A tumor-targeting protein nanoparticle based on Tat peptide and enhanced green fluorescent protein†
Xingang Guan*ab,
Chun Lic,
Dan Wangc,
Weiqi Sund and
Xiaodong Gai*c
aLife Science Research Center, Beihua University, Jilin 132013, P. R. China. E-mail: guanxg@ciac.ac.cn
bState Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China
cSchool of Basic Medical Sciences, Beihua University, Jilin 132013, P. R. China. E-mail: bhdxgaixiaodong@126.com
dSchool of Public Health, Beihua University, Jilin 132013, P. R. China
First published on 18th January 2016
Abstract
A protein-based nanoparticle containing cell penetrating peptides (CPPs) and enhanced green fluorescent protein (EGFP) was developed through a genetic engineering method. In addition to high stability and good biocompatibility, this fluorescent protein nanoparticle also displayed preferential tumor accumulation, indicating its potential application in tumor imaging and anti-cancer drug delivery.
Due to the enhanced permeability and retention (EPR) effect of nanoparticles, a great number of nanoparticles for tumor targeting and bioimaging have been developed for diagnosis and therapy during the past decade.1 For tumor-targeting, the nanoparticles were usually equipped with targeting molecules (aptamer, peptides, antibodies and others).2 While for tumor imaging, fluorescent molecules, contrast agents and other imaging moiety were loaded or conjugated to various nanoparticles.3 However, the decoration or conjugation usually requires complicated chemical synthetic and purification steps. Thus it is challenging to develop fluorescent nanoparticles in a straightforward way for tumor targeting and imaging simultaneously.4
Protein nanoparticles have attracted great interest in drug delivery and tumor imaging owing to their nature source, good biocompatibility, high tuning ability and easy production.5 Although the potential immunotoxicity could be caused by some protein material, no immune response was reported until now in the studies of albumin, gelatin, zein and casein protein nanoparticles.6 Up to now, the researchers have successfully developed various nanostructures including virus-like particles (VLPs), nanocages, nanodiscs, and others based on natural or synthetic protein materials.7,8 For example, Flenniken and his colleagues showed that small heat shock protein from Methanococcus jannaschii could self-assemble into nanocage with a diameter of 12 nm.9 Liang et al. had developed a cell-specific targeting nanoparticle based on human ferritin.10 Vazquez and his co-workers had showed a protein nanodisk assembled from a fusion protein including green fluorescent protein (GFP) and nine arginines (R9).8 Cespedes et al. reported a protein nanoparticle with high stability in vitro and in vivo, which was self-assembled by T22-GFP-H6.11
Cell-penetrating peptides (CPPs), also named protein transduction domains (PTDs), are a class of short peptides (less than 30 amino acids) which could transport efficiently across cell membrane into cells.12 The first discovered CPPs, Tat sequence, derived from HIV-1 encoded TAT protein (48–60). Tat peptides have been used as vectors for small molecules, nucleotides and proteins.13 Moreover, Tat peptides are also used to improve the cellular uptake of some nanoparticles such as liposomes, polymer micelles, mesoporous silica, gold nanostars, and others.14 Recently we developed a fluorescent protein nanoparticle from Tat peptide, enhanced green fluorescent protein (EGFP), and a hexahistidine. These protein nanoparticles were prepared from the fusion protein of H6-TatEGFP.15 Tat peptides and hexahistidine, fused at N-terminal of EGFP protein, confer protein monomers with a strong positive charge which promote self-organization of monomers into monodisperse protein nanoparticles. In this study, we investigated the stability of this protein nanoparticle and the biodistribution in vivo after injection intravenously.
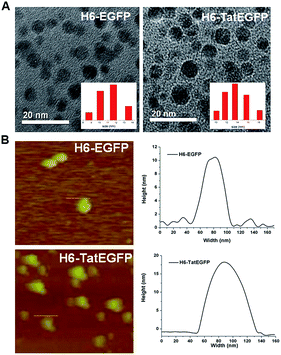
The nanoparticles were prepared by gene engineering method and obtained in dilution solution after protein purification. The size and morphology of nanoparticles were analysed by transmission electron microscope (TEM). As shown in Fig. 1A and Fig. S1,† the particle sizes of H6-EGFP and H6-TatEGFP were 11.7 nm and 13.5 nm, respectively. The structures of the protein nanoparticles were further confirmed by atomic force microscopy (AFM) in Fig. 1B. These results indicated the well-organized protein nanoparticles from H6-EGFP and H6-TatEGFP proteins.
In our previous work, we had shown the siRNA condensing activity and siRNA delivery abilities of H6-TatEGFP protein nanoparticles in vitro.15 Considering the EPR effect of nanoparticles, it is interesting to study the behaviors of these fluorescent nanoparticles in vivo. We first investigated the stability of H6-EGFP and H6-TatEGFP proteins in mouse plasma and serum by monitoring the fluorescence emission as used in Ce'spedes's report.11 The fluorescence intensity of both H6-EGFP and H6-TatEGFP proteins decreased to about 40% in mouse plasma after 24 h of incubation. While more than 60% of fluorescence decreasing was observed in mouse serum after 24 h (show in Fig. 2). The decreased fluorescence may be attributed to the proteolytic activities in serum and plasma, which did great damage to the structure of protein monomer. The failure in maintaining monomer structure may finally lead to the decreased fluorescent intensity and disruption of nanostructures. This result shows two proteins nanoparticles displayed moderate stability even in serum and plasma.
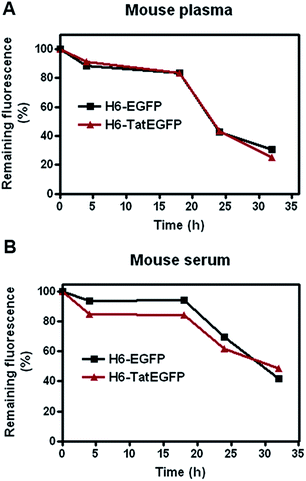 | ||
| Fig. 2 Stability analysis of H6-EGFP and H6-TatEGFP proteins in mouse plasma (A) and serum (B) by monitoring fluorescent emission. | ||
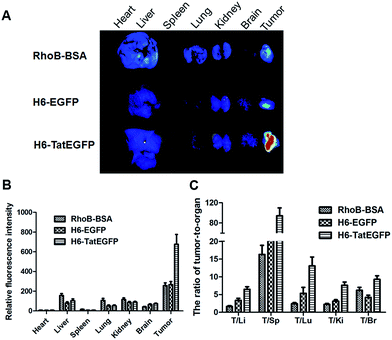
Nowadays Tat peptides have been widely used in promoting cell delivery of various types of cargoes. Tat peptides, which were used as targeting moiety, usually decorated in surface of nanoparticles to improve nuclear translocation.16 In this study, we next studied if Tat domain were involved in the biodistribution of protein nanoparticles. The biodistribution of H6-EGFP and H6-TatEGFP protein nanoparticles in vivo after tail vein administration was carried out by using xenograft-bearing mice. Rhodamine-B labelled bovine serum albumin (RhoB-BSA) was used as a negative control for EPR effect. The emission spectra of RhoB-BSA, H6-EGFP and H6-TatEGFP proteins are showed in Fig. S2.† Due to the different molecular weight of three proteins, 500 μg H6-EGFP and H6-TatEGFP proteins and 2 mg RhoB-BSA were injected into xenograft-bearing mice. After 2 h of administration, main organs including heart, liver, spleen, lung, kidney, brains and tumor were carefully excised and their fluorescent signals were recorded by fluorescent imaging system (CRI Maestro 500FL). As shown in Fig. 3A, nearly no signal of three proteins in heart and spleen was detected; while obvious fluorescent signals of RhoB-BSA, H6-EGFP and H6-TatEGFP were observed in liver and kidney, indicating the uptake by reticular-endothelial system (RES) and renal clearance of three proteins. Interestingly, there were significant differences in tumor accumulation of three proteins. H6-TatEGFP and H6-EGFP protein nanoparticles displayed preferential tumor distribution as shown in Fig. 3B and C (especially the ratio of tumor-to-organ), confirming the EPR effect of nanoparticles. Considering the Tat peptides (YGRRARRRRRR) difference between H6-TatEGFP and H6-EGFP protein nanoparticles, we speculated that Tat sequence may play a key role in promoting tumor accumulation of nanoparticles.
Tat peptides had been widely used in improving the cellular uptake of nanoparticles during the past decade.17 However, to the best of our knowledge, no report about the tumor-targeting property of Tat was found. Tat sequence was just decorated on the surface of nanoparticles in most studies, which was not involved in self-assembling process of nanoparticles. In our study, the Tat sequence plays a key role in formation of nanostructures.15 The mechanism of Tat sequence in tumor-targeting remained to be uncovered.
In summary, we had developed a tumor-targeting protein nanoparticle based on H6-TatEGFP fusion protein. This protein nanoparticle displayed high stability in mouse plasma and serum. More importantly, our protein nanoparticle could preferentially accumulated in tumor after tail vein administration. Our study highlights the potential application of our fluorescent protein nanoparticle for tumor imaging and drug delivery.
Acknowledgements
This work was financially supported by Science and Technology Department of Jilin province (No. 20130206050YY), Grant from Health Department of Jilin Province (No. 20122120 and 20122072), National Natural Science Foundation of China (Project No. 51503003) and Open Research Fund of State Key Laboratory of Polymer Physics and Chemistry (201501), Changchun Institute of Applied Chemistry, Chinese Academy of Sciences.Notes and references
- (a) A. Z. Wang, R. Langer and O. C. Farokhzad, Annu. Rev. Med., 2012, 63, 185–198 CrossRef CAS PubMed; (b) P. Couvreur, Adv. Drug Delivery Rev., 2013, 65, 21–23 CrossRef CAS PubMed; (c) H. Maeda, H. Nakamura and J. Fang, Adv. Drug Delivery Rev., 2013, 65, 71–79 CrossRef CAS PubMed.
- (a) L. Brannon-Peppas and J. O. Blanchette, Adv. Drug Delivery Rev., 2012, 64, 206–212 CrossRef; (b) S. D. Steichen, M. Caldorera-Moore and N. A. Peppas, Eur. J. Pharm. Sci., 2013, 48, 416–427 CrossRef CAS PubMed; (c) X. Hu, X. Guan, J. Li, Q. Pei, M. Liu, Z. Xie and X. Jing, Chem. Commun., 2014, 50, 9188–9191 RSC; (d) R. Wang, X. Hu, S. Wu, H. Xiao, H. Cai, Z. Xie, Y. Huang and X. Jing, Mol. Pharmaceutics, 2012, 9, 3200–3208 CrossRef CAS PubMed; (e) J. Ahn, Y. Miura, N. Yamada, T. Chida, X. Liu, A. Kim, R. Sato, R. Tsumura, Y. Koga and M. Yasunaga, Biomaterials, 2015, 39, 23–30 CrossRef CAS PubMed; (f) H. Li, L. Guo, A. Huang, H. Xu, X. Liu, H. Ding, J. Dong, J. Li, C. Wang and X. Su, Biomaterials, 2015, 63, 168–176 CrossRef CAS PubMed; (g) X. Guan, X. Hu, Z. Li, H. Zhang and Z. Xie, RSC Adv., 2015, 5, 22957–22964 RSC; (h) X. Guan, X. Hu, S. Liu, Y. Huang, X. Jing and Z. Xie, RSC Adv., 2014, 4, 55187–55194 RSC; (i) J. Yue, S. Liu, R. Wang, X. Hu, Z. Xie, Y. Huang and X. Jing, Mol. Pharmaceutics, 2012, 9, 1919–1931 CrossRef CAS PubMed; (j) X. Guan, X. Guan, H. Tong, J. Ma and X. Sun, J. Macromol. Sci., Part A: Pure Appl.Chem., 2015, 52, 401–406 CrossRef CAS; (k) X. Guan, R. Jiang, L. Sun, W. Chen, H. Tong, X. Sun, K. Feng and X. Gai, Dig. J. Nanomater. Bios., 2015, 10, 789–798 Search PubMed.
- (a) L. Quan, S. Liu, T. Sun, X. Guan, W. Lin, Z. Xie, Y. Huang, Y. Wang and X. Jing, Acs. Appl. Mater. Interfaces, 2014, 6, 16166–16173 CrossRef CAS PubMed; (b) Q. Wu, Q. Cheng, S. Yuan, J. Qian, K. Zhong, Y. Qian and Y. Liu, Chem. Sci., 2015, 6, 6607–6613 RSC; (c) S. Zhu, J. Zhang, J. Janjanam, J. Bi, G. Vegesna, A. Tiwari, F.-T. Luo, J. Wei and H. Liu, Anal. Chim. Acta, 2013, 758, 138–144 CrossRef CAS PubMed; (d) D. Ding, J. Liu, G. Feng, K. Li, Y. Hu and B. Liu, Small, 2013, 9, 3092–3092 CrossRef CAS.
- M. Zheng, S. Ruan, S. Liu, T. Sun, D. Qu, H. Zhao, Z. Xie, H. Gao, X. Jing and Z. Sun, ACS nano, 2015, 9, 11455–11461 CrossRef CAS PubMed.
- (a) M. Jahanshahi and Z. Babaei, Afr. J. Biotechnol., 2008, 7, 4926–4934 CAS; (b) W. Lohcharoenkal, L. Wang, Y. C. Chen, Y. Rojanasakul and Y. Rojanasakul, Biomed. Res. Int., 2014, 2014, 180549 Search PubMed; (c) K.-Y. Ahn, H. K. Ko, B.-R. Lee, E. J. Lee, J.-H. Lee, Y. Byun, I. C. Kwon, K. Kim and J. Lee, Biomaterials, 2014, 35, 6422–6429 CrossRef CAS PubMed; (d) Y.-T. Lai, E. Reading, G. L. Hura, K.-L. Tsai, A. Laganowsky, F. J. Asturias, J. A. Tainer, C. V. Robinson and T. O. Yeates, Nat. Chem., 2014, 6, 1065–1071 CrossRef CAS PubMed; (e) K. Fan, C. Cao, Y. Pan, D. Lu, D. Yang, J. Feng, L. Song, M. Liang and X. Yan, Nat. Nanotechnol., 2012, 7, 459–464 CrossRef CAS PubMed.
- A. O. Elzoghby, W. M. Samy and N. A. Elgindy, J. Controlled Release, 2012, 161, 38–49 CrossRef CAS PubMed.
- (a) L. Schoonen and J. C. M. van Hest, Nanoscale, 2014, 6, 7124–7141 RSC; (b) N. M. Molino and S. W. Wang, Curr. Opin. Biotechnol., 2014, 28, 75–82 CrossRef CAS PubMed; (c) Y. Ma, R. J. M. Nolte and J. J. L. M. Cornelissen, Adv. Drug Delivery Rev., 2012, 64, 811–825 CrossRef CAS PubMed; (d) K. Li, Z.-P. Zhang, M. Luo, X. Yu, Y. Han, H.-P. Wei, Z.-Q. Cui and X.-E. Zhang, Nanoscale, 2012, 4, 188–193 RSC; (e) D. Ren, F. Kratz and S. W. Wang, Small, 2011, 7, 1051–1060 CrossRef CAS PubMed.
- E. Vazquez, M. Roldán, C. Diez-Gil, U. Unzueta, J. Domingo-Espín, J. Cedano, O. Conchillo, I. Ratera, J. Veciana and X. Daura, Nanomedicine, 2010, 5, 259–268 CrossRef CAS PubMed.
- M. L. Flenniken, D. A. Willits, S. Brumfield, M. J. Young and T. Douglas, Nano Lett., 2003, 3, 1573–1576 CrossRef CAS.
- M. Liang, K. Fan, M. Zhou, D. Duan, J. Zheng, D. Yang, J. Feng and X. Yan, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 14900–14905 CrossRef CAS PubMed.
- M. V. Cespedes, U. Unzueta, W. Tatkiewicz, A. Sanchez-Chardi, O. Conchillo-Sole, P. Alamo, Z. Xu, I. Casanova, J. L. Corchero, M. Pesarrodona, J. Cedano, X. Daura, I. Ratera, J. Veciana, N. Ferrer-Miralles, E. Vazquez, A. Villaverde and R. Mangues, ACS Nano, 2014, 8, 4166–4176 CrossRef CAS PubMed.
- (a) S. M. Farkhani, A. Valizadeh, H. Karami, S. Mohammadi, N. Sohrabi and F. Badrzadeh, Peptides, 2014, 57, 78–94 CrossRef CAS PubMed; (b) H. Margus, K. Padari and M. Pooga, Mol. Ther., 2012, 20, 525–533 CrossRef CAS PubMed.
- (a) M. Rizzuti, M. Nizzardo, C. Zanetta, A. Ramirez and S. Corti, Drug Discovery Today, 2015, 20, 76–85 CrossRef CAS PubMed; (b) D. Kim, C. Jeon, J.-H. Kim, M.-S. Kim, C.-H. Yoon, I.-S. Choi, S.-H. Kim and Y.-S. Bae, Exp. Cell Res., 2006, 312, 1277–1288 CrossRef CAS PubMed; (c) M. Rafat, C. A. Cleroux, W. G. Fong, A. N. Baker, B. C. Leonard, M. D. O'Connor and C. Tsilfidis, Biomaterials, 2010, 31, 3414–3421 CrossRef CAS PubMed.
- (a) V. P. Torchilin, Adv. Drug Delivery Rev., 2008, 60, 548–558 CrossRef CAS PubMed; (b) L. Pan, Q. He, J. Liu, Y. Chen, M. Ma, L. Zhang and J. Shi, J. Am. Chem. Soc., 2012, 134, 5722–5725 CrossRef CAS PubMed; (c) H. Yuan, A. M. Fales and T. Vo-Dinh, J. Am. Chem. Soc., 2012, 134, 11358–11361 CrossRef CAS PubMed; (d) M. Malhotra, C. Tomaro-Duchesneau and S. Prakash, Biomaterials, 2013, 34, 1270–1280 CrossRef CAS PubMed.
- X. Guan, X. Hu, F. Cui, Y. Li, X. Jing and Z. Xie, Macromol. Biosci., 2015, 15, 1484–1489 CrossRef CAS PubMed.
- (a) X.-h. Tian, F. Wei, T.-x. Wang, D. Wang, J. Wang, X.-n. Lin, P. Wang and L. Ren, Mater. Lett., 2012, 68, 94–96 CrossRef CAS; (b) M. Malhotra, C. Tomaro-Duchesneau, S. Saha, I. Kahouli and S. Prakash, Int. J. Nanomed., 2013, 8, 2041 CrossRef PubMed.
- (a) L. Pan, Q. He, J. Liu, Y. Chen, M. Ma, L. Zhang and J. Shi, J. Am. Chem. Soc., 2012, 134, 5722–5725 CrossRef CAS PubMed; (b) H. Yuan, A. M. Fales and T. Vo-Dinh, J. Am. Chem. Soc., 2012, 134, 11358–11361 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra27411g |
| This journal is © The Royal Society of Chemistry 2016 |