Facile construction of novel imidazolidine-spirooxindoles via diastereoselective cycloaddition of N-acylhydrazine-derived imines with 3-isothiocyanato oxindoles†
Abstract
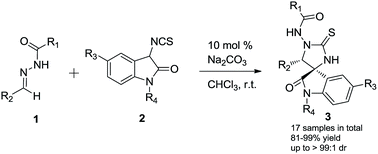
In the presence of 10 mol% of Na2CO3, the desired imidazolidine-spirooxindoles were obtained in 81–99% yield with up to 99 : 1 dr by means of the diastereoselective [3 + 2] cycloaddition of N-acylhydrazine-derived imines with 3-isothiocyanato oxindoles. Single-crystal X-ray structure analysis was conducted to determine the relative stereochemistry of the imidazolidine-spirooxindoles. Diastereoselective access to the imidazolidine-spirooxindoles was hypothesized by the proposed mechanism.

 Please wait while we load your content...
Please wait while we load your content...