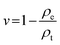Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
3-Dimensional porous nanocomposite scaffolds based on cellulose nanofibers for cartilage tissue engineering: tailoring of porosity and mechanical performance†
Narges
Naseri
a,
Jean-Michel
Poirier
a,
Lenart
Girandon
b,
Mirjam
Fröhlich
b,
Kristiina
Oksman
a and
Aji P.
Mathew
*ac
aDivision of Materials Science, Luleå University of Technology, 97187 Luleå, Sweden. E-mail: aji.mathew@ltu.se; aji.mathew@mmk.su.se; Fax: +46 920 49 1399; Tel: +46 920 49 2024
bEducell ltd., Prevale 9, 1236 Trzin, Slovenia
cDivision of Materials and Environmental Chemistry, Stockholm University, SE-10691 Stockholm, Sweden
First published on 8th January 2016
Abstract
Fully bio-based 3-dimensional porous scaffolds based on freeze-dried cellulose nanofibers (70–90 wt%) stabilized using a genipin crosslinked matrix of gelatin and chitosan were prepared. Morphology studies using scanning electron microscopy showed that the scaffolds have interconnected pores with average pore diameters of 75–200 μm and nanoscaled pore wall roughness, both favorable for cell interactions with cartilage repair. X-ray tomography confirmed the 3-dimensional homogeneity and interconnectivity of the pores as well as the fibrillar structure of the scaffolds. The compression modulus of the scaffolds (1–3 MPa) at room conditions was higher than natural cartilage (≈1 MPa). The lowered compression modulus of 10–60 kPa in phosphate buffered saline (PBS) at 37 °C was considered favorable for chondrogenesis. The current study therefore successfully addressed the challenge of tailoring the pore structure and mechanical properties simultaneously for cartilage regeneration. Furthermore, the scaffolds' high porosity (≈95%), high PBS uptake and good cytocompatibility towards chondrocytes are considered beneficial for cell attachment and extracellular matrix (ECM) production.
1 Introduction
Articular cartilage is an avascular, non-innervated tissue composed mostly of extracellular matrix (ECM) with a sparse population of chondrocytes distributed throughout the tissue and 70–85 wt% of water.1 Due to its poor cell density and lack of blood vessels, cartilage has a very limited capacity to repair itself from defects caused by trauma or aging. In this respect, developing new tissue engineering approaches to repair cartilage defects and to restore cartilage function are of great interest.2,3Due to good biocompatibility, natural biomaterials such as chitosan, gelatin, alginate and collagen have been used as raw materials for scaffolds in soft tissue engineering.3–9 These natural polymers support the cell growth and regeneration. However, their use is limited because of their lower mechanical properties when compared to synthetic polymers, especially load behaviour requirements necessary to allow proper cell proliferation.10–12
In cartilage tissue engineering, scaffolds are expected to imitate the functions of damaged cartilage and provide a 3-dimensional (3D) environment for cell growth and ECM production.3,4 Typically, the tailoring of scaffold porosity with pore sizes in the range of 200–300 μm is considered a benchmark for cartilage regeneration.4,13 Such porous scaffolds can be prepared using many different processes, such as freeze-drying, CO2 foaming, electrospinning or cryogelation, with a wide variety of polymers as found in literature.5,14–16
Chitosan and gelatin based scaffolds have been reported to be beneficial for soft load bearing tissues. Positive charges on the surface of chitosan can promote chondrocyte growth thus beneficial for cartilage repair.3,4 Gelatin is partially derived from collagen, which is the main protein component of connective tissues as cartilage, skin and bone. Furthermore, a blend of these polymers absorbs water and forms a hydrogel whereby allowing fluid to be retained in the scaffold structure leading to a higher compression modulus similar to natural soft tissue.4,13,17,18 A recent study on chitosan/gelatin blends with random and aligned pore structures prepared with a freeze-drying process showed a compression modulus in the range of 5 kPa (for randomly aligned scaffolds) to 30 kPa (in the vertical direction of the aligned scaffold), when tested at 37 °C after conditioning in PBS medium.18
To enhance the mechanical performance of biopolymers and their blends, different types of reinforcements obtained from natural materials have been used; the most important examples being derivatives of cellulose and chitin.8,9,14,15 Our earlier studies have also demonstrated that solution cast fibrous nanocomposite structures with high cellulose nanofibers concentrations (75 wt%) and collagen provide mechanical performance suitable for ligaments.8,9 Also, our previous studies have demonstrated the non-cytotoxicity of nanocellulose from different sources and their potential in medical applications.15,19,20
In the current study, 3D nanocomposite scaffolds for cartilage regeneration were processed via freeze-drying technique, where porous cellulose nanofiber structures were bound together and mechanically/dimensionally stabilized using low amounts of crosslinked chitosan/gelatin blend system. The pore structure developed during the processing is expected to impact the moisture uptake and mechanical performance of the resultant scaffolds. Moreover, the pore structure and sizes, which favor the movement of fluids through the scaffold, creating drag forces,21 as well as the development of ECM, is known to contribute to the load bearing under in vivo conditions. Though tailoring of mechanical properties using nanocellulose have received some attention in tissue engineering,8,9,22 limited studies are available on tailoring the pore structure in bionanocomposites and understanding the effect of porosity on the mechanical performance. It is highly challenging to tailor the pore structure and mechanical properties required for tissue regeneration, simultaneously, as increase in porosity generally decreases the mechanical properties and vice versa. The pore structure and porosity of the scaffold was tailored in the current study by varying the suspension concentrations, fibers/matrix ratio and crosslinking with the aim to obtain optimal mechanical properties and chondrocyte attachment and proliferation to obtain extracellular matrix of optimal quality. It was expected that these 3D porous structures could act as templates for the formation of new tissue and act as guidance for cell growth while facilitating nutrient and oxygen transport.
The structural morphology of the produced scaffolds was observed by scanning electron microscopy (SEM) and X-ray tomography. Mechanical properties in room condition and in PBS medium, moisture uptake, density and porosity, as well as in vitro biodegradation and cytocompatibility towards chondrocytes were also investigated.
2 Experimental
2.1 Materials
High-purity cellulose from softwood fibers (Norwegian spruce) with high cellulose content (95% cellulose, 4.5% hemicellulose and 0.1% lignin content as provided by Domsjö Fabriker AB, Sweden) was used as starting material for the production of cellulose nanofibers. Medium Mw chitosan (DD ≈ 75–85%), acetic acid, gelatin, as well as phosphate buffered saline (PBS) and genipin were purchased from Sigma-Aldrich, Germany.2.2 Methods
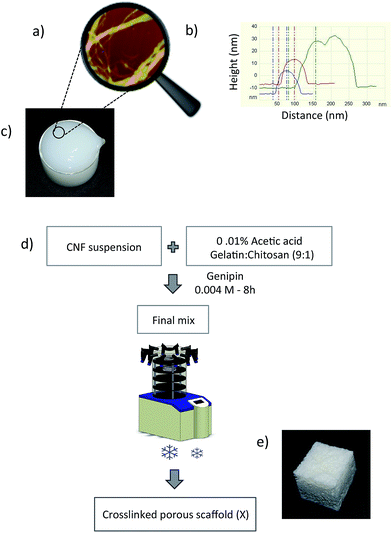
The atomic force microscopy of the prepared nanofibers (Fig. 1a) showed nanosized fibers with diameters in the range of 19–38 nm (Fig. 1b), based on the measurements using the Nanoscope V software (Santa Barbara, CA, USA). The diameter of nanofibers was measured from the height to compensate the tip broadening effect. The photograph of the gels of CNF obtained after grinding is also shown (Fig. 1c).
The suspension was centrifuged for 30 min and the rotor speed was 1500 rpm to remove water and obtain highly concentrated gel, typically between 7 and 10 wt%, which was used for scaffold processing.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was dissolved in 0.01% acetic acid medium containing 0.004 M genipin solution. Gelatin/chitosan mixture in the ratio of 9
1) was dissolved in 0.01% acetic acid medium containing 0.004 M genipin solution. Gelatin/chitosan mixture in the ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is referred to as the matrix (M) throughout the manuscript. The nanofiber suspensions were mixed with this solution in appropriate amounts to obtain final nanocomposites with different compositions. All samples were placed in plastic Petri dishes, frozen at −30 °C and freeze-dried at −70 °C in a vacuum (0.0026 mbar). The complete process with a photograph of the produced scaffold is shown in Fig. 1d and e, respectively. All scaffolds prepared in this work and their compositions are listed in Table 1.
1 is referred to as the matrix (M) throughout the manuscript. The nanofiber suspensions were mixed with this solution in appropriate amounts to obtain final nanocomposites with different compositions. All samples were placed in plastic Petri dishes, frozen at −30 °C and freeze-dried at −70 °C in a vacuum (0.0026 mbar). The complete process with a photograph of the produced scaffold is shown in Fig. 1d and e, respectively. All scaffolds prepared in this work and their compositions are listed in Table 1.
| Sample | CNF (g) | M (g) | Sample code | Density (g cm−3) | Porosity (%) | Average pore size (μm) |
|---|---|---|---|---|---|---|
a
M: matrix composed of gelatin/chitosan (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), X: crosslinked. 1), X: crosslinked.
|
||||||
| 1 | 4 | 0.0 | CNF4 | 0.069 | 95.5 | — |
| 2 | 4 | 0.4 | CNF4-M0.4 | 0.062 | 95.6 | 153 ± 53 |
| 3 | 4 | 0.4 | X-CNF4-M0.4 | 0.066 | 95.3 | 68 ± 49 |
| 4 | 4 | 1.1 | X-CNF4-M1.1 | 0.061 | 95.1 | 58 ± 35 |
| 5 | 4 | 2.2 | X-CNF4-M2.2 | 0.065 | 94.2 | 59 ± 18 |
| 6 | 5 | 0.5 | X-CNF5-M0.5 | 0.076 | 94.6 | 90 ± 71 |
| 7 | 6 | 0.6 | X-CNF6-M0.6 | 0.093 | 93.4 | 65 ± 51 |
2.3 Characterization
For high-resolution images, MAGELLAN 400 XHR-SEM (FEI Company, Eindhoven, The Netherlands) was used. The samples were placed on carbon tape, coated with tungsten, and observed under the SEM at an acceleration voltage of 3 kV.
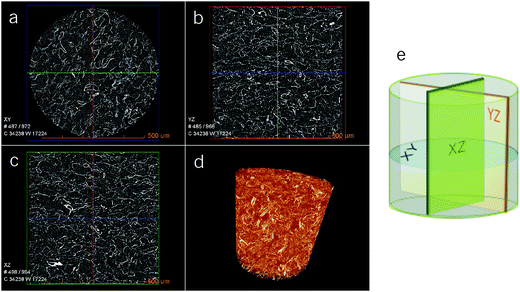
1where ρe is the experimental density of the scaffold and ρt is the theoretical density of a non-porous scaffold. The densities of CNF, gelatin and chitosan were taken as 1.54, 0.98 and 0.235 g cm−3, respectively.
Nanoscaled pores were measured using a Micromeritics ASAP 2000 instrument and the average pore diameters were determined from nitrogen adsorption measurements at 77 K using the BET method. The measurements were performed after degassing the samples at 100 °C for 48 h in dry N2 flow.
| Moisture uptake (%) = [(Wt − Wd)/Wd] × 100 | (2) |
In order to assess the swelling of the matrix phase, samples were dried in the vacuum oven and weighed as described above and placed in a 95% moisture desiccator. The samples were weighed every two days for two weeks in order to follow the weight gain until equilibrium. The moisture uptake was calculated using the same equation as above.
For tests in PBS, the sample thickness was about 5 mm and the cut samples were immersed in PBS for 24 h before testing. The displacement rate for moduli calculation was 400 μm min−1 and the initial contact force was 0.02 N, slightly adapted according to the specific rigidity of each type of sample (up to 0.05 N). Compression tests were also performed in PBS at varying strain rates ranging from 100 μm min−1 to 400 μm min−1 to evaluate viscoelasticity. Each test was performed at least five times and the average values were reported.
| Weight loss (%) = [(W0 − Wt)/W0] × 100 | (3) |
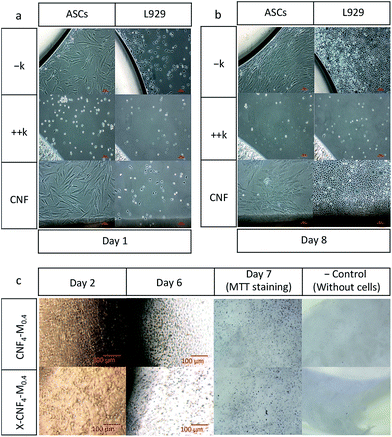
Cytocompatibility of the CNF. CNF films were fixed to cell culture dishes and the cells (adipose derived stem cells (ASCs) and L929 cell line) were seeded evenly throughout the cell culture dish. The impact of the biomaterial on cell growth and morphology was monitored and documented with photographs.
Cytocompatibility of the scaffolds. Cytocompatibility of the scaffolds was monitored in a direct contact testing system according to ISO 10993. The biomaterials were fixed in cell culture vessels and cells, namely chondrocytes, were seeded on the biomaterial, (0.5 × 106 cells per ml) in cell culture media supplemented with 10% FBS (Fetal Bovine Serum). The scaffolds with cells were incubated at 37 °C in 5% CO2 for 7 days. After 7 days the biomaterials were stained with MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) and inspected for the presence of live cells on the upper surface of the scaffolds. Biomaterial with no seeded cells was regarded as a negative control.
3 Results and discussion
3.1 Morphology and pore structure of scaffolds
The SEM images were evaluated to understand the effect of crosslinking and suspension composition on scaffold morphology. CNF4-M0.4 and X-CNF4-M0.4 (Fig. 2a and b) showed the pore sizes in the micrometer range in both cases. Uncrosslinked scaffolds had a wide distribution in pore size (Fig. 2a) while crosslinking slightly enhanced the overall homogeneity of the structure and decreased the pore size (Fig. 2b).The morphologies of X-CNF4-M0.4, X-CNF4-M1.1, and X-CNF4-M2.2 with varying the matrix content are compared in Fig. 2c–e. Fig. 2c shows CNF bound by the matrix forming an interconnected pore structure with single and bundled fibers emerging from and embedded in the matrix. X-CNF4-M1.1 and X-CNF4-M2.2 (Fig. 2d and e) show flat and layered structures and resemble self-assembly behaviour similar to that of pure gelatin, as previously found in literature.13,25 As the matrix content increased, smoother and thicker wall structures and fewer pores were observed, which was not considered favorable for the pore sizes required for cartilage applications.
When comparing the scaffolds with different initial suspension concentrations, X-CNF4-M0.4 showed a pore structure which is relatively homogeneous and interconnected, whereas X-CNF5-M0.5 and X-CNF6-M0.6 showed more of a layered structure than a fibrillar structure and formed denser structures with fewer pores and cellulose nanofibers coated with matrix compared to X-CNF4-M0.4 (images given in ESI, S1†). Earlier studies also have demonstrated the controlling the cell size and foam density by changing the suspension concentration.26,27
The pore sizes measured from SEM for all developed scaffolds are summarized in Table 1. In all cases the standard deviations are high and pore sizes in the range of 20 μm to 200 μm were observed. The highest average pore size (100–200 μm) was with the uncrosslinked system and the pore size decreased with crosslinking (20–120 μm), increased matrix content (20–75 μm) and increased suspension concentration (40–115 μm).
The X-CNF4-M0.4 system was considered optimal for the cartilage tissue engineering based on pore structure, homogeneity of pores and average pore sizes. When examined using high-resolution microscopy (Fig. 2f), X-CNF4-M0.4 showed a highly entangled network of CNF on the pore walls. These fibrous nanostructures and the pore wall roughness is expected to aid cell fixation and extracellular matrix (ECM) development after implantation because the rougher surface improves vascularization, diffusion rates to and from the scaffold for oxygen/nutrients supply and removal of waste.23,28 (For this system, the nanoscaled pores measured from micromeritics porosity analyser were in the range of 12–14 nm).
All of the materials were highly porous (>93%) and had low densities, shown in Table 1, and which agree with literature values.27 The crosslinking as well as the increase in matrix content had limited influence on porosity. These samples had a similar density irrespective of the matrix content (samples 1–5), most likely due to the higher bulk density of cellulose (1.54 g cm−3) as cellulose is the main component in all of the samples. When the concentration of the freeze-drying suspension increased (samples 3, 6 and 7) while keeping the fibers to matrix ratio constant (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the density increased from 0.066 to 0.093 g cm−3 due to denser packing of fiber network and as expected led to a decrease in porosity.26,27
1), the density increased from 0.066 to 0.093 g cm−3 due to denser packing of fiber network and as expected led to a decrease in porosity.26,27
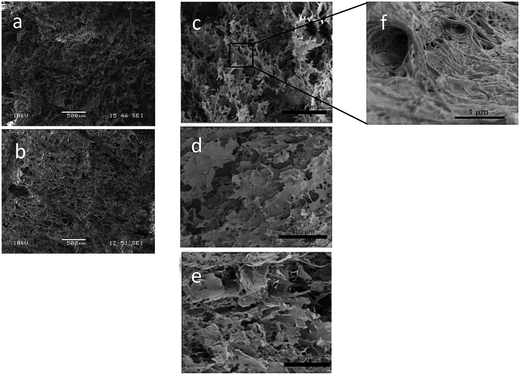
Fig. 3a–c shows cross-sectional images of the porous scaffold for the X-CNF4-M0.4 system obtained using X-ray tomography. It can be observed that the scaffold has high porosity (confirming the porosity data in Table 1) as well as pores are uniformly distributed in the horizontal as well as vertical sections of the scans. Furthermore, X-ray tomography confirmed the interconnectivity of the pores as well as the fibrillar structure of the scaffolds.
The 3D interconnectivity of pores (Fig. 3d) throughout the scaffold, the high degree of porosity, the hierarchical pore structure with micron sized pores in the bulk and the nanoscaled pores on the walls of scaffolds were considered optimal for the cartilage tissue engineering.26
3.2 Moisture uptake
As 70–85% of the weight of natural cartilaginous tissues is water,1 it is important to understand the water uptake by the scaffolds. The PBS uptake values of the submerged samples were in the range of 1000–1677%, depending on the composition. The results of the moisture uptake measurements are shown in Fig. 4. The initial moisture uptake was instantaneous (30 s) and remained constant after 3 days in PBS and the materials showed hydrogel behaviour.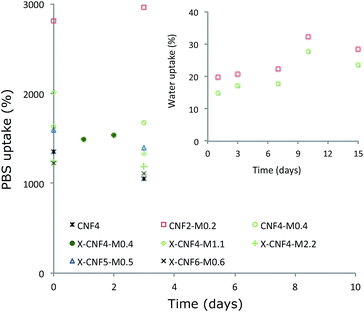 | ||
| Fig. 4 Effect of fibers/matrix ratio, initial concentration and crosslinking on the PBS and water uptake by the scaffolds. | ||
It can be inferred that the open pore structure of the materials (shown in SEM) plays a role in the fast and high moisture uptake and moisture susceptibility in the scaffolds. It was found that the amount of matrix does not significantly affect the uptake, as X-CNF4-M0.4, X-CNF4-M1.1 and X-CNF4-M2.2 showed similar water uptake. This indicates that a significant proportion of the water uptake is due to the porous structure of the scaffolds. A higher CNF concentration in the suspension resulted in a decrease in the uptake of the resultant scaffolds due to a tighter, denser network with smaller pores, as expected. However, it was noted that porous CNF scaffolds without matrix (CNF4) absorbed PBS quickly and disaggregated easily when manipulated since there is no matrix to bind the fibers together. The uncrosslinked samples also proved to be unstable after 3 days in PBS, while the crosslinked samples remained stable in moist conditions during the whole duration of the experiment.
The water uptake by the same scaffolds was monitored in 95% RH conditions (without immersion) and the maximum uptake was 30% of its original weight, even after 15 days (shown by Fig. 4 in the inset). This shows that the adsorption due to the scaffold swelling is negligible. No difference has been observed regardless of whether the samples are crosslinked or not, and the total uptake showed a tendency to decrease as the initial concentration increased, which is in correlation with the density of the material.
3.3 Mechanical properties
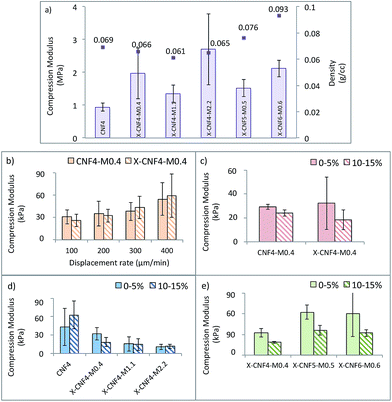
Compression is the preferred mode of mechanical testing for cartilage materials because the role of natural cartilage is to bear loads in compression1,13 (see ESI for the representative load displacement curves, S2†). In dry conditions and at 37 °C (Fig. 5a), the compression modulus was in the range 1–3 MPa which agree with earlier reports of anisotropic CNF based foams.26,27 No clear trend can be observed when varying the total concentration or the CNF/matrix ratio and the values do not follow the density as reported in some earlier literature,26 most likely due to the crosslinking effect which is not considered in density calculation. Nonetheless, the presence of the matrix enhanced the mechanical properties in dry conditions when compared to CNF alone. However, high standard deviations were observed and may be due to the wide distribution of the pore sizes.The aggregate compression modulus of articular cartilage is reported to be around 0.9 MPa by Martin et al.29 and 0.5–0.1 MPa by Guilak et al.30 Also, a wide range of values varying between 0.1 MPa and 2 MPa are reported as compression moduli for healthy cartilage31–34 depending on the source and testing conditions. The values of compression moduli of the current scaffolds are slightly higher than that of natural cartilage, but it may be noted that the moisture content in natural cartilage is greater.
The performance of the scaffolds was evaluated in simulated body conditions (PBS medium and 37 °C) to understand the effect of compression rate, crosslinking, fibers/matrix ratio and initial suspension concentration; (see Fig. 5b–e). The compression modulus showed a clear tendency to increase as the compression rate increased from 100 to 400 μm min−1 (Fig. 5b), a sign of the viscoelasticity of the scaffolds. In dry conditions, the scaffolds exhibited an elastoplastic behaviour, but when submerged in PBS, viscoelastic behaviour was evident. The viscoelastic behaviour in PBS medium is partly due to the swelling of the matrix phase as well as fluid flow through the pores of the scaffolds during compression. This tendency was reported for natural cartilage tissues when tested in compression mode and therefore considered favorable for load bearing by the scaffolds.21
The influence of crosslinking on compression modulus was evaluated for CNF4-M0.4 and X-CNF4-M0.4, tested in PBS at 400 μm min−1 (Fig. 5c) in the strain region of 0–5% and 10–15%. No significant improvement on the mechanical properties was achieved by crosslinking and the compression modulus was around 30 kPa. The low amount of matrix material available for crosslinking as well as the similar density and porosity observed for these scaffolds explains this trend.
The compression moduli of CNF4, X-CNF4-M0.4, X-CNF4-M1.1 and X-CNF4-M2.2 in the strain region of 0–5% and 10–15% are given in Fig. 5d. The values decreased from 62 kPa for pure CNF to 10 kPa for nanocomposites with the highest matrix concentration (X-CNF4-M2.2). The results show that the scaffolds become weaker in wet conditions when the matrix content increases. One possible reason is that gelatin and chitosan are mechanically weaker in wet conditions than the CNF network. Nevertheless, a minimum concentration of matrix phase was necessary to bind the fibers together and ensure the stability of the scaffold in wet medium. Furthermore, CNF4 collapsed completely during compression tests in submersion mode after 15% strain, while scaffolds with matrix phase showed better mechanical and dimensional stability in spite of the lower compression moduli. Fig. 5e shows scaffolds prepared by increasing suspension concentrations (X-CNF4-M0.4, X-CNF5-M0.5 and X-CNF6-M0.6) but the same ratio of fibers/matrix (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The compression modulus increased from 18 to 60 kPa as the concentration increased from 2.2 to 5.5 but stabilized thereafter. The increase in density of the scaffolds did not affect the mechanical properties significantly, especially in PBS medium.
1). The compression modulus increased from 18 to 60 kPa as the concentration increased from 2.2 to 5.5 but stabilized thereafter. The increase in density of the scaffolds did not affect the mechanical properties significantly, especially in PBS medium.
In general, it can be seen that the compression modulus in wet conditions is lower (10–60 kPa) than in dry conditions (1–3 MPa). The decreased mechanical properties in wet conditions were expected due to the swelling and plasticisation of the scaffold pore walls with water. The hydrophilicity of the nanocellulose, gelatin and chitosan as well as the high water-binding capability of gelatin significantly impacts the performance in aqueous medium. The compression moduli of the scaffolds with CNF as reinforcement in the chitosan/gelatin matrix is however significantly higher than those reported for the chitosan/gelatin blend,18 indicating that CNF acts as reinforcements in the porous scaffold.
The values presented here are lower than reported for natural cartilage tested in wet conditions. It may be noted that the current compression studies are performed submerged in PBS and at 37 °C which can weaken the scaffold in comparison to conditioned natural cartilage tested at 37 °C. More importantly, it was demonstrated earlier that in in vivo conditions, chondrocytes sense the mechanical properties of the substrate and soft scaffolds (4 kPa) facilitate chondrogenesis where as stiffer scaffolds (≥40 kPa) are shown to favor bone regeneration.35,36
In the current study, X-CNF4-M0.4 scaffolds have shown favorable mechanical properties (18–32 kPa) for chondrogenesis, stability in moist conditions and favorable pore sizes and pore wall morphology for cell adhesion. Therefore, these scaffolds are expected to develop extracellular matrix (ECM) and regenerate cartilage with the right mechanical properties after implantation. The evaluation of the scaffold after ECM development will be required to understand the performance of the scaffold in in vivo conditions and may be addressed in future.
3.4 Biodegradability
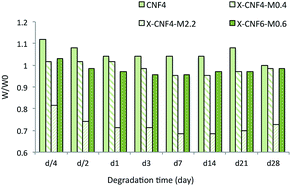
In vitro degradation tests of the scaffolds were investigated up to 28 days and the results are shown in Fig. 6. The scaffold, which contained only CNF4 (used as control), displayed significant morphological changes during the degradation time. In agreement with the observations during water uptake studies, these scaffolds did not have dimensional stability in order to bind the fibers together due to lack of matrix content. The scaffold comprising X-CNF4-M2.2 showed around 27% decrease in weight, while the scaffold comprising X-CNF4-M0.4 and X-CNF6-M0.6 demonstrated 1.5% weight loss after up to 4 weeks. Therefore, the greatest weight loss was obtained for the scaffold containing the higher amount of matrix.An optimal rate of degradation crucial for cartilage regeneration is one which balances stable 3D structures that provide support with gradual development of ECM.37
The short-term biodegradation studies showed that the rate of degradation of nanofibrous scaffolds could be controlled by the fibers/matrix ratio. The slow biodegradation tendency of biologically stable scaffolds in the current study, which provides an enduring support for the patient while also favoring ECM formation, can be considered beneficial. However, further long-term investigation needs to be done to evaluate ECM development in correlation with biodegradation of the scaffold to ensure the potential of the produced scaffold.
3.5 Cytocompatibility studies
4 Conclusions
Nanocomposites of cellulose nanofibers bound in a gelatin and chitosan matrix were prepared via freeze-drying and crosslinked using genipin to obtain highly porous (≈95% porosity) 3D scaffolds with optimal pore size, porosity, pore interconnectivity as well as mechanical performance, moisture stability and cell interactions. The freeze-drying route resulted in isotropic scaffolds and the pore structure was most homogenous for nanocomposites with a low amount of crosslinked matrix that acted as binding phase and dimensional stabilizer in moist conditions. The compression moduli of the optimal scaffolds (X-CNF4-M0.4) in dry conditions, at 37 °C, were around 1 MPa and considered comparable to natural cartilaginous tissue. These scaffolds showed viscoelasticity when immersed in PBS, similar to that of natural cartilage, but lower compression modulus (18–32 kPa) which was considered favorable for soft tissue regeneration. We have successfully tailored fully bio-based scaffolds with a high degree of bulk porosity, hierarchical pore structure, nanoscaled roughness and fibrillar structure of the pore walls combined with good mechanical properties and cytocompatibility with high potential for cartilage regeneration. Furthermore, the possibility to develop ECM and trap moisture in the interconnected pore structure is expected to bring the mechanical properties closer to those of natural cartilage after implantation.Acknowledgements
The authors gratefully acknowledge VINNOVA for financial support under the MNT-ERANET project n-POSSCOG (2011-02071). The authors would also like to thank Iftekhar Uddin Bhuiyan and Stephen Hall for help with high-resolution SEM images and X-ray tomography, respectively. Thushara Thomas is acknowledged for the help with water sorption studies.Notes and references
- J. M. Mansour, Kinesiology: the mechanics and pathomechanics of human movement, 2003, pp. 66–79 Search PubMed.
- E. B. Hunziker, Osteoarthritis Cartilage, 2002, 10, 432–463 CrossRef CAS PubMed.
- J. K. Francis Suh and H. W. T. Matthew, Biomaterials, 2000, 21, 2589–2598 CrossRef CAS.
- S. W. Whu, K. Hung, K. Hsieh, C. Chen, C. Tsai and S. Hsu, Mater. Sci. Eng., C, 2013, 33, 2855–2863 CrossRef CAS PubMed.
- S. Bhat, A. Tripathi and A. Kumar, J. R. Soc., Interface, 2011, 8, 540–554 CrossRef CAS PubMed.
- N. S. Sambudi, M. Sathyamurthy, G. M. Lee and S. B. Park, Compos. Sci. Technol., 2015, 106, 76–84 CrossRef CAS.
- Y. Zhao, S. Gao, S. Zhao, Y. Li, L. Cheng, J. Li and Y. Yin, Mater. Sci. Eng., C, 2012, 32, 2153–2162 CrossRef CAS.
- A. P. Mathew, K. Oksman, D. Pierron and M. Harmand, Macromol. Biosci., 2013, 13, 289–298 CrossRef CAS PubMed.
- A. P. Mathew, K. Oksman, D. Pierron and M. Harmad, Cellulose, 2012, 19, 139–150 CrossRef CAS.
- K. Tomihata, K. Burczak, K. Shiraki and Y. Ikada, in Polymers of Biological and Biomedical Significance, nonymous, ACS, 1993, pp. 275–286 Search PubMed.
- J. Hodde, Tissue Eng., 2002, 8, 295–308 CrossRef CAS PubMed.
- Z. Huang, Y. Z. Zhang, M. Kotaki and S. Ramakrishna, Compos. Sci. Technol., 2003, 63, 2223–2253 CrossRef CAS.
- M. Sarem, F. Moztarzadeh and M. Mozafari, Carbohydr. Polym., 2013, 93, 635–643 CrossRef CAS PubMed.
- N. Naseri, C. Algan, V. Jacobs, M. John, K. Oksman and A. P. Mathew, Carbohydr. Polym., 2014, 109, 7–15 CrossRef CAS PubMed.
- N. Naseri, A. P. Mathew, L. Girandon, M. Fröhlich and K. Oksman, Cellulose, 2015, 22, 521–534 CrossRef CAS.
- D. W. Hutmacher, Biomaterials, 2000, 21, 2529–2543 CrossRef CAS PubMed.
- V. Chiono, E. Pulieri, G. Vozzi, G. Ciardelli, A. Ahluwalia and P. Giusti, J. Mater. Sci.: Mater. Med., 2008, 19, 889–898 CrossRef CAS PubMed.
- A. Arora, A. Kothari and D. S. Katti, J. Mech. Behav. Biomed. Mater., 2015, 51, 169–183 CrossRef CAS PubMed.
- A. P. Mathew, K. Oksman, Z. Karim, P. Liu, S. A. Khan and N. Naseri, Ind. Crops Prod., 2014, 58, 212–219 CrossRef CAS.
- M. Colic, D. Mihajlovic, A. Mathew, N. Naseri and V. Kokol, Cellulose, 2014, 22, 763–778 CrossRef.
- H. N. Chia and M. L. Hull, J. Orthop. Res., 2008, 26, 951–956 CrossRef PubMed.
- H. Sehaqui, Q. Zhou, O. Ikkala and L. A. Berglund, Biomacromolecules, 2011, 12, 3638–3644 CrossRef CAS PubMed.
- A. Thorvaldsson, H. Stenhamre, P. Gatenholm and P. Walkenström, Biomacromolecules, 2008, 9, 1044–1049 CrossRef CAS PubMed.
- T. Telemeco, C. Ayres, G. Bowlin, G. Wnek, E. Boland, N. Cohen, C. Baumgarten, J. Mathews and D. Simpson, Acta Biomater., 2005, 1, 377–385 CrossRef CAS PubMed.
- J. S. Mao, L. G. Zhao, Y. J. Yin and K. de Yao, Biomaterials, 2003, 24, 1067–1074 CrossRef CAS PubMed.
- H. Sehaqui, M. Salajková, Q. Zhou and L. A. Berglund, Soft Matter, 2010, 6, 1824–1832 RSC.
- H. Sehaqui, Q. Zhou and L. A. Berglund, Compos. Sci. Technol., 2011, 71, 1593–1599 CrossRef CAS.
- H. Cheung, K. Lau, T. Lu and D. Hui, Composites, Part B, 2007, 38, 291–300 CrossRef.
- R. B. Martin, D. B. Burr and N. A. Sharkey, in Skeletal Tissue Mechanics, ed. R. B. Martin, D. B. Burr and N. A. Sharkey, Springer, New York, 1998, pp. 278–289 Search PubMed.
- F. Guilak, W. R. Jones, H. P. Ting-Beall and G. M. Lee, Osteoarthritis and Cartilage, 1999, 7, 59–70 CrossRef CAS PubMed.
- J. Jurvelin, I. Kiviranta, J. Arokoski, M. Tammi and H. Helminen, Eng. Med., 1987, 16, 15–22 CrossRef CAS PubMed.
- M. Laasanen, J. Töyräs, R. Korhonen, J. Rieppo, S. Saarakkala, M. Nieminen, J. Hirvonen and J. Jurvelin, Biorheology, 2003, 40, 133–140 CAS.
- A. J. Almarza and K. A. Athanasiou, Ann. Biomed. Eng., 2004, 32, 2–17 CrossRef PubMed.
- K. L. Spiller, J. L. Holloway, M. E. Gribb and A. M. Lowman, J. Tissue Eng. Regener. Med., 2011, 5, 636–647 CrossRef CAS PubMed.
- E. Schuh, J. Kramer, J. Rohwedel, H. Notbohm, R. Müller, T. Gutsmann and N. Rotter, Tissue Eng., Part A, 2010, 16, 1281–1290 CrossRef CAS PubMed.
- W. Zhong, Y. Li, L. Li, W. Zhang, S. Wang and X. Zheng, J. Mol. Histol., 2013, 44, 587–595 CrossRef CAS PubMed.
- J. Raghunath, J. Rollo, K. M. Sales, P. E. Butler and A. M. Seifalian, Biotechnol. Appl. Biochem., 2007, 46, 73–84 CrossRef CAS PubMed.
- L. Flynn, G. D. Prestwich, J. L. Semple and K. A. Woodhouse, Biomaterials, 2007, 28, 3834–3842 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra27246g |
| This journal is © The Royal Society of Chemistry 2016 |