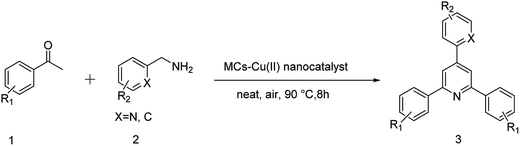
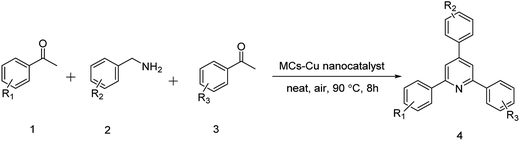
Copper(II) supported on magnetic chitosan: a green nanocatalyst for the synthesis of 2,4,6-triaryl pyridines by C–N bond cleavage of benzylamines
Ahmad Shaabani*,
Mahmoud Borjian Boroujeni and
Mohmmad Sadegh Laeini
Faculty of Chemistry, Shahid Beheshti University, G. C., P. O. Box 19396-4716, Tehran, Iran. E-mail: a-shaabani@sbu.ac.ir
First published on 4th March 2016
Abstract
In this paper, Cu/magnetic chitosan has been synthesized and used as a new green nanocatalyst for highly efficient synthesis of 2,4,6-triaryl pyridines via C–N bond cleavage of benzylamines under aerobic oxidation at 90 °C. The chemical and structural properties of the synthesized catalyst were determined by scanning electron microscopy, energy-dispersive X-ray, X-ray powder diffraction, thermogravimetric analysis and flame atomic absorption spectroscopies. It is found that the catalyst can be easily separated from the reaction mixture by an external magnetic field and recycled several times without a significant loss in activity.
1. Introduction
In recent years, developing routes to produce materials based on green chemistry, which minimizes pollution, have been focused on.1 To reach these goals, the development of heterogeneous catalysts in the synthesis of organic compounds is heavily investigated due to their ease of handling, reusability and simple work-up.2 Immobilization of precious-metal and nonprecious-metal catalysts on various inorganic, petrochemical derived polymers and other synthetic polymers to produce heterogeneous catalytic systems has been widely reported.3–9 Nevertheless the enthusiasm for a cleaner and sustainable chemistry has resulted in exploiting abundant natural polymers such as chitosan (Cs) for catalytic applications due to its high surface area, low cost, biocompatibility, biodegradability, and non-toxicity.10–12Chitin is the second most abundant natural polymer after cellulose which can be found in insects, fungi, shrimps and crabs.13 Chitosan (Cs), a chemically stable, non-toxic and biodegradable polysaccharide prepared from chitin, is an excellent candidate to be used as a support for copper,14–17 ruthenium,18 rhodium,19 cerium20 and other transition metals due to its insolubility in organic solvents and the presence of functionalizable amino groups in the structure. In addition, mesoporous, nitrogen-containing carbon materials obtained directly from pure chitin are used as an absorbent to remove toxic heavy metals and as a catalyst for epoxidation of styrene.21 Although chitin and chitosan have been used in industrial chemistry such as textile, cosmetics and biomedicine, they also have a significant potential for a sustainable production of small nitrogen-containing chemicals such as ethanolamine.22 Nowadays, various investigations have been carried out to develop new protocols for an efficient conversion of chitin to value-added chemicals.23–26
In recent decade, the synthesis of supported magnetic nanoparticles has attracted a considerable attention in catalyst science due to their availability, low toxicity, high catalytic activity, excellent surface area and magnetic separability thereby eliminating the filtration process after the end of reactions.27 Immobilization of iron oxide nanoparticles on biodegradable polymers such as cellulose and chitosan has produced highly efficient adsorbents for the removal of metals, drug carriers and reusable nano catalysts.28–32 To the best of our knowledge, there is no report in which copper(II) magnetic chitosan nanocomposite has been used for an organic transformation.
The C–N bond cleavage is an important synthetic strategy that has been widely investigated.33 It is known that transition metals, such as palladium,34 ruthenium,35 and copper36 are catalysts which promote the C–N bond cleavage and the C–N bond formation. Specially, the application of copper-based catalysts in this reaction is remained as a considerable topic due to the low toxicity and cost of copper compared to other more common noble metals. Recently Wang et al. reported the C–N bond cleavage and the formation for the synthesis of benzimidazo[1,2-a]quinazoline derivatives using CuI/L-proline.37 Jiang et al. developed an efficient copper-catalyzed C–N bond cleavage of aromatic methylamines to construct pyridine derivatives.38
Pyridine rings are one of the most important heterocyclic moieties which have many applications in natural products, organic and medical chemistry and functional materials.39 Pyridines synthesis is an interesting topic in modern synthetic chemistry among which, 2,4,6-trisubstituted pyridine derivatives have been used in supramolecular chemistry due to their p-stacking ability along with H-bonding capacity.40 The development of new heterogeneous catalysts for the synthesis of multi-substituted pyridines is in high demand.41,42
In continuation of our interest in the sustainable benign pathway for organic transformations and nanocatalysis,43–48 we wish to introduce the synthesis of 2,4,6-triaryl pyridine derivatives by the C–N bond cleavage of benzyl amines in the presence of Cu(II) immobilized on magnetic chitosan as a nanocatalyst. The obtained catalytic system, Cu/magnetic chitosan, exhibits a high activity and selectivity for the synthesis of 2,4,6-triaryl pyridine derivatives under aerobic conditions. The catalyst can be separated from the reaction mixture using an external magnetic field and be reused for five consecutive reaction times without a significant decrease in the catalytic activity.
2. Experimental
2.1. Materials and methods
Chitosan (molecular weight: 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–300
000–300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was purchased from Acros Organics. Iron(III) chloride hexahydrate, iron(II) chloride tetrahydrate, copper(II) chloride dehydrate and sodium hydroxide were purchased from Sigma Aldrich. Dimethylsulfoxide (DMSO), dimethylformamide (DMF), toluene, acetophenone, 4′-methylacetophenone, 4′-chloroacetophenone, 4′-bromoacetophenone, benzylamine, 4-methylbenzylamine, 4-chlorobenzylamine, 4-methoxybenzylamine, and 2-picolylamine were purchased from Sigma Aldrich. All chemicals were used without further purifications.
000) was purchased from Acros Organics. Iron(III) chloride hexahydrate, iron(II) chloride tetrahydrate, copper(II) chloride dehydrate and sodium hydroxide were purchased from Sigma Aldrich. Dimethylsulfoxide (DMSO), dimethylformamide (DMF), toluene, acetophenone, 4′-methylacetophenone, 4′-chloroacetophenone, 4′-bromoacetophenone, benzylamine, 4-methylbenzylamine, 4-chlorobenzylamine, 4-methoxybenzylamine, and 2-picolylamine were purchased from Sigma Aldrich. All chemicals were used without further purifications.
The FT-IR spectra were recorded on a Bomem MB-Series FT-IR spectrometer. The thermal analysis (TGA-DTA) was carried out using a Bahr STA-503 instrument at a heating rate of 10 °C min−1 in air. The X-ray powder diffraction (XRD) pattern was recorded on a STOE diffractometer with Cu Kα radiation (λ = 015418 nm). The scanning electron microscopy (SEM) and the energy dispersive X-ray spectroscopy (EDS) characterization of catalyst were performed using an electron microscopy Philips XL-30 ESEM. All the samples were sputtered with gold before observation. The 1H NMR spectra were recorded on a BRUKER DRX-300 AVANCE spectrometer. The NMR spectra were obtained in CDCl3 and DMSO-d6 using the tetramethyl silane (TMS) as internal standard. The melting points of the products were measured by an electrothermal 9100 apparatus. The concentrations of copper and iron were estimated using Shimadzu AA-680 flame atomic absorption spectrophotometer and an inductively coupled plasma optical emission spectrometer (ICP-OES) Varian Vista PRO Radial. The XPS analysis was performed using a Gammadata-scientifica ESCA 200 hemispherical analyzer equipped with an Al Kα (1486.6 eV) X-ray source.
2.2. General procedure for synthesis of magnetic chitosan nanoparticles (MCs)
The synthesis of Fe3O4 nanoparticles with Cs was carried out according to the literature which has described procedures.32 An aqua solution of FeCl3·6H2O (27.0 mg) and FeCl2·4H2O (9.95 mg) with a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in 5 ml distilled water was prepared and added to a suspension of the Cs (30.0 mg) in 20 ml distilled water under a vigorous stirring under nitrogen atmosphere. After stirring for 10 min, the sodium hydroxide solution was added to the mixture and the stirring was strongly continued for 4 h at 80 °C. After cooling the suspension to the room temperature, the MCs was separated by the magnet decantation, was washed with distilled water, ethanol and, finally was dried under the vacuum at the room temperature.
1 in 5 ml distilled water was prepared and added to a suspension of the Cs (30.0 mg) in 20 ml distilled water under a vigorous stirring under nitrogen atmosphere. After stirring for 10 min, the sodium hydroxide solution was added to the mixture and the stirring was strongly continued for 4 h at 80 °C. After cooling the suspension to the room temperature, the MCs was separated by the magnet decantation, was washed with distilled water, ethanol and, finally was dried under the vacuum at the room temperature.
2.3. General procedure for synthesis of MCs–Cu(II) nanocatalyst
The MCs (0.1 g) was suspended in 20 ml of distilled water and 0.03 g of CuCl2·2H2O was added to the mixture and stirring was continued for 3 h. The catalyst was separated using a magnet and was dried under the vacuum at 50 °C.2.4. General procedure for synthesis of copper nanoparticles@magnetic chitosan
The copper nanoparticles@magnetic chitosan was synthesized according to the pervious report.49 Briefly, 0.5 g of the magnetic chitosan was dispersed in 25 ml of ethanol in a 100 ml round-bottomed flask, after which 25 ml (0.25 mmol) of the ethanolic solution of the copper(II) acetate was added dropwise under vigorous stirring. After 4 h of stirring, the copper ions were completely adsorbed by chitosan and the MCs–Cu(CH3COO)2 was separated by an external magnet and was dried in a desiccator. The dried MCs–Cu(CH3COO)2 was again dispersed in 50 ml of methanol and 15 ml of NaBH4 (5 mmol) solution was added slowly under vigorous string under nitrogen atmosphere. The copper nanoparticles@magnetic chitosan were separated by an external magnet and washed several times with methanol and then dried in the desiccator.2.5. General procedure for preparation of 2,4,6-triaryl pyridines
A ketone (2 mmol), benzylamine (1.2 mmol), MCs–Cu(II) nanocatalyst (0.064 g, 5 mol%) were added to a 25 ml tube. The mixture was stirred for 8 h at 90 °C. Upon completion; acetone was added to the mixture and the catalyst was separated by an external magnet. The solid product was recrystallized from acetone-water 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) to produce the pure product.
1 (v/v) to produce the pure product.
2.6. Product characterization data of 2,4,6-triaryl pyridines
2,4,6-Triphenylpyridine (3a), yield: 90, C23H17N, white solid, FT-IR (KBr) cm−1: 1450–1600, 758, 692 1H NMR (300.13 MHz, CDCl3), δ 8.23 (d, J = 7.5 Hz, 4H), 7.9 (s, 2H), 7.7 (d, J = 6.9, 2H), 7.2–7.9 (9H, m, H-Ar), 13C NMR (75.47 MHz, CDCl3): 117.02, 127.40, 128.72, 129.68, 129.73, 139.19, 139.26, 150.06, 157.02.2,6-Diphenyl-4-(p-tolyl)pyridine (3b), yield: 91, C24H19N, white solid, FT-IR (KBr) cm−1: 3020, 2950, 1450–1600, 759, 692. 1H NMR (300.13 MHz, CDCl3), δ 8.2 (d, 4H), 7.9 (s, 2H), 7.7 (d, 2H), 7.2–7.9 (9H, m, H-Ar), 13C NMR (75.47 MHz, CDCl3): 21.25, 116.66, 127.54, 129.17, 129.63, 130.14, 135.18, 139.41, 156.96.
4-Phenyl-2,6-di-p-tolylpyridine (3c), yield: 90, C25H21N, white solid, FT-IR (KBr) cm−1: 3022, 2950, 1450, 1600, 757, 695. 1H NMR (300.13 MHz, CDCl3), δ 8.13 (d, J = 7.8 Hz, 4H), 7.87 (s, 2H), 7.76 (d, J = 7.2 Hz, 2H), 7.47–7.58 (m, 3H), 7.34 (d, J = 7.8 Hz, 4H), 2.46 (s, 6 Hz), 13C NMR (75.47 MHz, CDCl3): 21.30, 116.42, 126.90, 127.12, 128.82, 129.10, 129.35, 136.80, 138.92, 139.20, 149.93, 157.32.
2,4,6-Tri-p-tolylpyridine (3d), yield: 92, C26H23N, white solid, FT-IR (KBr) cm−1: 3022, 2950, 1450, 1600, 757, 695. 1H NMR (300.13 MHz, CDCl3), δ 8.12 (d, J = 7.8 Hz, 4H), 7.86 (s, 2H), 7.78 (d, J = 7.8 Hz, 2H), 7.33–7.36 (m, 3H), 2.46 (s, 9 Hz), 13C NMR (75.47 MHz, CDCl3): δ 21.35, 21.43, 116.44, 127.07, 127.12, 129.48, 129.87, 136.20, 136.83, 139.04, 139.07, 150.03, 150.326.
2′,6′-Diphenyl-2,4′-bipyridine (3e), yield: 95, C22H16N2, yellow solid, FT-IR (KBr) cm−1: 3059, 1594, 1488, 1321, 830, 770, 690. 1H NMR (300.13 MHz, CDCl3), δ 8.18–8.28 (m, 6H), 7.85–7.93 (m, 2H), 7.76 (t, J = 7.5 Hz, 2H), 7.47–7.59 (m, 6 Hz), 13C NMR (75.47 MHz, CDCl3): δ 119.13, 126.43, 127.20, 127.52, 127.70, 128.92, 129.49, 129.54, 129.87, 137.09, 139.40, 148.01, 157.33.
2,6-Bis(4-chlorophenyl)-4-phenylpyridine (3f), yield: 90, C23H15Cl2N, white solid, FT-IR (KBr) cm−1: 3052, 1489, 1486, 1597, 1524, 760, 694, 1H NMR (300.13 MHz, DMSO-d6), δ 8.36 (d, J = 8.4 Hz, 4H), 8.23 (s, 2H), 8.04 (d, J = 7.6 Hz, 2H), 7.50–7.61 (m, 7H), 13C NMR (75.47 MHz, DMSO-d6): δ 117.31, 127.87, 129.20, 129.55, 129.71, 129.91, 134.65, 137.86, 150.31, 155.75.
4-(4-Chlorophenyl)-2,6-diphenylpyridine (3g), yield: 95, C23H16ClN, white solid, FT-IR (KBr) cm−1: 3061, 1599, 1545, 1489, 775, 692, 1H NMR (300.13 MHz, DMSO-d6), δ 8.12 (d, J = 7.2 Hz, 4H), 8.14 (s, 2H), 7.91 (d, J = 8 Hz, 2H), 7.41 (d, J = 6.8 Hz, 2H), 7.28–7.36 (m, 6H), 13C NMR (75.47 MHz, DMSO-d6): δ 116.88, 127.44, 129.17, 129.47, 129.64, 134.70, 136.94, 139.18, 148.64, 157.05.
4-(4-Methoxyphenyl)-2,6-diphenylpyridine (3h), yield: 85, C24H19NO, white solid, FT-IR (KBr) cm−1: 3035, 1596, 1547, 1486, 750, 690, 1H NMR (300.13 MHz, CDCl3), δ 8.30 (d, J = 7.2 Hz, 4H), 8.15 (s, 2H), 7.93 (d, J = 8 Hz, 2H), 7.45–7.55 (m, 6H), 7.34–7.36 (d, J = 8 Hz, 2H), 3.36 (s, 3H), (13C NMR 75.47 MHz, CDCl3): δ 55.80, 114.80, 118, 127.60, 128.40, 129.30, 130.20, 152, 155.20, 161.10.
3. Results and discussion
The magnetic chitosan nanoparticles were prepared by the chemical co-precipitation of Fe3+ and Fe2+ ions with a molar ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.32 Then, the immobilization of copper on the magnetic chitosan was carried out in an aqueous solution of copper(II) chloride for 3 h under neutral conditions.17
1.32 Then, the immobilization of copper on the magnetic chitosan was carried out in an aqueous solution of copper(II) chloride for 3 h under neutral conditions.17
Fig. 1 shows the XRD patterns of the chitosan and the MCs–Cu(II) nanocatalyst. There are six characteristic peaks at 2θ = 30.04°, 35.5°, 43.12°, 53.44°, 57.04° and 62.8° in the XRD pattern of the MCs–Cu(II) nanocatalyst that confirm the standard pattern with a spinel structure for the crystalline magnetite (JCPDS card no. 01-1111). Because of the diffraction peaks of Fe3O4 are stronger than the diffraction peaks of chitosan, the peaks related to the chitosan were not observed.32 According to the Scherrer's equation the mean crystallite size calculated was 23 nm.
The Cu(II) loading on the MCs–Cu(II) nanocatalyst was determined as 5 wt% based on the FAAS analysis. The Fe loading level of catalyst was measured by ICP-AES and it was obtained 16.67 wt%.
Thermogravimetric analysis (TGA) was used in order to obtain information on the thermal stability of the chitosan and the MCs–Cu(II) nanocatalyst (Fig. 2). The TGA results confirm that the Cs was stable over 200 °C and there were three steps of mass losses for the catalyst in the temperature range of 50–260 °C, 260–315 °C and 315–520 °C. The weight loss of the catalyst is about 74% that corresponding to the thermal decomposition of the chitosan.
The SEM analysis was used to study the morphology and the structure of the MCs–Cu(II) nanocatalyst. The SEM images show an excellent dispersity of Fe3O4 nanoparticles on the Cs (Fig. 3). Also, the energy dispersive spectroscopy (EDS) analysis clearly illustrates the presence of iron and copper in the nanocatalyst (Fig. 4).
To show the efficiency of the MCs–Cu(II) as a nanocatalyst, we investigated the reaction of acetophenone (2 mmol) and benzylamine (1.2 mmol) for the synthesis of 2,4,6-triaryl pyridine derivates in toluene for 12 h under aerobic oxidation in the presence of various amounts of the catalyst (Table 1). The reaction in the absence of the catalyst did not result in the forementioned product (Table 1, entry 1). In addition, in the presence of the magnetic chitosan, no reaction occurred even after 8 h (Table 1, entry 2). As the amount of the catalyst was increased (5 mol%), the reaction went to completion at 90 °C (Table 1, entries 3–6). This reaction in the presence of CuCl2 as a homogeneous catalyst in the air gave the 2,4,6-triaryl pyridines in 40% yield (Table 1, entry 7). However, the separation of the catalyst from the reaction mixture was difficult. The yield of reaction in oxygen atmosphere was not increased (Table 1, entry 8).
| Entry | Catalyst (mol%) | Solvent | Oxidant | Temperature (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: acetophenone (2.00 mmol), benzylamine (1.2 mmol), catalyst, air (1 atm), 8 h.b Isolated yield. | |||||
| 1 | None | Toluene | Air | 90 | 0 |
| 2 | MCs | Toluene | Air | 90 | 0 |
| 3 | MCs–Cu(II) nanocatalyst (1) | Toluene | Air | 90 | 30 |
| 4 | MCs–Cu(II) nanocatalyst (3) | Toluene | Air | 90 | 60 |
| 5 | MCs–Cu(II) nanocatalyst (5) | Toluene | Air | 90 | 90 |
| 6 | MCs–Cu(II) nanocatalyst (8) | Toluene | Air | 90 | 90 |
| 7 | CuCl2 (5) | Toluene | Air | 90 | 40 |
| 8 | MCs–Cu(II) nanocatalyst (5) | Toluene | O2 | 90 | 90 |
| 9 | Copper nanoparticles@magnetic-chitosan (5) | Toluene | Air | 90 | 90 |
| 10 | MCs–Cu(II) nanocatalyst (5) | Neat | Air | 90 | 90 |
| 11 | MCs–Cu(II) nanocatalyst (5) | Toluene | Air | 90 | 88 |
| 12 | MCs–Cu(II) nanocatalyst (5) | Water | Air | 90 | 60 |
| 13 | MCs–Cu(II) nanocatalyst (5) | DMSO | Air | 90 | 70 |
| 14 | MCs–Cu(II) nanocatalyst (5) | DMF | Air | 90 | 40 |
| 15 | MCs–Cu(II) nanocatalyst (5) | Neat | Air | 70 | 60 |
| 16 | MCs–Cu(II) nanocatalyst (5) | Neat | Air | 110 | 90 |
For more investigation, copper nanoparticles@magnetic chitosan were prepared49 and used for this reaction. As shown in Table 1, the yield of the reaction in the presence of the copper nanoparticles@magnetic-chitosan was not significantly changed compared to that in the presence of the MCs–Cu(II) nanocatalyst. Therefore, the MCs–Cu(II) nanocatalyst was found to be a better catalyst than the copper nanoparticles@magnetic chitosan due to its simple preparation (Table 1, entry 9). Next, various solvents were used to study their effect on the yield of product. Surprisingly, the reaction did not show a strong solvent dependence. Also, using toluene had no efficient influence on the transformation (Table 1, entry 10–14). Thus, the reaction was carried out under solvent free conditions. After screening different temperatures, 90 °C was obtained as the best temperature for the reaction. The optimized conditions for the synthesis of the 2,4,6-triaryl pyridines were 5 mol% of the catalyst in the absence of any additive or solvent under the air at 90 °C.
With the optimized reaction conditions established, the scope of benzylamines and methyl ketones was examined to explore the generality of the 2,4,6-triaryl pyridines synthesis under the aerobic conditions.
Acetophenones derivatives with a wide array of functional groups such as chloro, bromo and methyl on the aryl group were employed to synthesis of the 2,4,6-triaryl pyridines. Phenyl methyl ketones with electron-withdrawing or electron-donating substituents on the aromatic ring result in the forementioned product. Using nonmethyl ketones such as cyclohexanone did not led to the desired product. Substituted benzyl amines were employed to give the desired pyridines in good yields. According to the previous report,38 under the homogeneous copper-catalysed system, 2-pyridylmethylamine with methyl ketone could not produce the 2,4,6-triaryl pyridines.38 However, in the case of our reaction conditions, using the MCs–Cu(II) nanocatalyst, the 2,4,6-triaryl pyridines were produced from the reaction of 2-pyridyl methylamines with methyl ketones (Table 2, entry 5).
| Entry | R1 | R2 | Product | Yieldb, % | Melting point |
|---|---|---|---|---|---|
| a Reaction conditions: 1 (2 mmol), 2 (1.2 mmol), MCs–Cu(II) nanocatalyst (0.064 g, 5 mol%), air, at 90 °C for 8 h.b Isolated yield. | |||||
| 1 | H | H | 3a | 90 | 135–138 |
| 2 | H | CH3 | 3b | 85 | 118–120 |
| 3 | CH3 | H | 3c | 85 | 154–156 °C |
| 4 | CH3 | CH3 | 3d | 80 | 174–176 °C |
| 5 | H | H, X = N | 3e | 90 | 98–100 °C |
| 6 | Cl | H | 3f | 80 | 175–178 |
| 7 | H | Cl | 3g | 85 | 127–128 |
| 8 | H | OMe | 3h | 80 | 99–101 |
| 9 | Cl | OMe | 3i | 75 | 190–192 |
| 10 | Br | H | 3j | 70 | 98–100 |
It is important to note that the cross reactions with two different methyl ketones provided asymmetric 2,4,6-triaryl pyridines as a major product. For example, when benzylamine (1.2 mmol) and p-methylacetophenone (1 mmol) were treated with acetophenone (1 mmol), the 2,4,6-triaryl pyridines were synthesized in a moderate yield. The results are summarized in Table 3.
Based on the previous reports,38,50–58 a plausible mechanism was proposed in Scheme 1. At first, single-electron transfer oxidation of benzylamine occurred in the presence of the MCs–Cu(II) nanocatalyst and then imine was produced from the aminolysis reaction. Next the hydrolysis of imine as a reversible process could provide benzylamine and benzaldehyde. The condensation of benzaldehyde, ketone, and benzylamine in the presence Cu(II) as a Lewis acid produced 1,4-dihydropyridine intermediate. Finally, the 2,4,6-triaryl pyridine was produced from the oxidation of 1,4-dihydropyridine intermediate under the catalytic aerobic conditions.
The stability of the MCs–Cu(II) nanocatalyst toward the leaching of copper ions was examined, as well. Briefly, a mixture of ketone and benzylamine in the presence of catalyst was stirred in toluene at 90 °C for 15 min, next the catalyst was separated by an external magnet from the reaction mixture and the reaction was continued by catalyst-free mixture under the same conditions for an additional 8 h. The yield of the reaction was 10% before the filtration of the catalyst and the final yield was 11%. Also, according to the FAAS analysis results, the leaching of the copper ions in to a solution phase was less than 0.5%. This means that the leaching of copper should be negligible.
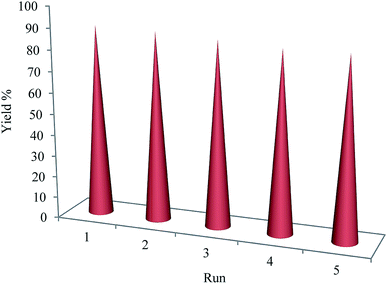
The recyclability of the MCs–Cu(II) nanocatalyst was surveyed for the synthesis of the 2,4,6-triaryl pyridines under the optimized conditions at 90 °C. After the reaction time, the solid catalyst was separated by an external magnet, was washed and dried at 80 °C and was used in the next run without further treatment. It was observed that in the next five consecutive uses of the MCs–Cu(II) nanocatalyst, the catalytic activity and selectivity did not significantly decrease (Fig. 5).
The XPS analysis of the catalyst after five runs of the reaction was shown in Fig. 6. The Cu 2p3/2 and Cu 2p1/2 were clearly observed at 934.05 and 954.12 eV, respectively, that confirm the Cu(II) ions in the catalyst were stable under the reaction conditions.59
4. Conclusion
The MCs–Cu(II) nanocatalyst was synthesized by a uniform distribution of Fe3O4 nanoparticles on the chitosan matrix. The new catalyst successfully promoted the synthesis of the 2,4,6-triaryl pyridines via a C–N bond cleavage of benzylamine under aerobic oxidations with fairly good yields. The reaction was carried out under environmentally benign, solvent-free and aerobic conditions. The structure of the catalyst was confirmed by the XRD, TGA, EDS and the SEM. The MCs–Cu(II) nanocatalyst showed advantages such as magnetic separability, recyclability, good chemical stability, low solubility in organic solvents, and a uniform distribution of the nanoparticles on the chitosan. The leaching tests indicated that the reaction was mainly heterogeneous in nature.Acknowledgements
We gratefully acknowledge financial support from the Catalyst Center of Excellence (CCE) of Shahid Beheshti University and the Iran National Elites Foundation (INEF).References
- R. A. Sheldon, I. Arends and U. Hanefeld, Green Chemistry and Catalysis, VCH, Weinheim, 2007 Search PubMed.
- G. Ertl, H. Knzinger and J. Weitkamp, Handbook of Heterogeneous Catalysis, VCH, Weinheim, 1997 Search PubMed.
- Y. Uozumi, Recent Progress in Polymeric Palladium Catalysts for Organic Synthesis, in Immobilized Catalysts, ed. A. Kirschning, Springer Berlin Heidelberg, 2004, pp. 77–112 Search PubMed.
- Z. Dong and Z. Ye, Appl. Catal., A, 2015, 489, 61–71 CrossRef CAS.
- E. Gross, F. Dean Toste and G. Somorjai, Catal. Lett., 2015, 145, 126–138 CrossRef CAS.
- K. Yamaguchi, T. Oishi, T. Katayama and N. Mizuno, Chem.–Eur. J., 2009, 15, 10464–10472 CrossRef CAS PubMed.
- A. Khalafi-Nezhad and F. Panahi, Green Chem., 2011, 13, 2408–2415 RSC.
- B. J. Gallon, R. W. Kojima, R. B. Kaner and P. L. Diaconescu, Angew. Chem., Int. Ed., 2007, 46, 7251–7254 CrossRef CAS PubMed.
- W. Zhang, Y. Sun and L. Zhang, Ind. Eng. Chem. Res., 2015, 54, 6480–6488 CrossRef CAS.
- Y. Wang, Y. Li, S. Liu and B. Li, Carbohydr. Polym., 2015, 120, 53–59 CrossRef CAS PubMed.
- D. J. Macquarrie and J. J. E. Hardy, Ind. Eng. Chem. Res., 2005, 44, 8499–8520 CrossRef CAS.
- E. Guibal, Prog. Polym. Sci., 2005, 30, 71–109 CrossRef CAS.
- V. K. Thakur and M. K. Thakur, ACS Sustainable Chem. Eng., 2015, 2, 2637–2652 CrossRef.
- B. S. P. A. Kumar, K. H. V. Reddy, K. Karnakar, G. Satish and Y. V. D. Nageswar, Tetrahedron Lett., 2015, 56, 1968–1972 CrossRef.
- B. Yang, Z. Mao, X. Zhu and Y. Wan, Catal. Commun., 2015, 60, 92–95 CrossRef CAS.
- M. Chtchigrovsky, A. Primo, P. Gonzalez, K. Molvinger, M. Robitzer, F. Quignard and F. Taran, Angew. Chem., Int. Ed., 2009, 48, 5916–5920 CrossRef CAS PubMed.
- R. B. N. Baig and R. S. Varma, Green Chem., 2013, 15, 1839–1843 RSC.
- R. B. N. Baig, M. N. Nadagouda and R. S. Varma, Green Chem., 2014, 16, 2122–2127 RSC.
- B. C. E. Makhubela, A. Jardine and G. S. Smith, Green Chem., 2012, 14, 338–347 RSC.
- N. Ahmed and Z. N. Siddiqui, ACS Sustainable Chem. Eng., 2015, 3, 1701–1707 CrossRef CAS.
- Y. Gao, X. Chen, J. Zhang and N. Yan, ChemPlusChem, 2015, 80, 1556–1564 CrossRef CAS.
- N. Yan and X. Chen, Nature, 2015, 524, 155–157 CrossRef CAS PubMed.
- F. D. Bobbink, J. Zhang, Y. Pierson, X. Chen and N. Yan, Green Chem., 2015, 17, 1024–1031 RSC.
- X. Chen, S. L. Chew, F. M. Kerton and N. Yan, Green Chem., 2014, 16, 2204–2212 RSC.
- Y. Wang, C. M. Pedersen, T. Deng, Y. Qiao and X. Hou, Bioresour. Technol., 2013, 143, 384–390 CrossRef CAS PubMed.
- Y. Pierson, X. Chen, F. D. Bobbink, J. Zhang and N. Yan, ACS Sustainable Chem. Eng., 2014, 2, 2081–2089 CrossRef CAS.
- S. Shylesh, V. Schünemann and W. R. Thiel, Angew. Chem., Int. Ed., 2010, 49, 3428–3459 CrossRef CAS PubMed.
- X. Yu, S. Tong, M. Ge, J. Zuo, C. Cao and W. Song, ACS Sustainable Chem. Eng., 2013, 1, 959–965 CAS.
- N. A. Eltouny and P. A. Ariya, Ind. Eng. Chem. Res., 2012, 51, 12787–12795 CrossRef CAS.
- H. Qin, C. M. Wang, Q. Q. Dong, L. Zhang, X. Zhang, Z. Y. Ma and Q. R. Han, J. Magn. Magn. Mater., 2015, 381, 120–126 CrossRef CAS.
- A. Shaabani, H. Nosrati and M. Seyyedhamzeh, Res. Chem. Intermed., 2015, 41, 3719–3727 CrossRef CAS.
- Z. Zarnegar and J. Safari, RSC Adv., 2014, 4, 20932–20939 RSC.
- A. Maleki, N. Ghamari and M. Kamalzare, RSC Adv., 2014, 4, 9416 RSC.
- T. Li, Z. Wang, M. Zhang, H.-J. Zhang and T.-B. Wen, Chem. Commun., 2015, 51, 6777–6780 RSC.
- X. Zhao, D. Liu, H. Guo, Y. Liu and W. Zhang, J. Am. Chem. Soc., 2011, 133, 19354–19357 CrossRef CAS PubMed.
- T. Koreeda, T. Kochi and F. Kakiuchi, J. Am. Chem. Soc., 2009, 131, 7238–7239 CrossRef CAS PubMed.
- C. Li, W.-T. Zhang and X.-S. Wang, J. Org. Chem., 2014, 79, 5847–5851 CrossRef CAS PubMed.
- H. Huang, X. Ji, W. Wu, L. Huang and H. Jiang, J. Org. Chem., 2013, 78, 3774–3782 CrossRef CAS PubMed.
- J. A. Bull, J. J. Mousseau, G. Pelletier and A. B. Charette, Chem. Rev., 2012, 112, 2642–2713 CrossRef CAS PubMed.
- C. Allais, J.-M. Grassot, J. Rodriguez and T. Constantieux, Chem. Rev., 2014, 114, 10829–10868 CrossRef CAS PubMed.
- J. Safari, Z. Zarnegar and M. Borujeni, Chem. Pap., 2013, 67, 688–695 CAS.
- J. Safari, S. Gandomi-Ravandi and M. Borujeni, J. Chem. Sci., 2013, 125, 1063–1070 CrossRef CAS.
- A. Shaabani, Z. Hezarkhani and E. Badali, RSC Adv., 2015, 5, 61759–61767 RSC.
- A. Shaabani, S. Keshipour, M. Hamidzad and S. Shaabani, J. Mol. Catal. A: Chem., 2014, 395, 494–499 CrossRef CAS.
- M. Mahyari, M. S. Laeini and A. Shaabani, Chem. Commun., 2014, 50, 7855–7857 RSC.
- M. Mahyari and A. Shaabani, Appl. Catal., A, 2014, 469, 524–531 CrossRef CAS.
- S. Keshipour, S. Shojaei and A. Shaabani, Cellulose, 2013, 20, 973–980 CrossRef CAS.
- A. Shaabani, M. Seyyedhamzeh, A. Maleki and F. Rezazadeh, Appl. Catal., A, 2009, 358, 146–149 CrossRef CAS.
- M. Chetia, A. A. Ali, D. Bhuyan, L. Saikia and D. Sarma, New J. Chem., 2015, 39, 5902–5907 RSC.
- K. Ouyang, W. Hao, W. X. Zhang and Z. Xi, Chem. Rev., 2015, 115, 12045–12090 CrossRef CAS PubMed.
- C. Zhang, C. Tang and N. Jiao, Chem. Soc. Rev., 2012, 41, 3464–3484 RSC.
- H. Tian, X. Yu, Q. Li, J. Wang and Q. Xu, Adv. Synth. Catal., 2012, 354, 2671–2677 CrossRef CAS.
- Z. Shi, C. Zhang, C. Tang and N. Jiao, Chem. Soc. Rev., 2012, 41, 3381–3430 RSC.
- R. D. Patil and S. Adimurthy, RSC Adv., 2012, 2, 5119–5122 RSC.
- Z. Hu and F. M. Kerton, Org. Biomol. Chem., 2012, 10, 1618–1624 CAS.
- A. E. Wendlandt, A. M. Suess and S. S. Stahl, Angew. Chem., Int. Ed., 2011, 50, 11062–11087 CrossRef CAS PubMed.
- E. A. Lewis and W. B. Tolman, Chem. Rev., 2004, 104, 1047–1076 CrossRef CAS PubMed.
- R. D. Patil and S. Adimurthy, Adv. Synth. Catal., 2011, 353, 1695–1700 CrossRef CAS.
- Q. Zhao, C. Bai, W. Zhang, Y. Li, G. Zhang, F. Zhang and X. Fan, Ind. Eng. Chem. Res., 2014, 53, 4232–4238 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |