not only distort the preferred growth orientation of the nanocrystal but also modifies the chemistry of the nanostructure surface, the morphology of the Pt nanostructure transforms from nanocube in the absence of Au atom to cauliflower shape in the AuPt bimetal. Such modification in the nature of nanocrystal growth using the presence approach upon the introduction of foreign ion in the reaction has also been observed in the case of AgPt nanofern.12 The presence of foreign ion during the Pt nanocrystal growth may influence the galvanic-assisted reduction and binding selectivity to the Pt system that restrict or increase the crystallographic growth rate toward particular direction in the nanocrystal.27–29
3.2 Catalytic properties
The heterogeneous catalytic properties of bimetallic AuPt FNPs was examined in the heterogeneous catalytic hydrogenation of acetone to isopropyl alcohol under microwave irradiation. The procedure for the microwave assisted catalytic hydrogenation of acetone followed our previous reported result. Briefly, in typical process, we firstly examined the effect of microwave irradiation power on the acetone hydrogenation. Microwave power used was in the range of 110 to 1100 W with exposure time as long as 1 min. 10 mL of 0.1 mol L−1 aqueous acetone was used in this study. Successful hydrogenation process was determined by the decreasing of characteristic optical absorbance band of acetone that is centered at λ = 265 nm. As shown in our previous result,20 the optimum power that gives high-yield conversion is 110 W, namely the lowest microwave power. As has been well-known, the increasing of the microwave power causes excessive temperature increment during the reaction, promoting the formation of isobutyl ketone instead of isopropyl alcohol. Therefore, for further analysis, microwave power of 110 W is used.Similar to our previous work, the effective formation of isopropyl alcohol during the acetone hydrogenation process was examined using high-performance liquid chromatography (HPLC) method. Fig. 4 shows typical HPLC spectra of the reaction during 30 s of the acetone hydrogenation process. As the Fig. 6 shows, two separate peaks with retention times of 25.67 and 27.55 respectively, are appeared in the spectrum. These peaks belong to acetone and isopropyl alcohol respectively. This result confirms the successful hydrogenation of acetone to isopropyl alcohol. No other peaks are observed inferring the present catalytic hydrogenation reaction is very selective and produced isopropyl alcohol only.
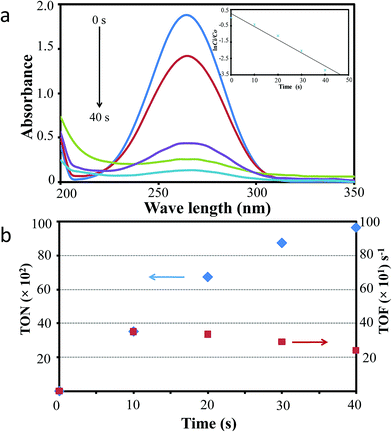
Fig. 5 shows the typical characteristic of catalytic hydrogenation of acetone in the presence of optimum AuPt FNPs sample (prepared using Au ion concentration of 0.28 mM) and microwave irradiation (110 W). As the optical absorbance dynamic of acetone during the catalytic hydrogenation as shown in Fig. 5a reveals, the acetone is effectively hydrogenated to form isopropyl alcohol as the presence of rapid decreasing in its absorbance at the characteristic peak (265 nm). For example, at the first 10 s of the reaction, the per cent yield of isopropyl alcohol obtained achieved up to 35% and it reaches the yield of 67% for a reaction time of as short as 20 s. The yield finally reached approximately 96% at the reaction time of 40 s. This phenomenon is clearly depicted by the acetone hydrogenation reaction kinetic shown in inset of Fig. 5a. Considering the nature of the catalytic reaction that uses catalyst system attached on a substrate surface and considering a limited surface reaction on the catalyst surface due to possible high liquid-surface hindrance, the present result is highly effective. By weighing the mass of the catalyst on the ITO substrate, the turnover number (TON) and the turnover frequency (TOF) of the reaction were calculated (see Experimental section for a details). The results are shown in Fig. 5b. As can be seen from the figure, the TON is exponentially increase with the increasing of the reaction time. As have been mentioned previously, the reaction was nearly vanished at the reaction time of 40 s with yield of the conversion of as high as approximately 96%. However, to evaluate the TON and TOF of the reaction, as widely adopted, the conversion yield at half-way reaction, i.e. 20 s in this case, was used. It was found that the TON as high as 67 × 102 was obtained at 20 s reaction time. This is equivalent with the TOF of as high as 3.5 × 102 s−1. This value is extremely high in term of heterogeneous catalytic reaction and this could be as the result of: (i) the large surface area of the structure due to being constructed by nanorods-networks. (ii) The effect of special surface chemistry properties upon bimetallisation. (iii) Judging from the TEM analysis result, the high-catalytic efficiency could also be related to the existence of high-energy surface, particularly (001) facet, in the AuPt FNPs. As also can be seen from the figure, the TOF value is slowly decrease with the increasing of the reaction time, for example decreases to 2.4 × 102 s−1 at 40 s reaction time. This reflects that the catalyst surface deactivation or poisoning are limited over this AuPt catalyst. This is probably due to the unique nature of the AuPt FNPs that possesses large-surface area, special surface chemistry properties and high-energy facet of (001).
In order to assess the performance of the AuPt FNPs nanocatalyst in the hydrogenation of acetone under a microwave irradiation, we compared the obtained performance with the currently reported results (see Table 2). For this purpose, we re-determined the catalytic efficiency postulation as η = (% yield of isopropyl alcohol)/(mass of catalyst (μg) × microwave power (W)) to eliminate the microwave irradiation effect in the acetone conversion. As can be seen from the Table 2, the present AuPt FNPs nanocatalyst is several order higher in efficiency than the recently reported results on the homogeneous and heterogeneous catalytic hydrogenation of acetone to isopropyl alcohol.5,18
| No. | Catalyst | Cat.mass | React. time | % of deg. | Conversion efficiency | Ref. |
|---|---|---|---|---|---|---|
| a Microwave (110 W) was applied.b Temperature of 80 °C and H2 were applied.c Temperature of higher than 100 °C and H2 was used.31 | ||||||
| 1 | Bimetallic AuPta | 35.5 μg | 40 s | 96 | 0.26% μg−1 W−1 | Present study |
| 2 | a-Pt nanofibera | 30 μg | 2 min | 40 | 0.01% μg−1 W−1 | 20 |
| 3 | PtGab | 50 mg | 12 h | 88 | 1.76 × 10−2% mg−1 °C−1 | 30 |
| 4 | Rhc | 200 mg | 13 h | 98 | 4.9 × 10−3% mg−1 °C−1 | 30 |
| 5 | RANEY® Nic | 2900 mg | 1.5 h | 99.8 | 4.31 × 10−5% mg−1 °C−1 | 18 |
While the AuPt FNPs bimetal shows enhanced-catalytic performance compared to the traditional RANEY® Ni32,33 or Rh34 catalysts, we then examined the effect of Au ions concentration in the AuPt FNPS on the catalytic performance. The catalytic performance result that have been normalized with the mass of catalyst on the surface is shown in Fig. 6. As the figure reveals, the catalytic performance linearly increases with the increasing of Au ion concentration in the AuPt FNPs. This could be attributed to the disruption of the electrons stability in d orbital of Pt system, which increase with the increasing of the Au ion concentration, as the result of intense lattice distortion due to the bimetallisation. As the result, the ability to catalyze and to promote the hydrogenation reaction is enhanced. This condition is consistent with the XPS results, where the increasing of the Au ion introduced into the bimetallic system will promote the possibility of Pt to reduce Au species or, in other words, accelerate the electrons transfer from the Pt to Au species across the Fermi levels at the interface. It will then promote the formation of the metallic Pt species than other species. The charge distribution of Pt and Au will therefore compensate the surface potential at their interface and the lattice mismatch between the two elements tends to favor the crystal strains, producing modification of nanoparticle electronic constellation and nanocrystal geometry. Moreover, this phenomenon will result peculiar surface atomic and electronic structure, favoring catalytic reaction.
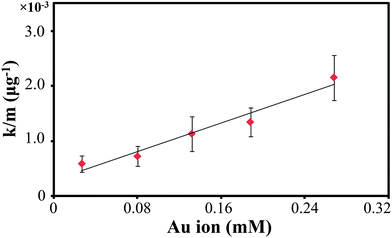 | ||
| Fig. 6 Kinetic rate (normalized over catalyst mass) of acetone hydrogenation over AuPt FNPs with different Au atom concentrations. | ||
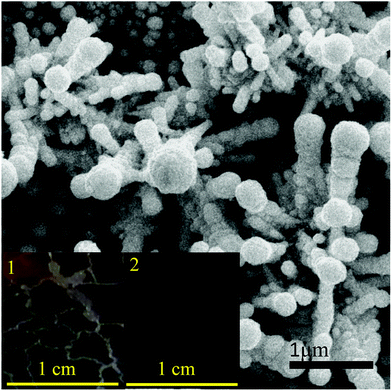
Unfortunately, the performance of the AuPt FNPs prepared using Au ion concentration higher than 0.28 mM could not be obtained due to the inconsistency of the catalyst mass on the substrate surface as the result of AuPt FNPs peel-off. As has been mentioned earlier, the increasing in the Au ion concentration in the reaction accelerated the growth of the AuPt FNPs on the surface, producing large size and thick AuPt FNPs layer on the surface. FESEM analysis on the sample indicated that the sample morphology was modified from quasi-spherical nanofibrous to branched-fibrous microrod structures (Fig. 7). Presumably because of their massive microstructured, they tend to peel off from the substrate surface (see inset in Fig. 7). Thus, their heterogeneous catalytic properties could not be accessed. Nevertheless, we predicts that the catalytic performance may reduce if the Au atom concentration in AuPt is further augmented. This is because of the modification of the structure and the surface atom and electronics properties. However, the investigation on the catalytic performance at higher Au atom concentration is in progress by finding the method to reduce the peel-off process during the growth, such as shortening the growth time.
The catalytic performance of this novel AuPt FNPs is absolutely high in term of both homogeneous and heterogeneous reaction. We assumed that this is the result of large surface area that provides facile reactant and product diffusion during the reaction. The existence of unique physico-chemical properties on the surface of the FNPs due to the existence of nanorod that construct the AuPt FNPs with high-energy facet of (001) and (110), bimetallisation process are also considered as the main factor for such high catalytic performance. As has been discussed earlier, the nanorod that construct the AuPt FNPs contains large portion of (001) and (110) facets, the first two highest energy facet in fcc Au or Pt. The presence of large-scale nanorod with this properties in FNPs may produce an incredibly high-surface reactivity for catalytic reaction. Meanwhile, the bimetallisation process has been well-known to modify the density of state of d-orbital of both metals that results in the lowering of the d bonding orbital energy in the bimetal system. This process may enhance the surface reactivity of the bimetal system.35 To understand the surface chemistry properties of the AuPt FNPs, such as the oxidation state and the charge transfer characteristic between Au and Pt in the bimetallic nanoparticles, an X-ray photoelectron spectroscopy (XPS) analysis were carried out on the sample. The result is shown in Fig. 8. Based on the wide energy scan analysis (Fig. 8A), it can be recognized the presence of Pt4f and Au4f as well as the background elements, such as C1s, In3d and O1s. A high-resolution scanning and curve fitting carried out on the Pt4f (Fig. 8B) and Au4f (Fig. 8C) peaks indicated that the metallic state of Pt and Au shifted toward the negative and positive site, respectively. For the case of Pt, the metallic state, if compared to the binding energy (71.3 eV)36 of bulk metallic Pt, it shifted as high as 0.51 eV. This infers that the intermetallic interaction during the bimetallisation has significantly perturbed the d-electron system,37 particularly, of the surface atoms. In normal case, such perturbation will decrease the electronic stability, generating a highly reactive surface properties. This certainly promises enhanced catalytic performance in application. In the present system, the Pt–O and O–Pt–O phases are also observed in the structure, namely approximately at binding energy of 71.8 and 73.5 eV, respectively. This could be the result of surface passivation of Pt surface atoms by the sodium dodecylsulphate surfactant. For the case of Au metallic state,38 its shifting is not as high as in the Pt, however, combinative effect of d-electron perturbation with Pt may generate a highly energetic surface electron, accelerating surface reaction and charge transfer. Thus, enhance the catalytic properties and surface reaction. This phenomenon is predicted to be more profound at higher concentration of Au in the bimetal system. Fig. 9 shows XPS spectra of Pt and Au taken from the AuPt FNPs sample with different Au atom concentration. It was found that the binding energy of metallic Pt and Au linearly shifted (negatively and positively, respectively) with the increasing of Au atom concentration. Table 3 summarizes a detailed properties of AuPt FNPs prepared using different Au ion concentrations. This reflects the kinetic rate of AuPt bimetallic formation increases39 with the increasing of Au concentration. This also reveals that Pt may have a greater tendency to lose electron than Au, increasing the instability of the d-electron system in AuPt FNPs, particularly Pt, and causes the possibility of the presence of Pt atom on the surface of AuPt FNPs is greater than the Au. This certainly produces AuPt with highly reactive properties for enhanced-catalytic reaction.
 | ||
Fig. 9 High-resolution scan of Au4f (upper panel) and Pt4f (lower panel) XPS spectra of AuPt with different Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt molar ratio. (a) Au Pt molar ratio. (a) Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt = 0.07 Pt = 0.07![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (b) 0.2 1, (b) 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (c) 0.33 1, (c) 0.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (d) 0.47 1, (d) 0.47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and (e) 0.67 1, and (e) 0.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
| No. | Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt molar ratio Pt molar ratio |
Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt atomic ratio Pt atomic ratio |
Au4f7/2 (eV) | Pt4f7/2 (eV) | Pt (0) (%) | Pt–O (%) | O–Pt–O (%) |
|---|---|---|---|---|---|---|---|
| 1 | 0.07![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | — | 71.06 | 41.06 | 36.37 | 22.57 |
| 2 | 0.20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
83.55 | 70.95 | 43.04 | 34.93 | 22.03 |
| 3 | 0.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
83.56 | 70.87 | 44.98 | 33.53 | 21.49 |
| 4 | 0.47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.3 6.3 |
83.61 | 70.82 | 46.83 | 32.21 | 20.96 |
| 5 | 0.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.1 5.1 |
83.62 | 70.80 | 48.65 | 31.01 | 20.34 |
From the XPS result, the atomic ratio between Au and Pt atoms can also be roughly obtained (see Table 3). Consistent with the original concentration of precursors, the atomic ratio between Au and Pt atoms linearly increases with the increasing of ionic ratio between the two metals in the reaction. This is a strong indication of facile control over the composition in the AuPt bimetal system. Nevertheless, the obtained atomic ratio between Au and Pt, as detected by the XPS result, is much different compared to the original ionic concentration ratio between the two metals. This could due to the limited bimetallisation process amongst the two metals as the result of lattice mismatch effect. The effect of the difference in the reduction potential between the Au and Pt ions during the reaction could also be the reason for this phenomenon.
It is true that the detailed physico-chemical properties of AuPt FNPs surface atoms, to which the high-performance can be associated with, are not yet obtained in the present stage due to the unavailability of the apparatus, including the low-energy electron diffraction (LEED) or Auger spectroscopies. Nevertheless, we are attempting to access the detailed properties of the surface and the result will be reported in separate publication.
4. Conclusions
Bimetallic AuPt FNPs constructed by a network of nanorods instead of a solid structure has been successfully synthesized directly on an ITO substrate via a liquid phase deposition method. It was found that the morphology and the surface atoms distribution in the AuPt FNPs critically depends on the Au ion concentration in the bimetallic system, where low and high concentration leads to a localized- and homogeneous atomic distribution of Au in the AuPt FNPs, inferring a fine control of the surface physico-chemical properties of the nanostructure. XPS analysis revealed that there is an improvement of electron transfer between the Pt and Au species with the increasing of Au ion, increasing the instability of electrons in d orbital system. This in turn promotes the formation of nanocrystal with unique morphology and highly reactive surface atom. Catalytic acetone hydrogenation, which was used as a model to evaluate the catalytic properties of the AuPt FNPs, proved that the bimetallic FNPs system is really a very efficient catalyst to convert acetone to isopropyl alcohol with the kinetic rate of the conversion increased with the increasing of the Au ion concentration in the AuPt FNPs. The TON and TOF as high as 67 × 102 and 3.5 × 102 s−1, respectively, were obtained from this system. Due to the procurement process that is easy, especially in controlling the atomic doping concentration as well as the exotic structure with highly fibrous structure and special surface chemistry feature, the present result should find potential application in catalysis, sensing and fuel cell.Acknowledgements
The authors acknowledge the financial support from the Ministry of Higher Education of Malaysia under the research fundamental FRGS/2/2013/SG02/UKM/02/8 and the Ministry of Science, Technology and Environment (MOSTE) of Malaysia under the Science Fund project no. 06-01-02-SF1157.References
- C. C. Liang and A. L. Juliard, Nature, 1965, 207, 629–630 CrossRef CAS.
- H. You, S. Yang, B. Ding and H. Yang, Chem. Soc. Rev., 2013, 42, 2880–2904 RSC.
- S. Cheong, J. D. Watt and R. D. Tilley, Nanoscale, 2010, 2, 2045–2053 RSC.
- G. Li, H. Kobayashi, J. M. Taylor, R. Ikeda, Y. Kubota, K. Kato, M. Takata, T. Yamamoto, S. Toh, S. Matsumura and H. Kitagawa, Nat. Mater., 2014, 13, 802–806 CrossRef CAS PubMed.
- G. M. R. van Druten and V. Ponec, Appl. Catal., A, 2000, 191, 153–162 CrossRef CAS.
- A. Balouch, A. Ali Umar, A. A. Shah, M. Mat Salleh and M. Oyama, ACS Appl. Mater. Interfaces, 2013, 5, 9843–9849 CAS.
- J. Wu, P. Li, Y.-T. Pan, S. Warren, X. Yin and H. Yang, Chem. Soc. Rev., 2012, 41, 8066–8074 RSC.
- F. Gao and D. W. Goodman, Chem. Soc. Rev., 2012, 41, 8009–8020 RSC.
- S. Yang and X. Luo, Nanoscale, 2014, 6, 4438–4457 RSC.
- C. Especel, D. Duprez and F. Epron, C. R. Chim., 2014, 17, 790–800 CrossRef CAS.
- N. S. Porter, H. Wu, Z. Quan and J. Fang, Acc. Chem. Res., 2013, 46, 1867–1877 CrossRef CAS PubMed.
- A. A. Umar, E. Rahmi, A. Balouch, M. Y. Abd Rahman, M. M. Salleh and M. Oyama, J. Mater. Chem. A, 2014, 2, 17655–17665 CAS.
- Y. Zhao, C. Ye, W. Liu, R. Chen and X. Jiang, Angew. Chem., Int. Ed., 2014, 53, 8127–8131 CrossRef CAS PubMed.
- S. Suzuki, T. Suzuki, Y. Tomita, M. Hirano, K.-I. Okazaki, S. Kuwabata and T. Torimoto, CrystEngComm, 2012, 14, 4922–4926 RSC.
- I. Banerjee, V. Kumaran and V. Santhanam, J. Phys. Chem. C, 2015, 119, 5982–5987 CAS.
- J. Luo, P. N. Njoki, Y. Lin, D. Mott, L. Wang and C.-J. Zhong, Langmuir, 2006, 22, 2892–2898 CrossRef CAS PubMed.
- S. Guo, L. Wang, S. Dong and E. Wang, J. Phys. Chem. C, 2008, 112, 13510–13515 CAS.
- N.-S. Chang, S. Aldrett, M. T. Holtzapple and R. R. Davison, Chem. Eng. Sci., 2000, 55, 5721–5732 CrossRef CAS.
- T. M. Yurieva, Catal. Today, 1999, 51, 457–467 CrossRef CAS.
- A. Balouch, A. Ali Umar, E. R. Mawarnis, S. K. Md Saad, M. Mat Salleh, M. Y. Abd Rahman, I. V. Kityk and M. Oyama, ACS Appl. Mater. Interfaces, 2015, 7, 7776–7785 CAS.
- A. Balouch, A. A. Umar, S. T. Tan, S. Nafisah, S. K. Md Saad, M. M. Salleh and M. Oyama, RSC Adv., 2013, 3, 19789–19792 RSC.
- K. X. Yao, X. Liu, Z. Li, C. C. Li, H. C. Zeng and Y. Han, ChemCatChem, 2012, 4, 1938–1942 CrossRef CAS.
- P. Strasser, S. Koh, T. Anniyev, J. Greeley, K. More, C. Yu, Z. Liu, S. Kaya, D. Nordlund and H. Ogasawara, Nat. Chem., 2010, 2, 454–460 CrossRef CAS PubMed.
- B. Goris, S. Bals, W. Van den Broek, E. Carbó-Argibay, S. Gómez-Graña, L. M. Liz-Marzán and G. Van Tendeloo, Nat. Mater., 2012, 11, 930–935 CrossRef CAS PubMed.
- Z. Peng and H. Yang, Nano Today, 2009, 4, 143–164 CrossRef CAS.
- F. Viñes, F. Illas and K. M. Neyman, Angew. Chem., Int. Ed., 2007, 46, 7094–7097 CrossRef PubMed.
- M. T. Schaal, M. P. Hyman, M. Rangan, S. Ma, C. T. Williams, J. R. Monnier and J. W. Medlin, Surf. Sci., 2009, 603, 690–696 CrossRef CAS.
- H. You, Z. Peng, J. Wu and H. Yang, Chem. Commun., 2011, 47, 12595–12597 RSC.
- W. Zhang, J. Yang and X. Lu, ACS Nano, 2012, 6, 7397–7405 CrossRef CAS PubMed.
- G. Van Druten and V. Ponec, Appl. Catal., A, 2000, 191, 153–162 CrossRef CAS.
- A. Balouch, A. Ali Umar, A. A. Shah, M. Mat Salleh and M. Oyama, ACS Appl. Mater. Interfaces, 2013, 5, 9843–9849 CAS.
- P. Fouilloux, Appl. Catal., 1983, 8, 1–42 CrossRef CAS.
- A. Rahman and S. S-Al-Deyab, Appl. Catal., A, 2014, 469, 517–523 CrossRef CAS.
- D. J. Cross, I. Houson, A. M. Kawamoto and M. Wills, Tetrahedron Lett., 2004, 45, 843–846 CrossRef CAS.
- J. A. Rodriguez and M. Kuhn, J. Phys. Chem., 1994, 98, 11251–11255 CrossRef CAS.
- S. De Miguel, O. Scelza, M. Román-Martınez, C. S.-M. de Lecea, D. Cazorla-Amorós and A. Linares-Solano, Appl. Catal., A, 1998, 170, 93–103 CrossRef CAS.
- J. Xu, T. Zhao and Z. Liang, J. Phys. Chem. C, 2008, 112, 17362–17367 CAS.
- J. Radnik, C. Mohr and P. Claus, Phys. Chem. Chem. Phys., 2003, 5, 172–177 RSC.
- X. Liu, D. Wang and Y. Li, Nano Today, 2012, 7, 448–466 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |