Preparation of water-soluble, PEGylated, mixed-dispersant quantum dots, with a preserved photoluminescence quantum yield†
C. Zaba‡
a,
O. Bixner‡ab,
F. Parta,
C. Zafiuc,
C.-W. Tana and
E.-K. Sinner*a
aInstitute for Synthetic Bioarchitectures, Department of Nanobiotechnology, University of Natural Resources and Life Sciences, Muthgasse 11, 1190 Vienna, Austria. E-mail: eva.sinner@boku.ac.at
bSchool of Materials Science and Engineering, Centre for Biomimetic Sensor Science, Nanyang Technological University, 50 Nanyang Drive, Singapore 637553
cICS-6 Structural Biochemistry, Forschungszentrum Jülich, Wilhelm-Johnen-Strasse, 52425 Jülich, Germany
First published on 3rd March 2016
Abstract
High quantum yields, colloidal stability and surfaces amenable to diverse chemical modifications are critical objectives for quantum dot (QD) synthesis. We present an approach of QD preparation developed for achieving these criteria. In addition, the nascent QDs are already water-soluble. We then partially exchanged the N-acetyl cysteine (NAC) ligands on our core–shell–shell CdTe/CdS/ZnS QDs for dithiol-poly(ethylene glycol) (dithiol-PEG). This resulted in mixed-dispersant QDs with photoluminescence properties rivaling those of QDs synthesized under high temperature conditions and modified by ligand exchange using dithiol-PEG. Optimization of the dithiol-PEG adsorption conditions not only retained efficient surface passivation by NAC as well as luminescence properties, but also resulted in sufficiently dense PEG-grafting to confer strong colloidal stability. Since particle properties such as solubility and protein resistance critically depend on the PEG conformation and density, we also evaluated the effects of various adsorption and grafting conditions on the polymer, using ATR-FTIR and TGA analysis. Leakage of cadmium ions from the core is prevented by the ZnS-shell, which is stabilized by the remnant NAC and physicochemically shielded by the PEG-layer. Furthermore, the residual NAC has carboxylic groups that can in principle still be chemically-modified with diverse functional groups. These characteristics, particularly their excellent solubility in water, render our QDs compatible for use in biomedical and environmental applications.
1. Introduction
Quantum dots (QDs) are photoluminescent semiconductor nanocrystals. They have attracted attention in almost all scientific fields after they were first discovered and described in the early 1980's by Alexei Ekimov.1 Since then, substantial focus has been directed to developing mono-disperse QDs with surfaces appropriate for use in biological samples.2 As such, QDs have become a viable alternative to organic fluorophores as labels in the life sciences, especially for single molecule analysis.3,4QDs have distinct advantages over organic fluorophores in optical properties.4 Their absorption spectra are notably broad, thereby facilitating excitation, while their emission spectra are narrow and tunable by controlling the size of the QDs.3–5 Moreover, QDs are substantially more photostable than organic fluorophores.4 Unlike organic fluorophores, the fluorescence properties of QDs are highly dependent on their surface defect concentration.5,6 Accordingly, their photoluminescence quantum yield (PLQY) can be enhanced by reducing surface defects via organic or inorganic surface modifications.6,7
Once optimized for performance, QDs were highly-anticipated alternatives as biological probes. However, their establishment had been severely hobbled by the potential leakage of toxic cadmium ions from the core into the surrounding biological environment. This cadmium leak induces tissue necrosis.15 This was a critical concern which was addressed by applying multiple shells of inorganic material, such as ZnS to the surface of QDs or irreversibly capping the QDs with organic material, such as high-affinity dithiols.
Furthermore, modifying the surface with high molecular weight dispersants can stabilize QDs sterically, rendering them colloidally stable in water. This is especially important for clinical applications where colloidally stable QDs with well-defined physicochemical and interfacial properties are required.8 In addition to stability, QDs also have to be targetable when used for fluorescence tracing in biological systems such as cells. To direct QDs to specific subcellular components, or to tag specific molecules, they would have to be modified with appropriate functional groups presented on their surface. This is facilitated by irreversibly exchanging typically-used shell material with reactive ligands which can then be conjugated to an appropriate biological molecule.3,4
Exchanging the shell with thiolated polyethyene glycol (PEG), or PEGylation, is a particularly appealing approach. Not only does the thiolated PEG confer anti-fouling properties to the QD surface under typical physiological conditions, they can also be used to covalently attach biological molecules.9 This method was shown to provide near-complete ligand exchange for trioctylphosphine/trioctylphosphine oxide (TOP/TOPO)-capped CdSe/ZnS QDs.10 Unfortunately, this was accompanied by a significant reduction in PLQY which has been explained to be because of quenching by the dithiol anchor used.10,11
In the case of QDs capped by monothiols, replacement of the ligand tends to be incomplete, compared to QDs capped by TOP/TOPO ligands. This is due to the relatively higher binding strength experienced by monothiols with the QDs, compared to TOP/TOPO. This incomplete ligand exchange is attendant with a less pronounced decrease in PLQY.
The ability of PEG to confer solubility and resistance to non-specific protein adsorption is dependent on its conformation, which in turn, is reported to be dependent on its grafting density.12–14 As such, a high grafting density, which is attainable with dithiol-PEG, is preferable. Furthermore dithiol-functionalized QDs have been shown to be considerably less toxic than mono-functionalized QDs.17
Our aim was to combine the advantages of each of the strategies outlined above, by synthesizing core–shell–shell QDs under aqueous conditions, and then modifying their surface with dithiol-PEG at high grafting density, without compromising on PLQY (Fig. 1). Adsorption of the thiolated PEG was achievable under a wide range of conditions. This allowed us to optimise the grafting density and PEG conformation. Using these methods, we produced cadmium leak-resistant QDs with a high grafting density of dithiol-PEG, yet with PLQY rivaling that of QDs previously produced by high temperature synthesis and capped by dithiol-PEG. Furthermore, we report careful characterisation of the nanocrystals obtained at successive steps of the synthesis scheme, thereby providing us with highly-characterized dithiol-PEGylated QDs of very defined architecture.
2. Experimental section
Methods that have been established in other reports have been relegated to the ESI.† The reader is encouraged to refer to the ESI† should more details be needed. In cases where these methods have been further validated by our work, we have included the relevant data in the main report. This is to allow us to highlight only those methods that we have developed, and for which detailed descriptions are necessary.2.1 Reagents
All reagents were purchased from Sigma-Aldrich, USA, and used as received without further purification. All reactions, especially ligand exchanges, were performed under inert gas conditions to prevent oxidation.2.2 Instruments
UV-Vis absorption spectra were collected at a scan speed of 400 nm min−1 on a Hitachi UV-2900 spectrophotometer equipped with a 1 cm quartz cell. The fluorescence spectra were recorded with a PerkinElmer LS 55 luminescence spectrometer at room temperature. The absorbance of each sample was adjusted to OD values below 0.1 to avoid homo-aggregation and self-quenching effects. The emission spectra were collected in the range between 500–750 nm at a scan speed of 400 nm min−1 and an excitation and emission slit-width of 4 nm. For a closer description of the evaluation of the PLQY please refer to the ESI.†Mid-IR powder spectra of the lyophilized samples were collected using a Bruker Tensor 37 FTIR spectrometer with a Bruker Platinum Diamond single reflection ATR equipment at a resolution of 4 cm−1 by averaging 32 scans.
Thermograms were recorded on a Mettler-Toledo TGA/DSC 1 STAR System in the temperature range 25–650 °C with a ramp of 10 K min−1 in synthetic air. 70 μl aluminum oxide crucibles were filled with 0.5–2 mg sample and the rest mass was evaluated by horizontal step setting.
TEM images were recorded on a FEI Tecnai G2 20 transmission electron microscope operating at 160 kV. Samples were prepared by dropping toluene dispersions onto 300 mesh carbon-coated copper grids and subsequently evaporating the solvent in air. Size distributions were evaluated using ImageJ.29
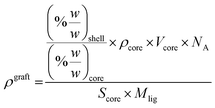
2.3 Grafting density calculations
The grafting densities (ρgraft) were calculated from TGA analysis using following equation according to Benoit et al.30:The relative mass loss of the ligand (wt% shell) and the residual mass of the quantum dot (wt% core) were found from the experimental TGA data in a range between 150–550 °C. −ΔmNAC [%w/w] was obtained from horizontal step setting in the interval 150–280 °C. −ΔmPEG [%w/w] was calculated by subtraction of −ΔmNAC [%w/w] from −Δmtot [%w/w] which corresponds to the remaining mass loss in the interval 280–550 °C. Corrected values for the lost mass fractions of the melt preparations (−ΔmNAC,corr) were obtained by accounting for the persistent NAC content above 280 °C (NAC residue) in addition to the primary mass loss [150–280 °C]. −ΔmPEG,corr [%w/w] corresponds to the difference −Δmtot − ΔmNAC,corr. Mligand for NAC was assumed to be that of NAC−Na+ (see ESI† for FTIR and EDX spectra). Corrected grafting densities (ρgraftcorr) refer to DHLA-PEG750-OMe ligands only, therefore −ΔmPEG,corr = −Δmtot,shell. It shall be noted that the calculation for both the surface area and volume were made under the assumption that particles are in a spherical shape (a detailed summary for the measured values is presented in the ESI Table S1†).
2.4 Preparation and purification of NAC-capped CdTe/CdS/ZnS and TOP/TOPO-capped CdSe/ZnS QDs
We began by synthesizing water-soluble CdTe/CdS/ZnS (core–shell–shell) QDs under aqueous conditions using a procedure adapted from Xiao et al.16 These conditions produced QDs that were capped by N-acetyl cysteine (NAC). As a reference, we synthesized organo-soluble TOP/TOPO-capped CdSe/ZnS QDs under high temperature conditions according to methods reported in literature6,18 (see ESI† for detailed descriptions of the syntheses).During synthesis, we exploited the fact, that QD emission wavelength is a function of the QD size, to determine the diameters of the CdTe QD cores. This was done spectroscopically using empirical sizing curves and calculated using data from Yu et al. and Rogach et al.21,22 The formation of CdS and ZnS shells was similarly monitored by recording progressive emission red-shifts. To validate this method of determining the QD diameter, we compared the results obtained spectroscopically with those obtained by evaluation of transmission electron micrographs.
2.5 Synthesis of DHLA-PEG750-OMe
DHLA-PEG750-OMe was synthesized as reported by Mei et al. and Uyeda et al.10,19 (Fig. 2). A detailed description of the synthesis can be found in the ESI.† NMR- and ATR-FTIR-characterization is in agreement with the original report.2.6 Ligand exchange reactions
Direct ligand exchange of the water-soluble CdTe/CdS/ZnS QDs was performed with an equimolar and a 10-fold excess of dithiol-PEG, with respect to the NAC content, at 100 °C for up to 12 hours. Briefly, 30 mg as-synthesized CdTe/CdS/ZnS QDs were dissolved in 25 mL ultra-pure water. The mixture was de-aerated and saturated with nitrogen several times at room-temperature. 52 mg DHLA-PEG750-OMe (5.52 × 10−5 mol) dissolved in 5 mL ultra-pure water were added through a septum under stirring and the mixture was heated at 100 °C for 12 hours. After cooling to room temperature the crude mixture was freeze-dried. To remove excess unbound DHLA-PEG750-OMe the crude reaction mixture was resuspended in 3–5 mL of ethanol. Next, 3–7 mL of hexane was added slowly until a monophasic turbid solution was formed. The DHLA-PEG750-OMe-capped QDs were collected by centrifugation at 3200 × g, for 15 min, at 20 °C. This was repeated 2–3 times. The clear supernatant was then discarded and the pellet was resuspended in 2 mL of ultra-pure water, filtered through a 0.2 μm PES syringe-filter (Millex, Merck-Millipore) and freeze-dried.The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 direct ligand exchange was performed as described above, with the following adjustment. 520 mg DHLA-PEG750-OMe (5.52 × 10−4 mol) dissolved in 5–10 mL N2-saturated ultra-pure water were quickly injected through a septum. Apart from that the procedure was kept the same.
10 direct ligand exchange was performed as described above, with the following adjustment. 520 mg DHLA-PEG750-OMe (5.52 × 10−4 mol) dissolved in 5–10 mL N2-saturated ultra-pure water were quickly injected through a septum. Apart from that the procedure was kept the same.
During melt ligand exchange as-synthesized QDs were reacted with a 6.5-fold excess of dithiol-PEG ligand, under inert atmosphere in various co-solvents, at 60 °C for 12 h. Preparation of DHLA-PEG750-OMe-capped CdTe/CdS/ZnS QDs was performed using a modified procedure of Uyeda et al. (2005).10 At first, 20 mg NAC-capped CdTe/CdS/ZnS QDs were resuspended in 200 mg DHLA-PEG750-OMe (2.13 × 10−4 mol) at room temperature and the flask was evacuated for 30 min. The container was then filled with inert gas and 200 μL of N2-saturated co-solvent (ultra-pure water, N-methyl-pyrrolidone (NMP), ethylene glycol (EG) or methanol (MeOH)) was added. The reaction mixture was stirred under an inert-atmosphere at 65 °C for 12 hours. After cooling to room temperature the solvent was removed under reduced pressure using a rotary evaporator. The crude reaction mixture was resuspended in 3–5 mL of ethanol. Approximately 3–7 mL of hexane was added slowly until a monophasic turbid solution was formed. The DHLA-PEG750-OMe-capped QDs were collected by centrifugation at 3200 × g, for 15 min, at 20 °C. This was repeated 2–3 times. The clear supernatant was then discarded and the pellet was resuspended in 2 mL of ultra-pure water, filtered through a 0.2 μm PES syringe-filter (Millex, Merck-Millipore) and freeze-dried.
TOP/TOPO-capped CdSe/ZnS QDs were prepared according to literature and subsequently melt-grafted as described above.6,18 A detailed description of the synthesis can be found in the ESI.† These QDs served as a reference material for characterization of the performance of the QDs prepared using our method.
3. Results and discussion
3.1 Development of ligand exchange conditions
Optimization of the DHLA-PEG750-OMe adsorption conditions involved the careful selection of solvents that would allow both effective QD dispersion as well as the collapsing of DHLA-PEG750-OMe sufficiently to minimize its radius of gyration (Table 1). This made it possible to maximize DHLA-PEG750-OMe adsorption onto the QD surface for the next step.| dinorgb [nm] | Preparation method | ρgraftcorr [nm−2] | −ΔΦ [%] | ξPEGa |
|---|---|---|---|---|
| a h – helical; a – amorphous.b Diameters obtained by experimental determination (std dev ±15% according to Yu et al.21).c Reference material for PLQY determination was fluorescein in 0.1 M NaOH (λex = 470 nm; error of measurement (n = 6): 2.6%). | ||||
| 3.2 | NAC-CdTe/CdS/ZnS | 4.3 | — | — |
| 3.2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 direct ligand exchange 10 direct ligand exchange |
0.9 | 23 | h |
| 3.2 | Aqueous melt | 1.3 | 2 | a |
| 3.2 | MeOH melt | 1.3 | 1 | a |
| 3.2 | EG melt | 2.1 | 11 | a |
| 3.2 | NMP melt | 1.3 | 72 | a |
| 4.2 | Reference melt10 | 2.2 | 78 | a |
Various methods for ligand exchange have been reported, although we have chosen to focus on two. The first is a method reported by Kim et al.,20 wherein CuInS2/ZnS QDs synthesized under organic conditions were modified with 11-mercapto-1-undecanol during the growth stage. We refer to this method as direct ligand exchange.
The other is melt ligand exchange as described by Uyeda et al.,10 where the authors had exchanged the dispersant for dithiol-PEG on organo-soluble TOP/TOPO-capped CdSe/ZnS QDs. Melt ligand exchange is a particularly effective method which retains PEG chain mobility and enhanced accessibility of the anchoring moiety for reaction.
We therefore aimed to adapt the direct ligand exchange method as well as the melt ligand exchange method for exchanging dithiol-PEG with the NAC shell of water-soluble CdTe/CdS/ZnS QDs synthesized under aqueous conditions. The resultant dithiol-PEGylated QDs were characterized and compared to evaluate the two preparation schemes.
The resultant dithiol-PEG-capped QDs were purified using ethanol/n-hexane precipitation, dried and characterized immediately afterwards. Successful ligand exchange was confirmed using attenuated total reflectance-Fourier transform infra-red (ATR-FTIR) spectroscopy and thermogravimetric analysis (TGA) (Table 1).
3.2 Size, size distribution and validation of spectroscopic method
The cores of CdTe QDs synthesized under aqueous conditions ranged from 2.1 to 2.3 nm (λabs 492 nm; λem 525 nm). The shell thicknesses were calculated to be 0.6 nm for CdS (λabs 538 nm; λem 564 nm) and 0.5 nm for ZnS (λabs 554 nm; λem 588 nm) respectively. This gave final QD diameters of 3.2 to 3.4 nm. Successful over-coating with higher band-gap semiconductor material, putatively the CdS and ZnS, was confirmed by compositional analysis of the resultant QDs using energy dispersive X-ray analysis (EDX) (see ESI Fig. S4†).Fig. 3 shows a transmission electron micrograph (TEM) of methanol melt ligand-exchanged QDs. We note that the cores, as synthesized according to previous reports, are spherical and well-dispersed. Measurement of the QD diameters using ImageJ gives a mean diameter of 3.3 ± 1.0 nm FWHM. The values obtained via spectroscopic analysis nicely match with this value. A similar agreement was verified for the TOP/TOPO-capped CdSe/ZnS QDs (see ESI S10†). We therefore highlight that spectroscopic analysis is an easier, yet sufficient method of determining QD size.
 | ||
| Fig. 3 TEM micrograph and its corresponding size histogram of DHLA-PEG750-OMe-capped CdTe/CdS/ZnS QDs prepared by MeOH-melt ligand exchange. | ||
Fig. 4 shows TEM micrographs of QDs prepared using various methods, at a lower magnification. This allowed us to assess the gross aggregating tendencies of each QD population. Pristine NAC-capped QDs exhibited aggregated clusters after drop-casting dispersion onto TEM grids (Fig. 4a). The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 direct ligand-exchanged DHLA-PEG750-OMe-capped QDs also showed agglomeration although there was a fraction of well-dispersed particles (Fig. 4b). In contrast, melt ligand-exchanged DHLA-PEG750-OMe-capped QDs yielded sterically-stabilized single particles (Fig. 4c and d). This was true after melt ligand exchange of both NAC-capped (Fig. 4c) and TOP/TOPO-capped (Fig. 4d) QDs.
10 direct ligand-exchanged DHLA-PEG750-OMe-capped QDs also showed agglomeration although there was a fraction of well-dispersed particles (Fig. 4b). In contrast, melt ligand-exchanged DHLA-PEG750-OMe-capped QDs yielded sterically-stabilized single particles (Fig. 4c and d). This was true after melt ligand exchange of both NAC-capped (Fig. 4c) and TOP/TOPO-capped (Fig. 4d) QDs.
3.3 Characterization of ligand exchanged QDs
Although our protocols were modified from reported methods, we considered it important to ensure that our QDs had been synthesized with the expected composition and structure and, subsequently, that they had been properly chemically-modified. To do this, we monitored the composition and molecular structure of the QD shell using EDX, ATR-FTIR and TGA. All NAC-capped surfaces that had undergone ligand exchange were composed of dithiol-PEG and un-exchanged NAC in co-existence. In contrast TOP/TOPO was not detectable after melt ligand exchange. EDX spectral analysis of the QDs after ligand exchange showed that the elemental composition remained unchanged and a C/O-ratio of 2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was obtained which suggests that DHLA-PEG750-OMe is the major mass fraction on the QD surface (see ESI Fig. S8†). This mixed-dispersant nature of the surface presents an outermost layer of PEG and a subjacent NAC seam (Fig. 1). We posit that the PEG layer confers steric stability on the QDs, preventing their aggregation and effectively passivates the QD surface against non-specific protein adsorption. The remnant NAC is yet useful since their carboxylic groups are in principle still addressable for chemical modification with various functional groups.
1 was obtained which suggests that DHLA-PEG750-OMe is the major mass fraction on the QD surface (see ESI Fig. S8†). This mixed-dispersant nature of the surface presents an outermost layer of PEG and a subjacent NAC seam (Fig. 1). We posit that the PEG layer confers steric stability on the QDs, preventing their aggregation and effectively passivates the QD surface against non-specific protein adsorption. The remnant NAC is yet useful since their carboxylic groups are in principle still addressable for chemical modification with various functional groups.
Monothiol exchange has been reported to attain efficiencies up to 80% during direct ligand exchange.20 However, when equimolar amounts of dithiol-PEG were used for direct ligand exchange in our case, replacement of the monothiol-NAC appeared to have failed. Furthermore, we had observed only a poor replacement of NAC despite the addition of 10-fold excess of dithiol-PEG and 12 h of incubation. In comparison, melt ligand exchange required only 6.5-fold excess of dithiol-PEG to achieve the same degree of ligand replacement. This agrees with the fact that ligand replacement is a mass-density driven process, since the dithiol-PEG adsorption kinetics would be reduced under the dilute conditions of direct aqueous ligand exchange.25 Furthermore, PEG is well-solvated in aqueous solution and exhibits a large radius of gyration. This might increase the influence of steric hindrance and also result in further shielding of the hydrophobic dithiol moiety against exposure. In addition, the poor direct ligand exchange rate may be due to insufficiently high temperatures, such as were used by Kim et al.20
QDs that had undergone aqueous-melt (Fig. 5f) ligand exchange displayed increased helical arrangements of the PEG C–C bonds. When melt ligand exchange was performed in N-methyl-2-pyrolidone (NMP), the corresponding IR spectrum (Fig. 5i) exhibited the sharpest bands, again suggesting a more ordered state. This is in contrast to noticeably amorphous signatures when melt ligand exchange was performed in methanol (MeOH) (Fig. 5g). The data suggest that aqueous solvents favour greater helical conformation of the PEG backbone due to water's inherent structure-promoting properties.
Furthermore, when aqueous and more protic solvents are used, the efficiency of melt ligand exchange is noticeably reduced. We posit that rearrangement of the PEG conformation during ligand exchange under aqueous conditions reduces exposure of the hydrophobic dithiol moiety to water as well as to the charged QD surface. These interactions would have been energetically unfavourable for the presentation of the dithiol anchor, resulting in the reduced efficiency of ligand exchange observed. In contrast, PEG chain mobility would be preserved when melt ligand exchange is performed.
The observations based on our various preparation methods suggest that dithiol-PEG structure is essentially determined in solution before adsorption to the QD surface and not due to lateral chain interactions of the grafted PEG. The reverse causal relationship might be argued, since inter-chain van-der-Waals interactions have been shown to promote PEG restructuring for end-grafted polymers.13 However, this counter-argument is not supported for our case by the observation that PEG conformation was independent of grafting density up to 2.2/nm2. Tight packing, and hence interaction, of the PEG chains on our QDs is likely to have been diminished by the interspersed, remnant NAC. Besides which, the low molecular weight of the DHLA-PEG750-OMe would allow for increased grafting densities before steric hindrance enforces a change in PEG conformation. Finally, the curvature of our QDs causes a reduction in effective PEG chain density in the outer volume, unlike the case of tightly-packed self-assembled monolayers (SAMs) on flat surfaces. In short, we find no evidence that inter-chain van der Waals interactions would have a significant influence on PEG conformation in our system.
Nonetheless, both helical and amorphous PEG conformations have been reported to be resistant to non-specific protein adsorption in SAMs on gold.13 This was observed to be true even under dilute conditions. However, the all-trans conformation was accessible to biofouling.13 As such, our QDs produced using both direct ligand as well as melt ligand exchange, would likely be able to resist non-specific protein adsorption. We note, however, that curvature of the QD surface would tend to reduce the effective PEG chain density, and increase interaction volume, toward the outer surface. Since this may compromise the anti-fouling effect,26 size may be an important parameter when designing or using QDs for purposes such as ours. This issue might also be addressed by the careful selection of the PEG-chain length.
A one-step TGA profile is characteristic for the loss of a single surface-bound species without fragmentation. This analysis would inform us of the purity and uniformity of the QD surfaces. ATR-FTIR spectra of QDs after combustion showed that only traces of organics were left after the TGA heat treatment and that all bands attributed to the ligands were no longer detectable. Minimal residues of decomposed ligand were indicated by bands at 3552 cm−1 (OH), 1641 cm−1 (OH), 1110 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S; S
S; S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 637 cm−1 (C–metal; C–S) and 616 cm−1 (C–metal; C–S). These are attributed to surface complexes (see ESI Fig. S9†).
O), 637 cm−1 (C–metal; C–S) and 616 cm−1 (C–metal; C–S). These are attributed to surface complexes (see ESI Fig. S9†).
To begin with, pristine NAC-capped QDs were characterized. The determined grafting density of ∼4/nm2 for the pristine QDs may suggest that they have formed a multi-layered shell (Fig. 6 red). However, multilayer formation via H-bonding of the amide groups is unlikely because of the highly negatively-charged state of NAC (νas,sCOO−) under the conditions, that mimic the physiological, which we have used. Furthermore, the IR spectra did not show signs of NAC multilayers, such as the presence of H-bonded carboxylic acid groups at about 1710 cm−1. As such, the data corresponds to a monolayer of the NAC ligand.
Likewise, characterization of the ligand-exchanged reference QDs (dithiol-PEG-capped CdSe/ZnS) showed a single decomposition profile, but at considerably higher onset temperature (Fig. 6 black). Comparison with the IR spectra confirmed that the particle surface is mainly composed of dithiol ligand with hardly any trace of the native TOP/TOPO capping agent (Fig. 5j).
In contrast, NAC-capped QDs that were subsequently ligand-exchanged exhibited multistep TGA profiles (Fig. 6 magenta; green; orange; blue and yellow). The corresponding IR spectra showed the presence of residual NAC (Fig. 5e–i). The first mass loss from 200 to 275 °C is attributed to NAC while the major step from 275 to 350 °C is mainly due to loss of surface-bound dithiol-PEG. Taken together, the data describe a surface comprising mixed dispersant species (Fig. 6). They also imply a lower binding energy for NAC to the QD surface, since NAC decomposition (bp ≈ 110 °C) starts considerably earlier than that of the dithiol-PEG (bp > 200 °C). When comparing QDs capped using melt ligand exchange, the relative step sizes were shown to differ (Fig. 6).
This was used to quantify the amount of NAC remaining on the different QDs that had undergone melt ligand exchange. Samples grafted in NMP (Fig. 6 magenta) and ethylene glycol (EG) (Fig. 6 green) showed the lowest thermal mass loss of NAC, with only approximately 15% (w/w) of the material persisting on the QD surface. In contrast, QDs that had undergone melt ligand exchange in methanol (Fig. 6 orange) and aqueous (Fig. 6 blue) conditions showed that 25–38% (w/w) of NAC had remained. Although there was little loss of NAC when NMP was used, this did not result in, nor was it due to, increased dithiol-PEG exchange, since the grafting efficiency was comparable to those when methanol and aqueous conditions were used. In contrast, ligand-exchanged reference QDs (dithiol-PEG-capped CdSe/ZnS) and those prepared via EG melt ligand exchange tended to show nearly doubled grafting densities. The grafting densities remained high after arithmetic correction, which translates to improved colloidal stability as observed in TEM (Fig. 4).27
Duplicate measurements of dithiol-PEG mass yielded values that varied by ±3% w/w. This uncertainty agrees with that reported by Benoit et al. and Bixner et al.26,30 This, in turn, yielded a standard deviation of ±12% w/w for the calculated grafting densities. To avoid batch-to-batch variation, all measurements were made using QDs that had been prepared from the same batch of core QDs. Similarly, all synthesized reagents, such as the DHLA-PEG750-OMe were used from a single batch.
The accuracy of the values obtained is dependent on two major factors: (i) experimental errors arising from the inherent error of the instruments used, as well as from varying sample purity; and (ii) theoretical errors arising from assumptions made regarding sample characteristics.
By ensuring sample purity using ethanol/n-hexane precipitation in our method instead of dialysis,17 using oxygenic instead of inert atmospheres for combustion,31 and keeping our sample masses below that at which mass effects begin to alter the onset temperature and thermogravimetric profiles, we were able to maintain the experimental error at ±3% w/w. The efficacy of ligand combustion was confirmed by ATR-FTIR analysis of the samples following TGA. In addition, the heating rate employed was carefully chosen to ensure that the thermal transitions were clearly discernible in order to ensure accurate evaluation.
However, this error was compounded by our assumption that the NAC species on the QDs was in the form of NAC−Na+ as opposed to protonated NAC. The difference in mass between the two species gave rise to an uncertainty of ±12% w/w when calculating dithiol-PEG grafting density. However, our assumption of NAC−Na+ being the species present was corroborated by analyses of the samples using ATR-FTIR and EDX.
We wish to point out, however, that the calculated surface area of the QDs depends on the shape they are assumed to have. The adoption of varying QD shapes, all with the same volume, could give rise to uncertainties of up to ±20% w/w when used to calculate grafting density. Therefore, we propose that the ramifications of this assumption far outweigh the errors arising from other factors. To validate our assumption of sphericity, we evaluated the shape of the QDs using the TEM micrographs. This assumption was further corroborated by the agreement with the measurements of QD size using spectroscopic analysis.
Fig. 7 depicts representative absorption and emission spectra of various DHLA-PEG750-OMe-capped QDs. The differences in dielectric relaxations observed between QDs synthesized under aqueous and high temperature conditions are attributed to disparate core and shell materials. The emission profiles of QDs synthesized under aqueous conditions were generally symmetrical but, with full-width at half maximum (FWHM) values of 57 nm, were broader than those of QDs synthesized under high temperature conditions whose FWHM were 29 nm. As explained, this broadening of the emission profiles is indicative of a broadening in the QD size distribution.
A broadening of the FWHM, and hence size distribution, was most pronounced after introducing the outermost protective ZnS layer. This is presumably due to remote anisotropic growth of the ZnS crystal lattice under the experimental conditions used (see ESI Fig. S5†).16 Nonetheless, the narrow FWHM obtained corresponds to a narrow QD size distribution. This appears to be a benefit when labelling biological samples.16
Both, QDs with and without dithiol-PEGylation yielded PLQYs up to 14% with respect to fluorescein. This is in accordance with reported values in the literature for similar QD preparations.23,24 In addition, we studied the effects of ligand exchange on the resultant PLQY. Whereas ligand-exchanged reference QDs (dithiol-PEG-capped CdSe/ZnS) showed a decrease in PLQY of more than 70% (see Table 1), NAC-capped QDs did not show any significant change in PLQY upon ligand exchange, except when NMP was used.
When considering the ATR-FTIR data for NAC-capped QDs, we note that aprotic solvents such as NMP (see Fig. 5i) were able to strip NAC most efficiently during melt ligand exchange, but protic solvents, such as methanol (see Fig. 5g), did not do so to the same extent. This is despite the fact that the dithiol-PEG grafting density obtained when NMP is used for melt ligand exchange is similar to that obtained using other solvents for melt ligand exchange.
We further note that non-protic solvents such as NMP showed FTIR spectra suggesting increased order in the PEG conformation. Finally, the use of non-protic solvents such as NMP for ligand exchange resulted in considerably reduced PLQY. This reduction could have resulted from the almost quantitative loss of surface passivation with the loss of NAC in NMP, as opposed to only partial loss of NAC in protic solvents. We posit that the remnant NAC retains the ability to protect the ZnS shell from loss of integrity, such as from oxidation, so preserving surface passivation of the QDs and, hence, PLQY.
It seems, therefore that the reduction in PLQY (−ΔΦ) is not due solely to the dithiol-PEG grafting density. We propose that loss of surface passivation has a dominant influence. In the case of melt ligand exchange in NMP, we further posit that changes in the protonation state of the dithiol-PEG, which corresponds to shifts of the thiol–thiolate equilibrium, leading to energetic reordering of ligand trap states, had contributed to the drastic PLQY reduction observed.28 Since we observed that the PLQY was permanently reduced, in our experiments, it might be that the shift in thiol–thiolate equilibrium, established prior to grafting, was maintained after grafting of the dithiol-PEG. To wit, protonation of the dithiol-PEG may be an essential parameter during ligand exchange, impacting surface composition and grafting efficiency as well as PLQY.
To summarise, we found that the PEG conformation (ξPEG) on highly curved, mixed-dispersant particles is not influenced by lateral chain interactions for low molecular weight PEG, but significantly determined by the adsorption conditions used, mainly the choice of solvent (see Table 1). Packing of the PEG chains is likely to be attenuated by additional free volume due to QD surface curvature, and disrupted by inter-dispersed remnant NAC.
We did not observe any relationship between dithiol-PEG grafting density and decrease in PLQY of mixed-dispersant QDs, when grafting densities of 1–2/nm2 were studied. Instead, it seems that surface oxidation, due to excessive loss of NAC-passivation, might have led to this loss. In the case of melt ligand exchange in NMP, we further propose that changes in the protonation state of the dithiol-PEG, leading to energetic reordering of ligand trap states, had resulted in the drastic PLQY reduction observed. We emphasize that complete exchange of NAC may not necessarily be desirable as the remnant NAC can still be chemically-modified to add chemical versatility to the QD surface. The presence of residual NAC per se does not present a health risk as NAC is an approved medical additive. More importantly, this would leave the protective PEG-layer, which others have chemically-modified for further function, undisturbed.
4. Conclusions
In conclusion, we have modified two reported methods for the purpose of exchanging a monothiol capping ligand, NAC, for a dithiolated one, DHLA-PEG750-OMe. In doing so we are able to provide QDs with high PLQY, anti-fouling ability, colloidal stability and a surface which might be further chemically-modified under appropriate conditions. In particular, the possibility of optimising the PEG chain density, especially in the outer volume, facilitates this surface modification. These are characteristics of critical advantage when QDs are applied to biological systems and when long-term imaging is required.In the process of studying two methods of ligand exchange, we have discovered that the choice of solvent for the ligand exchange reaction has a greater influence on PEG chain conformation than inter-chain van der Waal's interactions. Furthermore, we conclude that this conformational change occurs before the dithiol-PEG grafts onto the QD surface. Also, it appears that while the dithiol-PEG grafting density affects the PLQY, it is not the major factor leading to PLQY reduction. We interpret this data to indicate the importance of co-solvent choice on the loss of NAC from the QD surface as well as on the conformation of the dithiol-PEG. We further propose that the loss of NAC, in turn, has an impact on QD surface passivation and thus explains the loss of photoluminescence quantum yield. Loss of QD surface passivation might also lead to leakage of toxic cadmium ions from the core. Finally, we propose that QDs with a mixed-dispersant surface such as ours offers the advantage of choice wherein one may modify the more appropriate dispersant while leaving the other undisturbed to maintain its protective function.
Conflict of interest
The authors declare no competing financial interest.Acknowledgements
We would like to thank Prof. Dieter Baurecht, University of Vienna, for allowing us to use his ATR-FTIR facilities and Andrea Lassenberger, University of Natural Resources and Life Sciences, Vienna (Institute of Biologically Inspired Materials) for help with TEM microscopy.Notes and references
- A. I. Ekimov and A. A. Onushchenko, JETP Lett., 1981, 34, 345–349 Search PubMed.
- M. Bruchez, M. Moronne, P. Gin, S. Weiss and A. P. Alivisatos, Science, 1998, 281, 2013–2016 CrossRef CAS PubMed.
- E. R. Goldman, I. L. Medintz, A. Hayhurst, G. P. Anderson, J. M. Mauro, B. L. Iverson, G. Georgiou and H. Mattoussi, Anal. Chim. Acta, 2005, 534, 63–67 CrossRef CAS.
- U. Resch-Genger, M. Grabolle, S. Cavaliere-Jaricot, R. Nitschke and T. Nann, Nat. Methods, 2008, 5, 763–775 CrossRef CAS PubMed.
- A. P. Alivisatos, Science, 1996, 271, 933–937 CAS.
- B. O. Dabbousi, J. RodriguezViejo, F. V. Mikulec, J. R. Heine, H. Mattoussi, R. Ober, K. F. Jensen and M. G. Bawendi, J. Phys. Chem. B, 1997, 101, 9463–9475 CrossRef CAS.
- P. Reiss, M. Protiere and L. Li, Small, 2009, 5, 154–168 CrossRef CAS PubMed.
- I. L. Medintz, H. Mattoussi and A. R. Clapp, Int. J. Nanomed., 2008, 3, 151–167 CAS.
- D. S. Achilleos and M. Vamvakaki, Materials, 2010, 3, 1981–2026 CrossRef CAS.
- H. T. Uyeda, I. L. Medintz, J. K. Jaiswal, S. M. Simon and H. Mattoussi, J. Am. Chem. Soc., 2005, 127, 3870–3878 CrossRef CAS PubMed.
- B. Kundu, S. Chakrabarti and A. J. Pal, Chem. Mater., 2014, 26, 5506–5513 CrossRef CAS.
- R. Begum and H. Matsuura, J. Chem. Soc., Faraday Trans., 1997, 93, 3839–3848 RSC.
- P. Harder, M. Grunze, R. Dahint, G. M. Whitesides and P. E. Laibinis, J. Phys. Chem. B, 1998, 102, 426–436 CrossRef CAS.
- J. S. Kang and T. A. Taton, Langmuir, 2012, 28, 16751–16760 CrossRef CAS PubMed.
- C. Kirchner, T. Liedl, S. Kudera, T. Pellegrino, A. M. Javier, H. E. Gaub, S. Stolzle, N. Fertig and W. J. Parak, Nano Lett., 2005, 5, 331–338 CrossRef CAS PubMed.
- Q. Xiao, S. Huang, W. Su, W. H. Chan and Y. Liu, Nanotechnology, 2012, 23, 495717 CrossRef PubMed.
- Y. C. Yeh, K. Saha, B. Yan, O. R. Miranda, X. Yu and V. M. Rotello, Nanoscale, 2013, 5, 12140–12143 RSC.
- P. Reiss, J. Bleuse and A. Pron, Nano Lett., 2002, 2, 781–784 CrossRef CAS.
- B. C. Mei, K. Susumu, I. L. Medintz and H. Mattoussi, Nat. Protoc., 2009, 4, 412–423 CrossRef CAS PubMed.
- H. Kim, M. Suh, B. H. Kwon, D. S. Jang, S. W. Kim and D. Y. Jeon, J. Colloid Interface Sci., 2011, 363, 703–706 CrossRef CAS PubMed.
- W. W. Yu, L. H. Qu, W. Z. Guo and X. G. Peng, Chem. Mater., 2003, 15, 2854–2860 CrossRef CAS.
- A. L. Rogach, T. Franzl, T. A. Klar, J. Feldmann, N. Gaponik, V. Lesnyak, A. Shavel, A. Eychmuller, Y. P. Rakovich and J. F. Donegan, J. Phys. Chem. C, 2007, 111, 14628–14637 CAS.
- M. Grabolle, M. Spieles, V. Lesnyak, N. Gaponik, A. Eychmuller and U. Resch-Genger, Anal. Chem., 2009, 81, 6285–6294 CrossRef CAS.
- H. P. Zhu, M. Z. Hu, L. Shao, K. Yu, R. Dabestani, M. B. Zaman and S. J. Liao, J. Nanomater., 2014, 324972 Search PubMed.
- K. Susumu, B. C. Mei and H. Mattoussi, Nat. Protoc., 2009, 4, 424–436 CrossRef CAS PubMed.
- O. Bixner, A. Lassenberger, D. Baurecht and E. Reimhult, Langmuir, 2015, 31, 9198–9204 CrossRef CAS PubMed.
- T. Gillich, C. Acikgoz, L. Isa, A. D. Schluter, N. D. Spencer and M. Textor, ACS Nano, 2013, 7, 316–329 CrossRef CAS PubMed.
- S. Jeong, M. Achermann, J. Nanda, S. Lvanov, V. I. Klimov and J. A. Hollingsworth, J. Am. Chem. Soc., 2005, 127, 10126–10127 CrossRef CAS PubMed.
- W. S. Rasband, ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, 1997–2015, http://www.imagej.nih.gov/ij/ Search PubMed.
- D. Benoit, H. Zhu, M. Lilierose, R. Verm, N. Ali, A. Morrison, J. Fortner, C. Avendano and V. Colvin, Anal. Chem., 2012, 84, 9238–9245 CAS.
- M. Rudolph, J. Erler and U. A. Peuker, Colloids Surf., A, 2012, 397, 16–23 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, sample preparation, EDX spectra, TEM micrographs, TGA profiles, ATR-FTIR spectra, UV/Vis and fluorescence spectra. See DOI: 10.1039/c5ra26936a |
| ‡ C. Zaba and O. Bixner contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |