Multimodal theranostic assemblies: double encapsulation of protoporphyrine-IX/Gd3+ in niosomes†
F. Baris Barlasa,
Bilal Demira,
Emine Gulerab,
A. Murat Senisikc,
H. Armagan Aricand,
Perihan Unake and
Suna Timur*ab
aEge University, Faculty of Science, Biochemistry Department, 35100-Bornova, Izmir, Turkey. E-mail: sunatimur@yahoo.com
bEge University, Institute of Drug Abuse Toxicology & Pharmaceutical Sciences, 35100-Bornova, Izmir, Turkey
cSifa University Hospital, 35100 Bornova, Izmir, Turkey
dSifa University, Vocational School of Health Services, Radiotheraphy Department, Buca, Izmir, Turkey
eInstitute of Nuclear Sciences, Ege University, 35100 Bornova, Izmir, Turkey
First published on 11th March 2016
Abstract
Theranostic therapy is one of the most promising methods in cancer research, which simultaneously allows the treating and real-time monitoring of cancer. In the present study, a new method was developed to achieve advanced theranostic therapy by double encapsulation of gadolinium nanoparticles (GdNP) and protoporphyrin IX (PpIX) into niosomes. Hereby, niosomes are used for encapsulation of GdNP and PpIX, which will be called ‘Gd-PpIX-NI’. Niosomes are chosen as the encapsulation material owing to their high biocompatibility, physical and chemical stability, and fair price. On the other hand, GdNP and PpIX are good sensitizers for radiotherapy (RT) and, particularly, porphyrin structures are one of the most studied agents for photodynamic therapy (PDT). In this study, a multimodal treatment was performed with the combination of PDT and RT by using human alveolar type-II (ATII)-like cells (A549) and human cervical cancer cell line (HeLa). Moreover, ‘Gd-PpIX-NI’ serves as a dual cell imaging probe that provides both fluorescence and magnetic resonance imaging. Characterization of the sizes and zeta potential of the niosomal vesicles was carried out by dynamic light scattering and atomic force microscopy. To determine the cell viability after treatment with Gd-PpIX-NI, followed by PDT and RT application, the MTT method was used. The results showed that Gd-PpIX-NI assembling was homogeneous and consistent in terms of particle size, which is less than 100 nm. This material has potential as a good candidate for both PDT and RT, as well as diagnosis.
1. Introduction
Theranostically engineered nanoparticles (NPs) are a newly emerging topic of nanotechnology and have gained great attention. The term theranostic, which was coined by Funkhouser in 2002,1 is defined as multifunctional NPs that carry both therapeutic and diagnostic properties in a single NP structure. Typically, theranostic nano-platforms need to have biocompatibility as well as imaging and drug transport capabilities.2–4 Theranostics can be adapted to targeting strategies or designed for passive processes.5 Passive targeting processes are reliant on the size of the nanoparticles being smaller than the fenestrations of endothelial cells, enabling penetration of the interstitial fluid to allow accumulation in the tumor. Thereby, enhanced permeability and retention (EPR) effect can be increased in the case of the combination of leaky vasculature and poor lymphatic drainage.6 NPs as contrast agents have found widespread use in medical in vivo imaging, as can be seen from many medicinal and scientific reports.7–10 Among other imaging modalities, such as nuclear imaging (positron emission tomography/computed tomography/single-photon emission computed tomography), magnetic resonance imaging (MRI) has been a powerful medical imaging modality to demonstrate the anatomical structures of the body, particularly useful for the detection and characterization of diseased soft tissues, such as solid tumours.11 Spatial resolution, which is obtained with magnetic labels such as MRI agents, permits the contrasting of organ structures, but diagnostic imaging under subcellular conditions has its limits and has not been successfully achieved in medical operations. To overcome this problem, combined modalities were developed for the diagnosis of cancer. Within these approaches, the effect of imaging modalities could be enhanced using combinations such as fluorescence + MRI,12–15 fluorescence + radioactivity,16 MRI + radioactivity,17 MRI + CT,18,19 and MRI + PET.20Photodynamic therapy (PDT) is a non-invasive treatment modality in cancer therapy and has growing popularity in this field, especially in dermatology. Until the beginning of the 20th century, similar treatment techniques to PDT were used with sunlight and/or with a combination of salves for psoriasis, rickets vitiligo and skin cancer.21–23 There are three main requisites for PDT: light, oxygen and a photosensitizer (PS). In the presence of those elements, cells are exposed to reactive oxygen species (ROS), such as singlet oxygens, which are generated by the excitation of PSs to T1 state from a ground state with light.24 Recently, PSs used in clinical purposes are mainly originated from porphyrins, chlorophylls, and dyes.25 Porphyrins have a long evolutionary history25,26 and are extensively adopted for clinical applications; for instance, ‘Photofrin’ has been approved by FDA for the treatment of early and late endobronchial lesions.27 In addition, protoporphyrin IX (PpIX) has been utilized for fluorescence mediated surgery and PDT.28,29 It also has good potential for radiosensitivity under ionizing irradiation by increasing energy uptake in cancer cells in comparison with healthy cells.30 Over the last decade, tremendous attention has been paid to nanocarriers in order to develop theranostic structures.31–34 A huge amount of nanocarriers, such as liposomes,35 dendrimers,36 amphiphilic polymers,34 and metallic nanoparticles,37 has been reported in the literature. Among those carriers, lipid based carriers can be described as one of the oldest technologies that created an important change in cancer treatment. However, owing to their chemically unstable phospholipids, oxidative degeneration and decomposition of targeted liposomes by lysosomes with subsisting enzymes consisting of hydrolases and peptidases during the cell internalization process, their use has been restricted. In contrast to those mentioned carriers, niosomes have moved forward because of their enhanced stability and good solubility, which contribute to enhance the biocompatibility of encapsulated materials in organisms. Likewise liposomes, which are spherical but made up of non-ionic surfactants that construct a bilayer membrane consisting of a stabilizer molecule. The infrastructure of niosomes is mainly created by self-assembling of non-ionic surfactants and cholesterol. The vesicular shape and encapsulation properties are similar to liposomes, with the only difference that these materials are prepared by using non-ionic surfactants instead of phospholipids.38,39 Vesicular structures as functional carriers for PDT and/or radiotherapy (RT) have rapidly become popular in cancer research. Nevertheless, niosomes have not been employed enough in the construction of multifunctional carriers, unlike liposomes. There are a few reported studies that use niosomes as the key element of PDT. In those reports, methylene blue as a PS was used in PDT for the preparation of a niosomal gel, which was utilized in the treatment of hidradenitis suppurativa skin disorder.40 To our best knowledge, there are no studies that include multimodal combined therapy for cancer research over niosomal vesicles and double encapsulation of an imaging agent and a PS into them. The present study includes the synthesis, characterization and applicability of multimodal combined therapy by using encapsulated gadolinium nanoparticles (GdNP) as the MRI agent and PpIX as the PS element in niosomes. All through the study, HeLa and A549 were used as model cancer cells to test the proposed nanocarriers by means of passive targeting. After the detailed characterization steps with different techniques, niosome-based nanocarriers were tested as multimodal imaging (fluorescence and MR imaging) and combined therapy (RT and PDT) systems.
2. Experimental
2.1 Materials and apparatus
Magnevist (gadolinium nanoparticles (GdNPs), Bayer, Germany), protoporphyrin IX, cholesterol, Span 61, phosphate buffered saline (PBS, pH 7.4), MES buffer (pH 6.0), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), and sodium dodecyl sulphate (SDS) were purchased from Sigma (Germany). Chloroform (HPLC grade), methanol (HPLC grade) and 2-propanol and diamino-2-phenylindol (DAPI) were purchased from Sigma-Aldrich. Water was purified in a Milli-Q plus System (Millipore). Radiotherapy applications were carried out via a 6 MV linear accelerator system (LINAC, Siemens Primus, Germany) and an MR (Siemens, Germany) system was used for MRI studies at SIFA University Hospital (Izmir/TURKEY). Atomic force microscopy (AFM) (Nanosurf AG, Flex AFM Switzerland) was used for the examination of surface morphologies. Size and zeta potential measurements were carried out using dynamic light scattering (DLS, Malvern Zetasizer, Nano ZS model, Malvern Instruments Ltd., U.K.) method.2.2 Synthesis
The niosomes were prepared via a thin film method with sonication. Briefly, 20 mM of span 61 was mixed with cholesterol (at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) and dissolved in a chloroform and methanol mixture (2
1 molar ratio) and dissolved in a chloroform and methanol mixture (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mL) in a round bottom flask. The solvent was then evaporated by a rotary evaporator (Buchi R-3, Switzerland) to get a thin film and it was dried overnight in a vacuum desiccator. 50 mg mL−1 Magnevist and 240 μg mL−1 (100 μM) PpIX dissolved in 10 mL of phosphate buffer saline (PBS) were then added to the film and the dispersion was sonicated for 1 min at 130 W and 20 kHz (Sonics & Materials Inc. USA). Then, the samples were dialyzed against PBS to eliminate to un-encapsulated PpIX and GdNP.
1 mL) in a round bottom flask. The solvent was then evaporated by a rotary evaporator (Buchi R-3, Switzerland) to get a thin film and it was dried overnight in a vacuum desiccator. 50 mg mL−1 Magnevist and 240 μg mL−1 (100 μM) PpIX dissolved in 10 mL of phosphate buffer saline (PBS) were then added to the film and the dispersion was sonicated for 1 min at 130 W and 20 kHz (Sonics & Materials Inc. USA). Then, the samples were dialyzed against PBS to eliminate to un-encapsulated PpIX and GdNP.
2.3 Characterization
After preparing niosomes, un-entrapped PpIX was separated and the PpIX remained entrapped in niosomes was detected by complete disruption of the vesicles using chloroform and analysing of the resultant solution spectrophotometrically at 410 nm by using a thermo plate reader. It can be represented as:| Entrapment efficiency (EE%) = (amount entrapped/total amount) × 100 |
For determination of the GdNP content; positive control (commercial Magnevist in distilled water), negative control (distilled water) and the samples (niosomes containing PpIX and Magnevist) were added to Eppendorf tubes and their images were taken with magnetic resonance (MR, T1 weighted). Then, the difference in their contrasts on MR images was evaluated via Image J software. The sizes were analysed using a dynamic light scattering system, (DLS). All samples were measured 3 or 4 times. In addition, niosomes were imaged by AFM with a 10 μm high resolution scan head using non-contact mode.
2.4 Cell culture
A549, the human alveolar type-II (ATII)-like cell lines and human cervical cancer cell line (HeLa) were cultured in Dulbecco's Modified Eagle Medium (DMEM) with L-Glutamine (Lonza, Switzerland) supplemented with 10% (v/v) FCS (Lonza, Switzerland), 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U mL−1 penicillin and 10
000 U mL−1 penicillin and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U mL−1 streptomycin (Lonza, Switzerland). Cells were cultivated in 75 cm2 tissue culture flasks and maintained under standard cell culture conditions (5.0% CO2, 95% humidity and 37 °C in incubators). Cells were passaged two times weekly.
000 U mL−1 streptomycin (Lonza, Switzerland). Cells were cultivated in 75 cm2 tissue culture flasks and maintained under standard cell culture conditions (5.0% CO2, 95% humidity and 37 °C in incubators). Cells were passaged two times weekly.
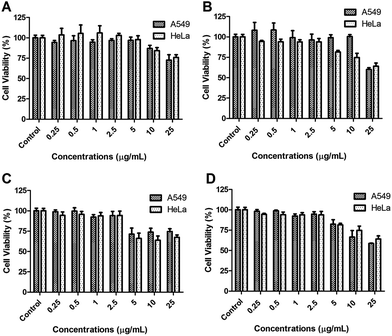
The dose-dependent cytotoxicity of the samples was investigated via 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay.41 Cells were transferred from the flasks to 96-well-tissue plates. Cultivation of the cells in the wells was continued until reaching confluence. The medium was removed and the cells were washed with PBS. After the cells were treated with the samples at varying concentrations for 2 h, the samples were removed by washing with PBS. After adding 10% MTT solution to the wells (110 μL per well) and incubation of 4 h with MTT reagent, to dissolve formazan, which was produced inside the cells as a result of MTT treatment, 100 μL of SDS (1.0 g of SDS in 10 mL of 0.01 M HCl) was added to each well. After 24 h incubation, the optical densities of each well were analysed with a spectrophotometric plate reader (Bio-Tek Instruments, Inc., Winooski, VT, USA) at 570 nm and 630 nm.
For the radiotherapy (RT) application, irradiation of the cells was performed as follows; 4 × 103 cells were seeded to 96 well plates and incubated overnight. Then, medium was removed and the samples were added after 2 h irradiation with various doses (2.5, 5.0 and 10 Gray (Gy)) of radiation (delivered by LINAC), as described previously.42 After irradiation, cells were incubated for 72 h in ideal cell culture conditions (37 °C, 5.0% CO2). The standard MTT procedure was then carried out to determine the cell viability.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells were seeded in 24 well plates and incubated for 48 under ideal cell culture conditions. Then, the cells were pre-treated with the samples: plain niosomes, Gd-NI, PpIX-NI and Gd-PpIX-NI. After 2 h, 5 min exposure between 400 and 700 nm wavelength ranges with a homemade LED lamp (5.1 J cm−2) was carried out. Cell viability was measured after 24 h incubation at the ideal conditions as mentioned above.
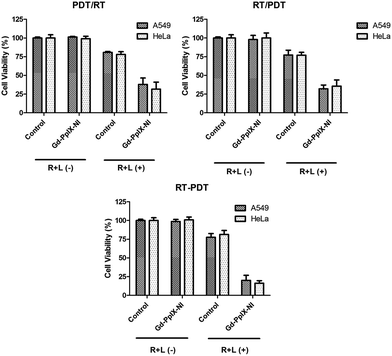
000 cells were seeded in 24 well plates and incubated for 48 under ideal cell culture conditions. Then, the cells were pre-treated with the samples: plain niosomes, Gd-NI, PpIX-NI and Gd-PpIX-NI. After 2 h, 5 min exposure between 400 and 700 nm wavelength ranges with a homemade LED lamp (5.1 J cm−2) was carried out. Cell viability was measured after 24 h incubation at the ideal conditions as mentioned above.In order to investigate the simultaneous effects of PDT and RT (2.5 Gy) on the cell viability, various alternatives were tested. First, PDT was given 24 h before RT (PDT/RT). Secondly, a subsequent application was performed and PDT was applied immediately after RT (PDT + RT). The third was PDT application 72 h after RT (RT/PDT). Cell viabilities were determined by MTT method and the therapeutic efficiencies were evaluated and compared with each other.
8 × 103 A549 and HeLa cells were seeded in a 6 well-plate and incubated 2 days before applying the samples. Subsequently, Gd-PpIX-NI was applied to cells for 2 hours then the medium was removed and cells were washed 2 times with PBS. After that, medium without any samples was added onto the cells and MR imaging was carried out at the T1-weighted state. Then, the amount of contrast was measured with Image J software by generating an MRI histogram. Negative control was formed with cells with only medium under the same conditions.
3. Results and discussion
3.1 Physicochemical characterization
Niosomes have gained a lot of attention as a novel promising material by researchers who are working on drug/nanoparticle carrier systems. Niosomes that have liposome-like structures were used for PpIX and GdNP encapsulation to generate an efficient nano-carrier system with diminished side effects of encapsulant materials in living cells. Beside the encapsulant materials, engineered niosome particles have to be characterized psysicochemically. In this scope, particle size and zeta potential analysis of the engineered particles were carried out. The hydrodynamic particle sizes of the synthesized vesicles were 37.51 ± 12 nm for plain niosomes, 40.18 ± 14 nm for Gd-NI, 37.1 ± 11 nm for PpIX-NI and 50 ± 10 nm for Gd-PpIX-NI (Table 1). Among several vesicle preparation methods like reverse-phase evaporation and ether injection, it can be seen that a further sonication and/or extrusion process is crucial for obtaining nano-sized samples in such vesicular systems. The particle size of the niosome vesicles is less than 100 nm. Moreover, zeta potential, which is one of the most important physicochemical properties of a particle, is a characteristic of the surface charge of the suspension or dispersion, knowledge of which for the niosome vesicles can help to predict and control the fate of these structures in cell media.44 The zeta potentials of niosome vesicles are −39 ± 8 mV for plain niosomes, −18 ± 5 mV for Gd-NI, −31 ± 5 mV for PpIX-NI and −25 ± 6 mV for Gd-PpIX-NI (Table 1). According to the surface potential results, PpIX encapsulation slightly changed the surface charge of vesicles. Moreover, GdNP encapsulation decreased the surface potential for both Gd-NI and GD-PpIX-NI samples. This effect might have occurred from the positive charge of GdNPs.45| Size (nm) ± S.D. | Zeta potential (mV) ± S.D. | |
|---|---|---|
| Plain niosome | 37.51 ± 12 | −39 ± 8 |
| Gd-NI | 40.18 ± 14 | −18 ± 5 |
| PpIX-NI | 37.1 ± 11 | −31 ± 5 |
| Gd-PpIX-NI | 50 ± 10 | −25 ± 6 |
Following the determination of the particle size and surface charge, the stability of the theranostically engineered vesicles (Gd-PpIX-NI) was investigated at two different temperatures via DLS (+4 °C and +25 °C) for 20 days with a 5 day interval. Fig. S1† illustrates that there was a difference between the particle sizes in the two storage conditions, especially for 25 °C. In the room temperature conditions, the size of Gd-PpIX-NI vesicles was increased to 78 nm from 50 nm after 20 days. Other researchers have also reported a similar effect of high temperature on the vesicles.46,47 On the other hand, the particle size did not change after 20 days at +4 °C. Furthermore, it is also known that niosomes are robust at +4 °C for 3 months.45,48
AFM is a typical characterization technique used to assess the morphology and shape of the engineered particles. Fig. 1 shows the AFM height images of vesicles formed by the spin-coating method. To compare the effects of the encapsulants in a morphological way, other vesicles were prepared beside the Gd-PpIX-NI sample. As can be seen from the figure, tiny spherical spots represent the niosomal vesicles. Moreover, the sizes of vesicles may be seen as smaller than from the DLS data because of the change of the medium condition from aqueous to dry and forming small aggregations during adsorption onto the analysis surfaces.49,50 A further data analysis in the way of particle height and width was carried out by picking seven spots from each figure. The particle height and width curve of each spot are illustrated in Fig. S2† as the raw data of AFM height images.
 | ||
| Fig. 1 AFM height images (2.5 × 2.5 μm) of plain niosome (a), Gd-NI (b), PpIX-NI (c) and Gd-PpIX-NI (d). | ||
3.2 Encapsulation efficiency
PpIX and GdNP encapsulation rates were measured by two different methods. The encapsulated PpIX ratio was determined spectrophotometrically (410 nm) using a calibration curve with a linear equation of y = 0.0067x + 0.167 (R2 = 0.985). The encapsulation efficiency of the engineered niosomes was 66% for both PpIX-NI and Gd-PpIX-NI vesicles. When compared to other carriers for PpIX, the encapsulation capacity of our formulation seems higher.51 Hence, Gd-PpIX-NI may be a good transporter to cancerous areas to perform radiotherapy and photodynamic therapy. Furthermore, Magnevist determinations have been identified in previous studies based on MRI (T1 weighted).52,53 Fig. 2 represents the MR images and histogram of the theranostically engineered vesicles. The histogram of GdNP provides the MRI density data via Image J processing of the image. The ratio between encapsulated GdNPs (Fig. 2 tube D) and positive control (beginning concentration of GdNPs, Fig. 2 tube A) is approximately 80%. As a result, it can be claimed that Gd-PpIX-NI vesicles have potential for the proposed combined therapy of PDT and RT.3.3 Cell culture
3.4 Cell imaging
4. Conclusion
With the growing need for effective and rapid methods in cancer therapy and diagnosis, newly designed modalities are required to remove the disadvantages of applied techniques in tumour treatments. In this concept, NP included systems have become advantageous and researchers are interested in generating novel platforms with many extreme functionalities. Theranostics that can carry many of those functionalities may be helpful to understand and treat cancerous tissues. Hence, in this work, theranostically engineered niosomes that incorporated PpIX for PDT and RT and GdNPs for MRI were synthesized in the first part of study. After characterization of niosomes in terms of size, surface charge and morphology by using DLS and AFM, the cytotoxicity was checked and non-toxic doses were selected for further use for treatment modalities. Separate applications of RT with niosomes could be a guide to decide which ionizing radiation dose was effective. In the final step of treatment modalities (RT and PDT), different combinations of RT and PDT techniques were introduced to A549 and HeLa cells. It was stated that the simultaneous application of RT and PDT (RT–PDT) had the best activity when compared to consecutive applications (RT/PDT and PDT/RT). On the other hand, use of PpIX as a component of the vesicular structure enables fluorescence imaging of the cells. This fact provides an additional functionality besides MR imaging. Therefore, dual imaging could be provided as a result of this approach. All the data obtained for Gd-PpIX-NI vesicles with greater stability at 4 °C indicates that this novel structure could be a versatile, functional theranostic candidate for possible combined therapy and multimodal diagnosis applications. In addition, this study has created a different application field for the encapsulation capabilities of niosomes and various strategies can be easily designed on the basis of this approach.Acknowledgements
This work was supported by Ege University Scientific Research Project (2011/FEN/055).Notes and references
- J. Funkhouser, Curr. Drug Discov., 2002, 2, 17 Search PubMed.
- H. Koo, M. S. Huh, I.-C. Sun, S. H. Yuk, K. Choi, K. Kim and I. C. Kwon, Acc. Chem. Res., 2011, 44, 1018 CrossRef CAS PubMed.
- M. E. Caldorera-Moore, W. B. Liechty and N. A. Peppas, Acc. Chem. Res., 2011, 44, 1061 CrossRef CAS PubMed.
- M. Liong, J. Lu, M. Kovochich, T. Xia, S. G. Ruehm, A. E. Nel, F. Tamanoi and J. I. Zink, ACS Nano, 2008, 2, 889 CrossRef CAS PubMed.
- M. Wang and M. Thanou, Pharmacol. Res., 2010, 62, 90 CrossRef CAS PubMed.
- H. Maeda, Bioconjugate Chem., 2010, 21, 797 CrossRef CAS PubMed.
- L. H. Reddy, J. L. Arias, J. Nicolas and P. Couvreur, Chem. Rev., 2012, 112, 5818 CrossRef CAS PubMed.
- T. Krasia-Christoforou and T. K. Georgiou, J. Mater. Chem. B, 2013, 1, 3002 RSC.
- E. Terreno, D. D. Castelli, A. Viale and S. Aime, Chem. Rev., 2010, 110, 3019 CrossRef CAS PubMed.
- A. J. L. Villaraza, A. Bumb and M. W. Brechbiel, Chem. Rev., 2010, 110, 2921 CrossRef CAS PubMed.
- Y. W. Jun, Y. M. Huh, J. S. Choi, J. H. Lee, H. T. Song, S. Kim, S. Yoon, K. S. Kim, J. S. Shin, J. S. Suh and J. Cheon, J. Am. Chem. Soc., 2005, 127, 5732 CrossRef CAS PubMed.
- J. Kim, H. S. Kim, N. Lee, T. Kim, H. Kim, T. Y. I. C. Song, W. K. Moon and T. Hyeon, Angew. Chem., Int. Ed., 2008, 47, 1 CrossRef.
- M. Liong, J. Lu, M. Kovochich, T. Xia, S. G. Ruehm, A. E. Nel, F. Tamanoi and J. I. Zink, ACS Nano, 2008, 2, 889 CrossRef CAS PubMed.
- R. Kumar, M. Nyk, T. Y. Ohulchanskyy, C. A. Flask and P. N. Prasad, Adv. Funct. Mater., 2009, 19, 853 CrossRef CAS.
- T. Nam, S. Park, S. Y. Lee, K. Park, K. Choi, I. C. Song, M. H. Han, J. J. Leary, S. A. Yuk, I. C. Kwon, K. Kim and S. Y. Jeong, Bioconjugate Chem., 2010, 21, 578 CrossRef CAS PubMed.
- T. Buckle, P. T. K. Chin and F. W. B. Van Leeuwen, Nanotechnology, 2010, 21, 482001 CrossRef PubMed.
- M. J. Hamamura, S. Ha, W. W. Roeck, D. J. Wagenaar, D. Meier, B. E. Patt and O. Nalcioglu, Cancer Res. Treat., 2010, 9, 21 CrossRef.
- K. Kłodowski, J. Kamiński, K. Nowicka and J. Tarasiuk, Computerized Medical Imaging and Graphics, 2014, 38, 458 CrossRef PubMed.
- S. W. Chou, Y. H. Shau, P. C. Wu, Y. S. Yang, D. B. Shieh and C. C. Chen, J. Am. Chem. Soc., 2010, 132, 13270 CrossRef CAS PubMed.
- K. Pinker, P. Brader, G. Karanikas, K. El-Rabadi, W. Bogner, S. Gruber, M. Reisegger, S. Trattnig and T. H. Helbich, Radiologe, 2010, 50, 1030 CrossRef CAS PubMed.
- R. Ackroyd, C. Kelty, N. Brown and M. Reed, Photochem. Photobiol., 2001, 74, 656 CrossRef CAS PubMed.
- M. D. Daniell and J. S. Hill, Aust. N. Z. J. Surg., 1991, 61, 340 CrossRef CAS PubMed.
- J. D. R. V. Spikes, G. Jori, E. J. Land and T. H. Truscott, Primary Photoprocesses in Biology and Medicine, ed. R. V. Bensasson, Springer US Press, NY, USA, 1985, pp. 209–227 Search PubMed.
- J. Moan, J. Photochem. Photobiol., B, 1990, 6, 343 CrossRef CAS.
- R. R. Allison, G. H. Downie, R. Cuenca, X.-H. Hu, C. J. H. Childs and C. H. Sibata, Photodiagn. Photodyn. Ther., 2004, 1, 27 CrossRef CAS PubMed.
- M. Ballico, V. Rapozzi, L. E. Xodo and C. Comuzzi, Eur. J. Med. Chem., 2011, 46, 712 CrossRef CAS PubMed.
- T. J. Dougherty, C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan and Q. Peng, J. Natl. Cancer Inst., 1998, 90, 889 CrossRef CAS PubMed.
- N. S. van den Berg, F. W. van Leeuwen and H. G. van der Poel, Curr. Opin. Neurol., 2012, 22, 846 Search PubMed.
- B. Zhao and Y. Y. He, Expert Rev. Anticancer Ther., 2010, 10, 1797 CrossRef CAS PubMed.
- Z. Luksiene, P. Juzenas and J. Moan, Cancer Lett., 2006, 235, 40 CrossRef CAS PubMed.
- J. H. Ryu, S. Lee, S. Son, S. H. Kim, J. F. Leary, K. Choi and I. C. Kwon, J. Controlled Release, 2014, 190, 477 CrossRef CAS PubMed.
- T. Krasia-Christoforou and T. K. Georgiou, J. Mater. Chem. B, 2013, 1, 3002 RSC.
- R. Kumar, W. S. Shin, K. Sunwoo, W. Y. Kim, S. Koo, S. Bhuniya and J. S. Kim, Chem. Soc. Rev., 2015, 44, 6670 RSC.
- C. Sanson, O. Diou, J. Thevenot, E. Ibarboure, A. Soum, A. Brulet, S. Miraux, E. Thiaudiere, S. Tan, A. Brisson, V. Dupuis, O. Sandre and S. Lecommandoux, ACS Nano, 2011, 5, 1122 CrossRef CAS PubMed.
- S. Li, B. Goins, L. Zhang and A. Bao, Bioconjugate Chem., 2012, 23, 1322 CrossRef CAS PubMed.
- S. T. Lo, A. Kumar, J. T. Hsieh and X. Sun, Mol. Pharm., 2013, 10, 793 CrossRef CAS PubMed.
- H. Sharma, P. K. Mishra, S. Talegaonkar and B. Vaidya, Drug Discovery Today, 2015, 20, 1143 CrossRef CAS PubMed.
- C. A. J. Hunter, J. Pharm. Pharmacol., 1988, 33, 161 CrossRef.
- R. Nur Un, F. B. Barlas, M. Yavuz, D. Ag Seleci, M. Seleci, Z. P. Gumus, E. Guler, B. Demir, M. Can, H. Coskunol and S. Timur, Int. J. Polym. Mater., 2015, 64, 927 CrossRef.
- M. A. Fadel and A. A. Tawfik, Clin. Exp. Dermatol., 2015, 40, 116 CrossRef CAS PubMed.
- M. Akin, R. Bongartz, J. G. Walter, D. Odaci Demirkol, F. Stahl, S. Timur and T. Scheper, J. Mater. Chem., 2012, 22, 11529 RSC.
- J. W. Chang, K. H. Park, H. S. Hwang, Y. S. Shin, Y. T. Oh and C. H. Kim, J. Radiat. Res., 2014, 55, 245 CrossRef CAS PubMed.
- K. Morimoto, T. Ozawa, K. Awazu, N. Ito, N. Honda, S. Matsumoto and D. Tsuruta, PLoS One, 2014, 9, 1 Search PubMed.
- J. Sabın, G. Prieto and F. Sarmiento, Soft Matter, 2012, 8, 3212 RSC.
- J. Sabín, G. Prieto, S. Sennato, J. M. Ruso, R. Angelini, F. Bordi and F. Sarmiento, Phys. Rev. E, 2006, 74, 031913 CrossRef PubMed.
- R. Agarwal, O. P. Katare and S. P. Vyas, Int. J. Pharm., 2001, 228, 43 CrossRef CAS PubMed.
- I. P. Kaur, A. K. Mitra and D. Aggarwal, J. Drug Delivery Sci. Technol., 2007, 17, 33 CrossRef CAS.
- A. Shahiwala and A. Misra, J. Pharm. Pharm. Sci., 2002, 5, 220 CAS.
- B. Ruozi, D. Belleti, A. Tombesi, G. Tosi, L. Bondioli, F. Forni and M. A. Vandelli, Int. J. Nanomed., 2011, 6, 557 CrossRef CAS PubMed.
- O. Teschke, Langmuir, 2002, 18, 6513 CrossRef CAS.
- E. K. Oh, S. E. Jin, J. K. Kim, J. S. Park, Y. Park and C. K. Kim, Eur. J. Pharm. Sci., 2011, 44, 149 CrossRef CAS PubMed.
- S. Li, B. Goins, L. Zhang and A. Bao, Bioconjugate Chem., 2012, 20, 1322 CrossRef PubMed.
- J. Guenoun, A. Ruggiero, G. Doeswijk, R. C. Janssens, G. A. Koning, G. Kotek, G. P. Krestin and M. R. Bernsen, Contrast Media Mol. Imaging, 2013, 8, 165 CrossRef CAS PubMed.
- T. Kitagawa, J. Yamamoto, T. Tanaka, Y. Nakano, D. Akiba, K. Ueta and S. Nishizawa, Oncol. Rep., 2015, 33, 583 CAS.
- M. Dizdaroglu, Free Radical Biol. Med., 1991, 10, 225 CrossRef CAS PubMed.
- F. Geng, K. Song, J. Z. Xing, C. Yuan, S. Yan, Q. Yang, J. Chen and B. Kong, Nanotechnology, 2011, 22, 285101 CrossRef PubMed.
- J. F. Hainfeld, D. N. Slatkin and H. M. Smilowitz, Phys. Med. Biol., 2004, 49, 309 CrossRef.
- W. Rima, L. M. Sancey, T. Aloy, E. Armandy, G. B. Alcantara, T. Epicier, A. Malchère, L. Joly-Pottuz, P. Mowat, F. Lux, O. Tillement, B. Burdin, A. Rivoire, C. Boulé, I. Anselme-Bertrand, J. Pourchez, M. Cottier, S. Roux, L. C. Rodriguez and P. Perriat, Biomaterials, 2013, 34, 181 CrossRef CAS PubMed.
- J. Yamamoto, S. Ogura, T. Tanaka, T. Kitagawa, Y. Nakano, T. Saito, M. Takahashi, D. Akiba and S. Nishizawa, Oncol. Rep., 2012, 27, 1748 CAS.
- K. Berg, Z. Luksiene, J. Moan and L. Ma, Radiat. Res., 1995, 142, 340 CrossRef CAS PubMed.
- J. Takahashi, M. Misawa, M. Murakami, T. Mori, K. Nomura and H. Iwahashi, SpringerPlus, 2013, 2, 602 CrossRef PubMed.
- M. K. Khaing Oo, Y. Yang, Y. Hu, M. Gomez, H. Du and H. Wang, ACS Nano, 2012, 6, 1939 CrossRef CAS PubMed.
- C. A. Robertson, D. H. Evans and H. Abrahamse, J. Photochem. Photobiol., B, 2009, 96, 1 CrossRef CAS PubMed.
- W. Ng, Q. Huang, X. Liu, M. Zimmerman, F. Li and C. Li, Transl. Cancer Res., 2013, 2, 442 CAS.
- E. Guler, F. B. Barlas, M. Yavuz, B. Demir, Z. P. Gumus, Y. Baspinar, H. Coskunol and S. Timur, Colloids Surf., B, 2014, 121, 299 CrossRef CAS PubMed.
- B. Demir, F. B. Barlas, E. Guler, P. Z. Gumus, M. Can, M. Yavuz, H. Coskunol and S. Timur, RSC Adv., 2014, 4, 34687 RSC.
- A. P. Valdes, A. Kim, M. Brantsch, C. Niu, Z. B. Moses, T. D. Tosteson, B. C. Wilson, K. D. Paulsen, D. W. Roberts and B. T. Harris, Neuro-Oncology, 2011, 13, 846 CrossRef PubMed.
- H. Xing, S. Zhang, W. Bu, X. Zheng, L. Wang, Q. Xiao, D. Ni, J. Zhang, L. Zhou, W. Peng, K. Zhao, Y. Hua and J. Shi, Adv. Mater., 2014, 26, 3867 CrossRef CAS PubMed.
- E. Peng, F. Wang and J. M. Xue, J. Mater. Chem. B, 2015, 3, 2241 RSC.
- N. Ding, Y. L. Robert, J. L. Chang, Y. Lei, H. Jian and G. L. Xiang, Int. J. Nanomed., 2011, 6, 2513 CrossRef CAS PubMed.
- H. Kobayashi, S. Kawamoto, M. Bernardo, M. W. Brechbiel, M. V. Knopp and P. L. Choyke, J. Controlled Release, 2006, 111, 343 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra26737d |
| This journal is © The Royal Society of Chemistry 2016 |