The cadmium binding characteristics of a lactic acid bacterium in aqueous solutions and its application for removal of cadmium from fruit and vegetable juices
Abstract
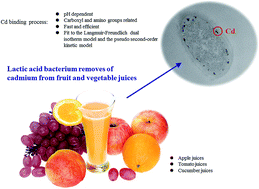
Heavy metal cadmium (Cd) is an environmental pollutant that causes adverse health effects in humans. This toxic metal has been detected in a wide range of fruit and vegetables. A strain of lactic acid bacteria, Lactobacillus plantarum CCFM8610, was screened out for its good ability to bind Cd, and this study was designed to investigate the Cd binding properties of this bacterium, and to evaluate its use for removal of Cd from fruit and vegetable juices. Electron microscopy observations and energy dispersive X-ray analysis confirmed that the majority of the Cd was bound to the surface of the bacterial cell. The Cd biosorption of L. plantarum CCFM8610 was strongly pH dependent, and carboxyl and amino groups of the bacterial surface molecules are important in the binding process. The biosorption was fast and efficient, and could be well explained by the Langmuir–Freundlich dual isotherm model (R2 = 0.99) and the pseudo second-order kinetic model (R2 = 0.99). After a 2 h incubation and a simple centrifugation, L. plantarum CCFM8610 treatment removed 67% to 82% of the Cd from nine types of fruit and vegetable juices. Long-period fermentation by L. plantarum CCFM8610 (36 h) also significantly decreased Cd concentrations in the juices (56% to 81%). Our results show that this food-grade bacterial strain could be used as a potential probiotic for Cd removal from fruit and vegetable juices.

 Please wait while we load your content...
Please wait while we load your content...